14
Emerging Functional Foods Derived from Almonds
Isabela Mateus Martins, Qianru Chen, and C. Y. Oliver Chen
Antioxidants Research Laboratory, Jean Mayer USDA Human Nutrition Research Center on Aging, Tufts University, USA
14.1 Introduction
The development of functional foods with constituents that exert beneficial bioactions in health promotion and prevention is a field in expansion. The increase in their developments is attributed mainly to the association of their consumption with reduced risk of chronic diseases (Shahidi 2009). This great attribute of functional foods in health promotion and prevention is very appealing to consumers with an awareness of maintaining/promoting health through foods. Thus, the demand for functional foods is increasing because consumers believe that foods can contribute directly to their health and life quality (Betoret et al. 2011; Siegrist et al. 2008; Siró et al. 2008).
Functional foods are spread among all different food sectors, from breakfast cereals to dairy products and from processed meats to beverages. Functional ingredients in both original and derivative forms can be incorporated into a wide range of food products. Popular functional ingredients include fibers, polyphenol‐rich extracts, and food with well‐known human health benefits such as berries and dark cocoa. In recent decades, tree nuts have been recognized as a food group with multiple health benefits, ranging from cholesterol reduction to blood glucose control. Among all tree nuts, almonds have been regarded as the epitome of healthy foods because they are a rich source of protein, monounsaturated fatty acids, dietary fiber, vitamin E, riboflavin, and essential minerals as well as phytosterols and polyphenols (Kendall et al. 2010; Yada et al. 2013). All of these nutrients/nonnutrients and other unidentified constituents work together in a synergistic manner to make almonds an ingredient ready for incorporation into functional foods. There is a great body of clinical evidence showing that almond consumption is inversely associated with several risk factors for chronic disease, i.e. dyslipidemia, hyperglycemia, oxidative stress, inflammation, and overweight/obesity. While the clinical evidence still needs to be gathered, fiber and polyphenols in almonds with bacteria‐modulating properties may also help maintain/promote gut health by serving as prebiotics.
This chapter will provide an overview of the diverse bioactions of almonds and their nutrients and discuss how almonds can be used in the development of functional products.
14.2 Overview of Almond Nutrients
Almonds are a nutrient‐dense food, as defined by the US Food and Drug Administration (FDA) because of the rich content of multiple nutrients (Chen et al. 2006). Almonds are an excellent source of magnesium and α‐tocopherol (containing >20% of the daily value (DV) (FDA 2013)) and a good source of protein, phosphorus, fiber, copper, and riboflavin (containing 10−20% DV in one serving – around 28 g) (Figure 14.1).
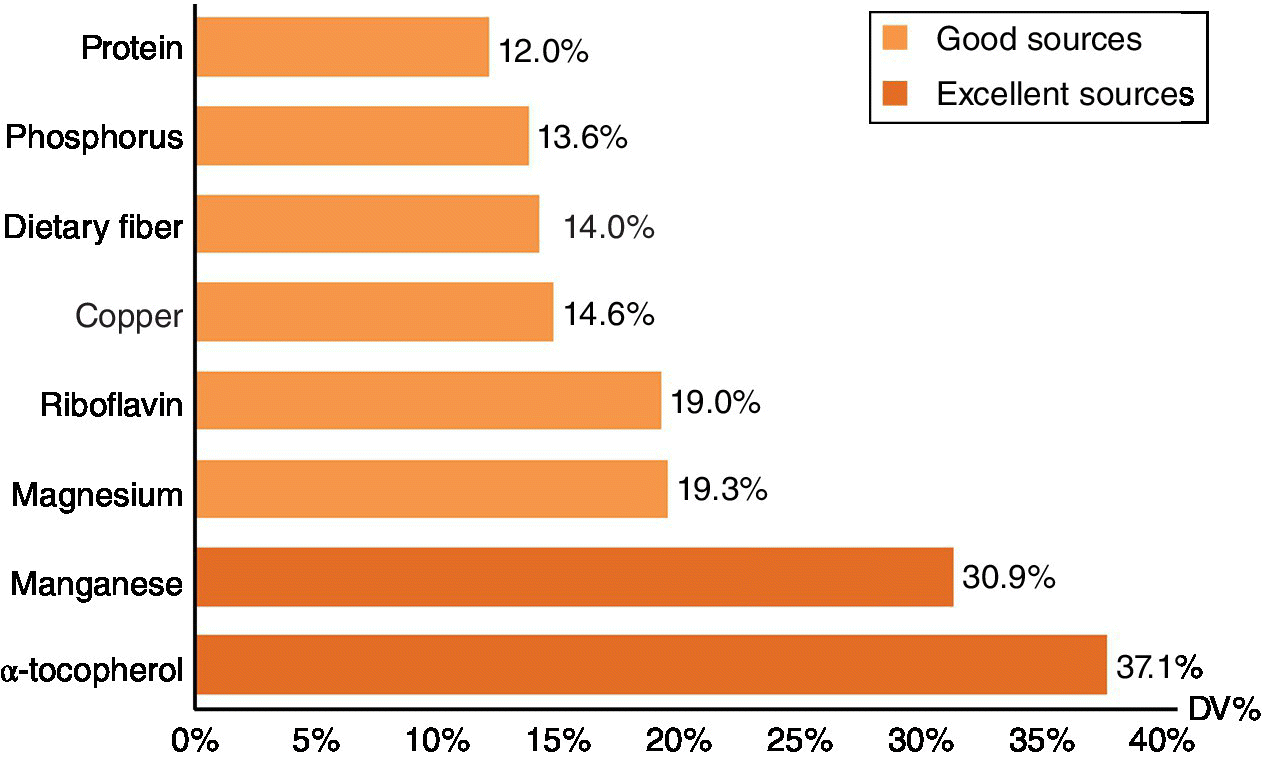
Figure 14.1 The percentage of daily value (DV) of the selected nutrients in 28 grams (1 serving) of almonds.
The energy provided by almonds is derived largely from the fat content, ranging from 25 to 66 g/100 g fresh weight (Yada et al. 2011). It is worth noting that fats in almonds comprise mainly monounsaturated fatty acids (MUFA) at 63.2%, and polyunsaturated fatty acid (PUFA) at 24.7% (USDA 2014). Further, almonds are free of cholesterol. Like olive oil, oleic acid is the predominant fatty acids in almonds. Also, almonds are appreciated as a vitamin‐rich food because one serving (28 g) can provide half the recommended daily amount (RDA) (Hellwig 2006) of α‐tocopherol (7.5 mg). Almonds are also packed with many B vitamins, e.g. riboflavin, niacin, thiamine, pantothenic acid, pyridoxine, and folates. Polyphenols, which display an array of bioactions, including antioxidation, antiinflammation, and glucoregulation, have been characterized in almonds (Milbury et al. 2006). Polyphenols are mainly present in the skins but the content varied widely between cultivars, ranging from 127 (Fritz) to 241 (Padre) mg gallic acid equivalent/100 g of fresh weight (Milbury et al. 2006). Among flavonoids, flavanols and flavonol glycosides were the most abundant, comprising up to 38–57% and 14–35% of the total quantified polyphenols, respectively (Monagas et al. 2007).
14.3 Health Benefits and Bioactions of Almonds
Almonds and other tree nuts and peanuts were previously considered unhealthy foods, mainly due to their high fat content, which may cause unwanted weight gain. Since the early 1990s, the health benefits of their consumption have been increasingly documented in clinical trials. Almonds are associated with a reduction in blood cholesterol and glucose, biomarkers of oxidative stress and inflammation. Further, they can be incorporated into dietary regimes for weight loss or maintenance. The putative mechanisms by which almonds and their constituents protect against risk factors of chronic diseases are demonstrated in Figure 14.2. All of this evidence supports the recommendation for their incorporation into functional foods.
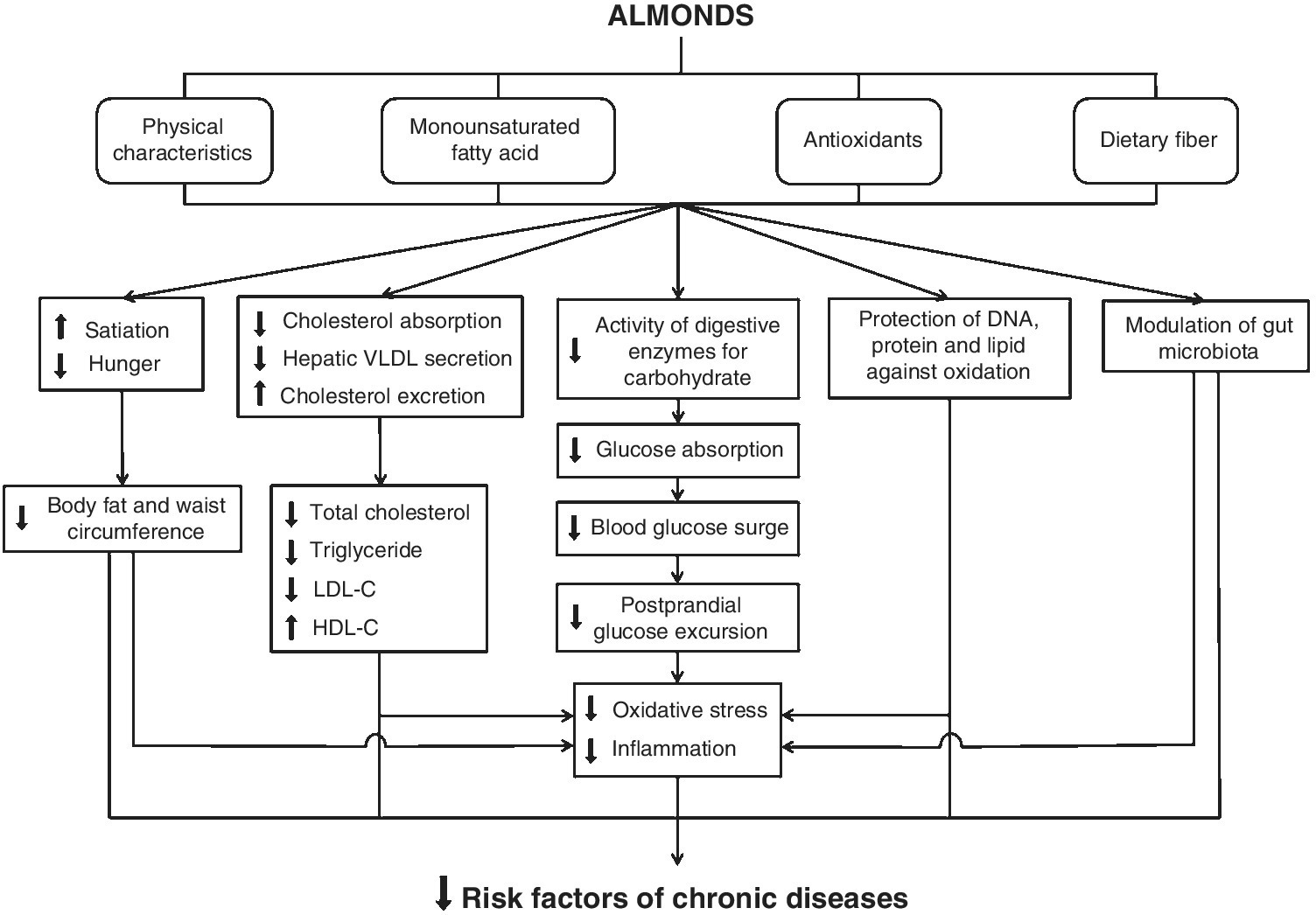
Figure 14.2 Putative mechanisms by which almonds and their constituents protect against risk factors for chronic diseases.
14.3.1 Cholesterol Reduction
In recent decades, many clinical trials have consistently demonstrated that almonds are beneficial to blood cholesterol control, lipid profile, and lipoproteins in different populations, including healthy individuals (Abbey et al. 1994; Berryman et al. 2015; Hyson et al. 2002; Jaceldo‐Siegl et al. 2011) and patients with hypercholesterolemia and diabetes (Damasceno et al. 2011; Jenkins et al. 2003, 2008; Li et al. 2011; Spiller et al. 1998, 2003; Tamizifar et al. 2005). The main results of these studies can be found in Table 14.1. This hypocholesterolemic effect of almonds in both free‐living and controlled study settings has been extensively reviewed by Berryman et al. (2011) and Kamil and Chen (2012). In summary, almonds lower low‐density lipoprotein cholesterol (LDL‐C) by 2.9% to 35.0% and total cholesterol (TC) by 1.5% to 35.0%, in a dose‐dependent manner. However, the effect on high‐density lipoprotein cholesterol (HDL‐C) is still uncertain. Some studies have showed an increase in HDL‐C by up to 8.1% (Foster et al. 2012) while others did not show any change (Abbey et al. 1994; Damasceno et al. 2011; Li et al. 2011; Spiller et al. 1998). The differences in subject ethnicity, study duration, background diet, and almond dosage could all contribute to the inconsistency.
Table 14.1 Effect of almonds on cholesterol and lipoprotein profile in clinical interventions.
| Design | Subjects | Duration (week) | Intervention | TG* | TC* | LDL‐C* | HDL‐C | Reference |
| Cross‐over | Healthy, elevated LDL‐C, 47.5 years, 26 F, 22 M |
6 | 42.5 g/day almonds vs isocaloric muffin substitution (no almonds/day) | ↓ 7.2% | ↓ 5.1% | ↓ 5.3% | ↑ 1.7% | Berryman et al. 2015 |
| Parallel | Overweight and obese, 37.5 years, 108 F | 12 | 50 g/day almonds vs baseline | ↓ 46.0% | ↓ 35.0% | ↓ 8.7% | – | Abazarfard et al. 2014 |
| Parallel | Overweight and obese, 46.8 years, 112 F, 11 M | 24 and 72 | Hypocaloric or almond‐enriched diet vs baseline | ↓ 12.6% ↓ 4.4% |
↓ 4.4% ↓ 1.2% |
↓ 4.7% ↓ 2.7% |
↑ 0.1% ↑ 8.1% |
Foster et al. 2012 |
| Cross‐over | Hypercholesterolemic, 50 years, 9 F, 9 M | 4 | Almonds (22% of total calories) vs baseline | – | ↓ 6.4% | ↓ 13.4% | – | Damasceno et al. 2011 |
| Parallel | Healthy, 49.4 years, 38 F, 43 M | 24 | Almond (15% of total calories) vs habitual diet | ↓ 1.0% | ↓ 1.5% | ↓ 2.9% | ↑ 1.4% | Jaceldo‐Siegl et al. 2011 |
| Cross‐over | T2DM, mild hyperlipidemia, 58 years, 11 F, 9 M |
4 | Almonds (20% of total calories) vs NCEP Step 2 diet | – | ↓ 6.0% | ↓ 11.6% | – | Li et al. 2011 |
| Cross‐over | Hyperlipidemic, 64 years, 12 F, 15 M |
4 | 73 g whole almonds or 37 g whole almonds vs no almonds |
↓ 12.2% ↓ 8.5% |
↓ 3.6% ↓ 2.9% |
↓ 2.8% ↓ 5.0% |
↑ 1.5% ↑ 2.8% |
Jenkins et al. 2008 |
| Cross‐over | Healthy, 41 years, 8 F, 8 M | 4 | Almonds (10% or 20% of calories) vs control diet | – | ↓ 4.5% | ↓ 7.1% | – | Jambazian et al. 2005 |
| Cross‐over | Hyperlipidemic, 56 years, 13 F, 17 M |
4 | 25 g/day almonds vs NCEP Step 1 diet |
↓ 7.2% | ↓ 5.9% | ↓ 12.3% | ↑ 1.5% | Tamizifar et al. 2005 |
| Cross‐over | Healthy and mildly hypercholesterolemic, 41 years, 11 F, 14 M | 4 | 68 g/day almonds vs NCEP Step 1 diet |
– | ↓ 4.4% | ↓ 7.0% | – | Sabaté et al. 2003 |
| Parallel | Hyperlipidemic, 60 years, 9 F, 16 M |
4 | Portfolio diet (with 16.6 g/1000 kcal almonds) vs NCEP Step 2 diet | – | ↓ 26.6% | ↓ 35.0% | – | Jenkins et al. 2003 |
| Cross‐over | Healthy, 43.5 years, 12 F, 10 M | 6 | 66 g/day whole almonds or 35 g/day almond oil vs baseline |
↓ 14.5% ↓ 15.3% |
↓ 4.3% ↓ 4.5% |
↓ 6.6% ↓ 7.0% |
↑ 4.3% ↑ 6.9% |
Hyson et al. 2002 |
| Cross‐over | Hypercholesterolemic, 64 years, 12 F, 15 M | 4 | 28 g/day or 56 g/day almonds vs baseline | – | ↓ 3.4% ↓ 5.6% |
↓ 4.4% ↓ 9.9% |
↑ 4.6% ↑ 3.8% |
Jenkins et al. 2002 |
| Parallel | Hypercholesterolemic, 53 years, 33 F, 12 M | 4 | 100 g/day almonds vs control diet |
– | ↓ 15.6% | ↓ 19.0% | – | Spiller et al. 1998 |
| Parallel | Healthy, 41.3 years, 16 M | 9 | 84 g/day raw almonds vs baseline |
– | ↓ 7.0% | ↓ 10.3% | – | Abbey et al. 1994 |
*– no change; ↑ increase ↓ decrease.
F, female; HDL‐C, high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; M, male; NCEP, National Cholesterol Education Program; T2DM, type 2 diabetes mellitus; TC, total cholesterol; TG, triglycerides.
The fatty acid profile of almonds has been generally accepted as the primary mechanism responsible for the improvement in lipid profile (Berryman et al. 2011; Chen et al. 2006; Griel & Kris‐Etherton 2007; Kamil & Chen 2012; Kris‐Etherton et al. 2009; Sabaté et al. 2010). This notion is supported by a study by Hyson et al. (2002) illustrating that replacing half of their habitual fat (approximately 29% energy) for six weeks with either whole almonds or almond oil decreased LDL‐C, TC, and triglycerides (TG) and increased HDL‐C by a similar degree in 22 normolipemic men and women. Nishi et al. (2014) reported that incorporating almonds into a National Cholesterol Education Program (NCEP) Step 2 diet to replace ~10% or 20% of energy increased oleic acid and other unsaturated fatty acid contents in serum, total triglycerides, and nonesterified fatty acid fractions in hyperlipidemic adults. They further suggested that these changes in fatty acid profile could contribute to reduced risk for coronary heart disease (CHD).
While the mechanism of action by which almonds improve the lipid profile has not been elucidated, it has been suggested that the positive effect of unsaturated fatty acids in almonds on hepatic very low‐density lipoprotein (VLDL) production or/and VLDL lipolysis might contribute to a downstream reduction in LDL‐C (Berryman et al. 2011; Foster et al. 2012; Spiller et al. 2003). Thus, the benefits of almonds on lipid profile can be ascribed mainly to their favorable lipid composition. Nevertheless, it should be noted that the magnitude of improvement is larger than the effect of almond lipids alone (Abbey et al. 1994; Berryman et al. 2015; Hyson et al. 2002; Jenkins 2002; Lovejoy et al. 2002; Sabaté et al. 2003; Spiller et al. 2003), suggesting that constituents other than lipids may make a contribution (Berryman et al. 2011; Griel & Kris‐Etherton 2007; Sabaté et al. 2010). A more recent controlled feeding trial confirmed that almonds (42.5 g/day for six weeks) decreased LDL‐C, TC, and TG and maintained HDL‐C in patients with hypercholesterolemia, compared with a cholesterol‐lowering control diet (Berryman et al. 2015),
In addition to the favorable lipid composition, other nutrients in almonds may play a role in the cholesterol lowering effect. As noted in the above nutrients section, almonds are a good protein source (Ahrens et al. 2005; Chen et al. 2006). Replacing dietary carbohydrates with proteins has been reported to be beneficial to LDL‐C in both normolipidemic and hypercholesterolemic individuals (Appel et al. 2005), probably through an inhibition on hepatic VLDL secretion (Berryman et al. 2011). Almonds are a good source for dietary fiber. Of all tree nuts, almonds have the highest dietary fiber content. Its cholesterol lowering benefit has been well appreciated. The insoluble fibers in almonds help reduce LDL‐C concentration by decreasing intestinal transit time and improving satiation (Hollis & Mattes 2007). Finally, phytosterols in almonds can help improve lipid profile by increasing cholesterol excretion and decreasing cholesterol absorption (Berryman et al. 2011).
14.3.2 Glucose Regulation
Almonds are regarded as a low glycemic index (GI) food because of their low available carbohydrate content, as well as their healthy lipid profile and high quantity of vegetable proteins, fibers, and magnesium. Therefore, almonds appear to be an appropriate food to be included in a diabetes management plan, and there have been some clinical trials examining the effect of almonds on glycemic control in healthy people and patients with diabetes (Table 14.2).
Table 14.2 Effect of almonds on glucose regulation and body weight control in clinical interventions.
| Design | Subjects | Duration (week) |
Intervention | Results* | Reference |
| Cross‐over | Healthy, elevated LDL‐C, 47.5 years, 26 F, 22 M | 6 | 42.5–g/day almonds vs isocaloric muffin substitution (no almond) |
|
Berryman et al. 2015 |
| Parallel | T2DM, 50 years, 12 F, 9 M | 12 | 42.5 g/day almonds vs no almond |
|
Sweazea et al. 2014 |
| Parallel | Increased risk for T2DM, 39 years, 89 F, 48 M | 4 | 43 g/day almonds with breakfast/ lunch vs 43 g/day almonds alone as a morning or afternoon snack vs no almonds |
|
Tan & Mattes 2013 |
| Cross‐over | Impaired glucose tolerant, 39 years, 14 subjects | 10 | 42.5 g whole almonds (WO), almond butter (AB), defatted almond flour (AF) or almond oil (AO) vs composite meal |
|
Mori et al. 2011 |
| Cross‐over | Diabetics, 3 F, 4 M, nondiabetics, 11 F, 2 M |
12 | 28 g almonds vs baseline |
|
Cohen & Johnston 2011 |
| Cross‐over | T2DM, mild hyperlipidemia, 58 years, 11 F, 9 M |
4 | Almonds (20% of total calories) vs NCEP Step 2 diet |
|
Li et al. 2011 |
| Parallel | Overweight and obese, 53 years, 65 adults | 24 | Low calorie diet + 84 g/day of almonds vs complex carbohydrates |
|
Wien et al. 2003 |
| Cross‐over | T2DM, 35 years, 17 F, 13 M | 4 | High‐fat, high‐almond (37% total fat, 10% from almonds) vs low‐fat, high‐almond (25% total fat, 10% from almonds) vs high‐fat control vs low‐fat control |
|
Lovejoy et al. 2002 |
* – no change; ↑ increase ↓ decrease.
BMI, Body Mass Index; F, female; HOMA, Homeostasis Model Assessment; LDL‐C, low‐density lipoprotein cholesterol; M, male; NCEP, National Cholesterol Education program; NEFA, nonesterified fatty acids; T2DM, type 2 diabetes mellitus.
Almonds are capable of modulating the GI of co‐consumed foods. Josse et al. (2007) found that almonds decreased the GI of white bread in a dose‐dependent manner. The GI‐modulating effect can be extended to meals containing a more complex nutrient profile than carbohydrate‐rich white bread. In a four‐week randomized, parallel designed trial with 137 healthy adults consuming almonds (43 g/day) with breakfast or lunch or alone as morning or afternoon snacks, Tan and Mattes (2013) observed a decrease in the postprandial glucose response 60 minutes after ingestion. Glycemic control is crucial to those who have impaired glucose regulation, e.g. patients with diabetes and metabolic syndrome. Cohen and Johnston (2011) reported in an acute trial that almonds (1 serving, 28 g) consumed immediately before a starchy meal significantly reduced postprandial glycemic response in patients with type 2 diabetes mellitus. They also found in a longer term study that after 12 weeks of almond consumption (28 g/day for five days/week), HbA1c was significantly reduced by 4% compared to the baseline. Li et al. (2011) also noted in a controlled feeding study that replacing 20% of dietary calories with almonds led to significant decreases in fasting blood glucose, insulin, and homeostatic model assessment (HOMA) in patients with type 2 diabetes. Interestingly, Lovejoy et al. (2002) did not find any change in glycemic status, insulin sensitivity, and HbA1c in patients with type 2 diabetes who consumed almonds to replace 10% daily energy need for four weeks. The conflicting results might be attributed to almond dose, diabetes duration, and study design.
The constituents in almonds contributing to the blood glucose‐modulating effect have not been fully elucidated. Mori et al. (2011) suggested that the modulation is most likely due to the high unsaturated fat content. This suggestion was based on the results of a human study showing that almond oil, rather than almond butter and defatted almond flour, exhibited the same degree of suppressive effect on postprandial glucose response as whole almond. The low and delayed postprandial blood glucose response might be a consequence of almond lipid‐mediated reduction in the breakdown rate of complex carbohydrates through its inhibitory effect on gastric emptying (Tan & Mattes 2013). In addition to the almond lipids, polyphenols and phytates in whole almonds can inhibit carbohydrate digestive enzymes, an action resulting in a decrease in overall glucose absorption and subsequent blood glucose surge (Lo Piparo et al. 2008; Yoon et al. 1983). Thus, almonds may help decrease the incidence of metabolic syndrome, type 2 diabetes, and cardiovascular disease via the bioactions of glucoregulation because lowering postprandial glucose excursion could decreases the risk of oxidative damage to lipids and proteins (Jenkins et al. 2006).
14.3.3 Antiinflammation
Inflammation is one of the mechanisms involved in the development and progression of atherosclerosis and insulin resistance (Danesh et al. 2004; Festa et al. 2002). Inflammatory markers, such as C‐reactive protein (CRP), interleukin‐6 (IL‐6), fibrinogen, vascular cell adhesion molecule‐1 (VCAM‐1), and intracellular adhesion molecule‐1 (ICAM‐1), have been identified as independent predictors for cardiovascular disease or type 2 diabetes (Asegaonkar et al. 2011; Luc et al. 2003; Pradhan & Ridker 2002; Pradhan et al. 2001; Soinio et al. 2006; Zhang et al. 2009). Due to their favorable nutrient profile, almonds have been shown to diminish inflammation via direct and indirect mechanisms (e.g. ameliorating glucose dysregulation).
Sweazea et al. (2014) determined the effect of almonds on the biomarkers of diabetes and cardiovascular disease in patients with type 2 diabetes in a randomized, parallel design study. They found that after consumption of 42.5 g almonds/day, 5 days/week for 12 weeks, CRP was reduced by ~30% (p = 0.029) compared to no dietary change, and IL‐6 and tumor necrosis factor (TNF)‐α were not affected. In agreement with this study, Liu et al. (2013) illustrated in a randomized, cross‐over, controlled feeding trial with Chinese patients with type 2 diabetes and mild hyperlipidemia that in comparison with the NCEP Step 2 diet, the incorporation of almonds to replace 20% daily calories significantly decreased IL‐6 and CRP and tended to decrease TNF‐α (Liu et al. 2013). In addition, Rajaram et al. (2010) reported in a randomized, controlled, cross‐over feeding study with 25 healthy Americans that compared to a nut‐free diet, almonds replacing 10% and 20% of daily calories lowered CRP and E‐selectin in a dose‐independent manner. In contrast, Estruch et al. (2006) found in a PREDIMED study of 772 free‐living asymptomatic adults that three months consumption of a Mediterranean diet including mixed nuts (30 g/day of walnuts, hazelnuts, and almonds) did not change CRP, but reduced circulating IL‐6, ICAM‐1, and VCAM‐1. More information about the results of these studies is given in Table 14.3. The apparent inconsistency in inflammatory responses to almond or nut consumption indicates the complexity of the inflammatory network and suggests inclusion of multiple inflammatory biomarkers to test hypotheses in clinical trials.
Table 14.3 Effect of almonds on inflammation and antioxidation in clinical interventions.
| Design | Subjects | Duration (week) | Intervention | Results* | Reference |
| Parallel | T2DM, 50 years, 9 M, 12 F | 12 | 42.5 g/day almonds vs no almonds |
|
Sweazea et al. 2014 |
| Cross‐over | T2DM, 58 years, 9 M, 11 F | 4 | 56 g/day almonds (20% of calories) vs control diet |
|
Liu et al. 2013 |
| Cross‐over | Healthy, 37.5 years, 14 F, 11 M | 4 | Almonds (10% or 20% of calories) vs baseline |
|
Rajaram et al. 2010 |
| Parallel | High cardiovascular risk, 67.5 years, 433 F, 339 M |
12 | Mediterranean diet, mixed nuts (30 g/day walnuts, hazelnuts, and almonds) vs baseline |
|
Estruch et al. 2006 |
| Parallel | 20 M (56 years), 20 M (22 years), 20 M (27 years) with two or more CV risk factors, 15 M above 18 years (control) | 4 | 50 g/day almonds vs habitual diet |
|
Choudhury et al. 2014 |
| Cross‐over | T2DM, mild hyperlipidemia, 58 years, 11 F, 9 M |
4 | Almonds (20% calories) vs NCEP Step 2 diet |
|
Li et al. 2011 |
| Cross‐over | Healthy soldiers, smokers, 21.5 years, 60 M |
4 | 84 g/day almonds vs 120 g/day pork |
|
Li et al. 2007 |
| Cross‐over | Healthy, 35.5 years, 8 F, 7 M |
5 study sessions, 4 h/session |
2 bread control meals 3 test meals: almonds and bread; parboiled rice; instant mashed potatoes |
|
Jenkins et al. 2006 |
| Cross‐over | Healthy, 41 years, 8 F, 8 M |
4 | Almonds (10% or 20% of calories) vs control diet |
|
Jambazian et al. 2005 |
* ‐ no change; ↑ increase ↓ decrease.
CRP, C‐reactive protein; CV, cardiovascular; F, female; ICAM, intracellular adhesion molecule; IL, interleukin; M, male; NCEP, National Cholesterol Education Program; T2DM, type 2 diabetes mellitus; TNF, tumor necrosis factor; VCAM, vascular cell adhesion molecule.
It is quite challenging to characterize in a whole food concept which nutrients are responsible for decreasing inflammation. Antioxidant vitamins, fiber, L‐arginine, magnesium, and phytochemicals in almonds may all work together to exert antiinflammatory actions (Calder et al. 2009; Casas‐Agustench et al 2010; Jiang et al. 2006; Lucotti et al. 2009; Salas‐Salvadó et al. 2008; Singh et al. 2005; Wells et al. 2005). Furthermore, the observed antiinflammatory effect may simply be secondary to the improvements in blood cholesterol and glucose in patients with metabolic disorders. Thus, more studies are warranted to elucidate the mechanism of action for the reductions in inflammatory biomarkers.
14.3.4 Antioxidation
Almonds contain a variety of antioxidant phytochemicals, including phenolic compounds and α‐tocopherol, which have been inversely linked to risk factors for chronic diseases, such as cardiovascular diseases and diabetes (Kendall et al. 2010; Ros 2009). However, it should be noted that, as for the above‐mentioned antiinflammatory benefits, the antioxidative effects of almonds may be due to the reduction in oxidative stress secondary to the overall improvement in wellbeing. The benefits of almonds in oxidative stress status have been demonstrated in healthy individuals and patients with chronic disease, and such evidence has been reviewed by Chen et al. (2006), Mirrahimi et al. (2011), and Kamil and Chen (2012). As almonds are one of the richest sources of vitamin E, their consumption has been linked to elevated α‐tocopherol status in a dose‐dependent manner (Jambazian et al. 2005).
As LDL oxidation plays a significant role in atherogenesis, one approach to enhancing the resistance of LDL to oxidation is to augment lipophilic antioxidants in LDL particles. As anticipated because of the high α‐tocopherol content, almond consumption is linked to elevated resistance of LDL to oxidation in hyperlipidemic and normolipidemic people (Kamil & Chen 2012). Polyphenols in almonds, mostly flavonoids, such as flavanols and flavonol glycosides in the skin, may also contribute to increasing the antioxidant defense network by acting as antioxidants or by modulating endogenous antioxidant defenses. The predominant flavonoid present in almonds is isorhamnetin rutinoside (Milbury et al. 2006). It is worth noting that absorbed polyphenols might work with other antioxidants such as vitamin C and E in a synergistic manner to protect susceptible molecules against radical attack (Chen et al. 2005).
Besides protection of DNA and lipid, almond nutrients can protect proteins against radical attack or conjugation with aldehydes. For example, Jenkins et al. (2006) observed that protein thiol concentration in serum was increased following almond ingestion, suggesting less oxidative protein damage. As almond consumption is associated with reduced glycemic excursion in healthy people and improved glucoregulation in patients with type 2 diabetes, amelioration of oxidative stress may be secondary to the improvement in hyperglycemia (Josse et al. 2007; Li et al. 2011).
Normal endothelial functions are important to prevent/protect against development and progression of atherosclerosis. Abnormalities in endothelial function originate from many factors, with oxidative stress and inflammation being the best established. Choudhury et al. (2014) reported for the first time that almonds (50 g/day for four weeks) improved endothelial function, which was assessed using flow‐mediated dilation technique in asymptomatic healthy young and middle‐aged men with two or more cardiovascular risk factors. They also noted that systolic blood pressure was improved by almonds. While the exact mechanism of action for the improvement remains to be elucidated, the effect of almond nutrients on oxidative stress and inflammation may have some contributions in this regard (see Table 14.3).
The overall data suggest that α‐tocopherol and polyphenols as the main antioxidants work together in an additive manner to protect lipid, DNA, and protein from oxidation. It should be noted that almonds may also help decrease oxidative stress status via improvements in hyperlipidemia and hyperglycemia which are associated with the production of reactive oxygen species.
14.3.5 Body Weight Control
Almonds have historically been perceived as a food causing unwanted weight gain because of their high fat content. However, this perception is changing because their consumption does not link with weight gain but rather is associated with reduced Body Mass Index (BMI) and their inclusion in weight‐maintaining diets is therefore recommended (Bes‐Rastrollo et al. 2009).
According to the reviews of Sabaté (2003), Rajaram and Sabaté (2006), and Kamil and Chen (2012), the inclusion of almonds in the diet without any advice or restriction resulted in no significant change in body weight or BMI. These results suggest that the additional energy derived from almonds was displaced by reduced consumption of other foods, a food displacement effect. This mechanism is further substantiated by a 12‐week randomized, parallel‐arm controlled clinical trial, in which patients with type 2 diabetes in the almond group tended to consume fewer carbohydrates (Sweazea et al. 2014). The displacement effect can be ascribed to increased satiety and fullness and decreased hunger after almond consumption, which are good attributes for weight loss and maintenance. Cassady et al. (2009) observed that as a hard food, almonds could extend mastication time, which in turn elevates satiety, suppresses hunger, and modulates the release of gut hormones such as cholecystokinin, glucagon‐like peptide‐1, and peptide YY. Similarly, Hull et al. (2015) found that adding almonds (28 or 42 g/day) as a mid‐morning snack for three days decreased the amount of foods consumed during lunch and dinner, whose calories were equivalent to the 173 and 259 kcals consumed as almonds. Further, the subjective appetite ratings measured between the snack and lunch were higher in a dose‐dependent manner. Although half the weight of almonds is lipids, these lipids are not so bioaccessible and remain unavailable during the whole digestion process because of the structural barriers of cell walls that impede the penetration of digestive enzymes (Berry et al. 2008; Grundy et al. 2015).
Although it was developed more than 100 years ago by Atwater and Bryant (1900), the Atwater factor system is still widely employed to estimate the energy value of foods. However, Novotny et al. (2012) has proved with very astonishing evidence that the Atwater factor, when applied to almonds, resulted in a 32% overestimation of their measured energy content, which resulted from unabsorbed lipids. All of these results suggest that almonds can delay nutrient absorption and maintain satiety/suppress hunger and have a low metabolized energy content.
The benefits of almonds on body weight can extend to body composition. During weight loss, the reduction in body fat, especially in the central abdominal area, is the most beneficial to health compared to the loss of subcutaneous fat. In a 24‐week trial with 65 overweight and obese adults, a low‐calorie diet enriched with 84 g/day of almonds reduced body weight/BMI, waist circumference, and fat mass significantly more than the low calorie control diet (Wien et al. 2003)., Reduction in body fat was consistently noted in Chinese patients with type 2 diabetes (Li et al. 2011). In a more recent randomized, cross‐over, controlled feeding study on 48 people with high LDL‐C, a cholesterol‐lowering diet with and without addition of almonds (42.5 g/day) decreased abdominal fat and leg fat, despite no difference in body weight between the two dietary groups (Berryman et al. 2015). While the mechanism(s) by which almond constituents reduced abdominal fat remains to be explored, MUFA in almonds may enable the redistribution of central body fat (Paniagua et al. 2007). More detailed information is presented in Table 14.2.
According to a World Health Organization report (WHO 2014), more than 1.4 billion adults (≥20 years) were overweight in 2008 and more than 40 million children (under age five) were overweight or obese in 2012. The epidemic of overweight and obesity is a global problem because they are closely associated with increasing prevalence of the metabolic syndrome, type 2 diabetes, and cardiovascular diseases, which have serious implications for healthcare systems and the financial burden for individuals and countries (Mozumdar & Liguori 2011). Health and nutrition educational programs based on the research data are vital to control obesity and its related diseases. With the growing body of evidence on health benefits and body weight loss/maintenance, almonds can be included in healthy diets to control and maintain body weight because of their low metabolized energy, hunger suppression, and appealing taste.
14.3.6 Prebiotics
Dietary fiber intake is continually encouraged in all populations because fiber intake remains low, insufficient to reach the level at which its maximum health benefits are achieved. Gut health has drawn a lot of public attention recently, especially with emerging evidence showing the link between gut health and diseases in organs distant from the gut. One of the main research areas is to establish the impact on the gut microbiome of prebiotics and probiotics which foster the growth of beneficial microbes and suppress harmful ones. A prebiotic is a nutrient, compound or food which is resistant to human digestive enzymes, can benefit the growth of beneficial bacteria, and promotes host wellness and health (Gibson et al. 2004; Pineiro et al. 2008). Dietary fibers and resistant starches (typically polysaccharides, such as pectins and xylans) are well recognized as prebiotics, and they are degraded by bacterial enzymes but resistant to pancreatic enyzmes (Gibson et al. 2010). Almonds are one of the most fiber‐rich foods. With 3.4 g fiber per 28 g serving, almonds provide a significant amount of fiber for microbial fermentation in the gut. The total fiber content of whole almonds is among the highest (12%) of all the edible nuts (Mandalari et al. 2008). The total dietary fiber of almond skin (by‐product of the almond‐processing industry) is approximately 45% wet weight (w/w), most of this being insoluble fiber with 3–4% soluble fiber (Mandalari et al. 2010a,b). Using an in vitro fermentation bioreactor, a high amount of almond skin cellulose was found at different stages of fermentation in the large bowel and the bifidobacteria and Eubacterium rectale populations were increased, suggesting that almond skins might have prebiotic properties (Mandalari et al. 2008).
Liu et al. (2014) demonstrated the prebiotic effect of almonds in a human study. They found that the six‐week consumption of roasted almonds (56 g/day) or almond skins (10 g/day) increased the populations of Bifidobacterium spp. and Lactobacillus spp. in 48 healthy adult volunteers and decreased the pathogen Clostridium perfringens. Further, almonds or almond skins increased activity of β‐galactosidase, which is mainly synthesized by bifidobacteria and lactobacilli, and decreased activities of β‐glucuronidase, nitroreductase, and azoreductase, which are synthesized by harmful bacteria, suggesting a favorable change in the microbial profile. In contrast, Ukhanova et al. (2014) did not find a marked impact of almond consumption (up to 85 g/day for 18 days) on microbiota, particularly in lactic acid bacteria, in a randomized, controlled, cross‐over feeding study with healthy adults, even though there was a decrease in Firmicutes bacterium DJF VP 44 and Clostridium sp. ASF 396. Thus, the prebiotic effect of almonds or their constituents may depend on the duration of consumption. The complexity of the gut’s microbial ecosystem and a lack of definitive means for its characterization are also underlying factors for the inconsistency (Di Bella et al. 2013).
In addition to dietary fibers, polyphenols present in almonds skins could have a prebiotic effect via their microbial modulating action. However, their impact on the gut microbiota remains to be elucidated. Nevertheless, gut bacteria are capable of metabolizing polyphenols, especially transforming larger polyphenols to simple phenolic acids. Such small phenolic acids derived from bacterially mediated metabolism of polyphenols in almond skins have been detected in plasma and urine (Urpi‐Sarda et al. 2009). This interplay between almond polyphenols and gut microbiota can have significant implications for health in the gut and the whole body because this relationship can be associated with reduction in harmful microbes, production of antimicrobial substances, and modulation of metabolic and autoimmune diseases (Round & Mazmanian 2009). Thus, the use of almonds in the development of prebiotic foods appears to be an appealing strategy for health prevention and promotion.
14.4 Development of Functional Foods with Almonds
Recently, there has been growing interest in the development of functional ingredients and foods, nutraceuticals, and dietary supplements (Shahidi 2009). The term “functional food” was first mentioned in Japan in 1984 in the context of a food related to nutrition, modulation of physiological systems, sensory satisfaction, and fortification. More recently, functional foods have been defined as food products with special constituents that promote biological, molecular, and physiological effects benefiting health (Bigliardi & Galati 2013; Hardy 2000). With the astronomical increase in healthcare costs and aging populations, functional foods may have a place in health maintenance and promotion (Shahidi 2009).
The actions of a functional food rely on the bioactives naturally present in the product, are artificially formulated using appropriate technologies, or both. Development of functional foods began initially with the fortification of essential nutrients, such as vitamin C and E, folic acid, zinc, iron, calcium, and so on. In the last decade, the focus has evolved to adding novel constituents, such as omega‐3 fatty acids, phytosterol, and soluble fiber (β‐glucan), and developing foods with multiple health benefits. Further, a wide range of food products, such as breakfast cereals, snacks, beverages, and supplements, have been employed as platforms to deliver functional ingredients or nutrients (Sloan 2000, 2002, 2004, 2014).
Development of functional foods is a complex, challenging process with a critical need to attain product acceptance by consumers and necessary approvals from regulatory authorities (Day et al. 2009; Jones & Jew 2007). Consumer acceptance is one of the top priorities in the development of functional foods because characteristics of quality attributes, like texture and flavor, can be affected even though the potential negative effects can be minimized with the use of other quality enhancement ingredients and techniques (Day et al. 2009). In particular, sensory attributes such as taste, color, aroma, and texture are important elements with a great influence on consumer acceptance. Stability of functional ingredients/constituents in finished products is also critical to success. Technologies that stabilize bioactive compounds are in development, such as microencapsulation (envelopment of small solid particles, liquid droplets or gases in a coating), edible films (carry active ingredients that can reduce the risk of pathogen growth on the food surface or specific nutrients beneficial to humans), and vacuum impregnation (introduces desirable solutes into the porous structures of foods) (Betoret et al. 2011; Bigliardi & Galati 2013).
In July 2003, the FDA approved a health claim stating, “Scientific evidence suggests but does not prove that eating 1.5 ounces per day of most nuts, such as almonds, as part of a diet low in saturated fat and cholesterol may reduce the risk of heart disease” (FDA 2014). Food products with health claims attesting to functional capacity and increased quality of life in the general population are well accepted by consumers (Jones & Jew 2007). Almonds and their processing by‐products can provide a wide range of ingredients for the development of functional foods because of their functional constituents (monounsaturated fat, magnesium, α‐tocopherol, fiber, polyphenols, riboflavin, and other micronutrients).
According to a review by Siró et al. (2008), the most notable platforms for functional products include prebiotics and probiotics, beverages, cereals, bakery products, spreads, meats, and eggs. We believe that almonds and their derivatives, such as almond paste, milk, and oil, could be added in most of these categories as ingredients to enhance functionality and bioactive content of functional foods. Bakery products present an ideal vehicle by which functionalities of bioactives or nutrients can be delivered to the consumer in an acceptable food (Siró et al. 2008). Almonds in different forms have been traditionally used in many bakery and confectionery products such as cakes, pies, cookies, and breads. The introduction of almonds into bakery products has grown by 13% in Europe and North America (California Almonds 2014), an increase most likely attributed to the health benefits substantiated by a growing body of clinical evidence. In addition to bakery and confectionery products, almonds have been formulated into breakfast cereals, snack bars, almond chocolate, and nut mixtures as a snack. Further, almond butter, which contains significantly more fiber, calcium, and potassium than sunflower seed or peanut butter, has become a new alternative for those who are allergic to peanuts (Thomas & Gebhardt 2010). It should be noted that almond butter should contain a minimum of 90% almonds and be prepared by grinding shelled, blanched or unblanched, raw or roasted almonds, to which salt, honey, evaporated cane syrup, corn maltodextrin, flax seed, wheatgerm, cocoa powder, cocoa butter or vanilla may be added as ingredients (USDA 2011). Palm or peanut oil can be used as a stabilizing agent.
Almond skins are rich in polyphenols and dietary fiber and could be considered a functional food ingredient, as well as a natural antioxidant preservative added to control oxidative processes in more oxidation‐prone foods (Garrido et al. 2008). During almond processing, skins are produced as a by‐product. While they are a valuable ingredient because of their polyphenol and fiber content, their true value has not been fully realized in the arena of foods, nutraceuticals, and pharmaceuticals. Dietary fibers have many roles in health promotion and prevention, e.g. increasing cholesterol elimination, maintaining glucose regulation, and modulating gut microbiota. Thus, the incorporation of almond skins into bakery and cereal products can enhance their functional characteristics. They can also be added to granola mix, rice, mashed potatoes, pastas, salads and salad dressings, cereal bars, crackers, yogurts, fermented beverages, juices, and bakery products such as muffins, cookies, pancakes, and waffles. Finally, they can be lyophilized, powdered, and then sold as a functional ingredient to food manufacturers or as a functional food to consumers. These functional foods made with almond skins could provide an array of health benefits, for example lowering blood cholesterol, maintaining/decreasing blood glucose, enhancing antioxidant defense and immunity, and modulating gut microbiota. Furthermore, the polyphenols present in the skins could be used as a potential natural antimicrobial agent in the food preservative market (Mandalari 2012). The photoprotective potential of polyphenols and other constituents in almond skins have also been reported, suggesting that almond nutrients can be used to develop products for skin health (Evans et al. 2013).
Almond oil displays many health benefits due to its rich content of α‐tocopherol and oleic acid in antioxidation and cholesterol reduction for CVD prevention. In addition, almond oil can serve as an ingredient in skin (as an emollient) and hair care products. It could be used in the production of salad dressings, mayonnaise, whipped cream, and cake fillings and toppings to improve their functional characteristics. However, one downside is that production costs will be augmented because of the relatively higher cost of almond oil compared to other vegetable oils on the market.
Almond flour is made with blanched almonds, whereas almond meal can be made with either whole or blanched almonds. Almond flour or meal can be used to replace wheat flour in products such as cakes, waffles, cookies, pancakes, and breads. This replacement benefits the products by reducing carbohydrate content whose consumption is generally linked to the development or progression of metabolic disorders and by gaining the health benefits of almonds. Further, patially replacing wheat flour with almond meal can add texture and flavor to the products. Almond meal can also be used in place of bread crumbs in meatballs or as a coating for fish and chicken. Almond meal is a gluten‐free product and thus is an interesting alternative for people with gluten‐related disorders.
Almond milk has become popular and is an alternative to dairy milk especially for consumers with lactose intolerance and dairy protein allergy. With its favorable taste and nutrition values, the demand for almond milk has been increasing. Similar to almond milk, the demand for soymilk is escalating. Almond milk could serve as the base ingredient for production of new nondairy fermented products with probiotic bacteria and functional features. Bernat et al. (2015) evaluated the fermentative process of almond milk using a mixed culture of L. reuteri and S. thermophilus and found that the fermentation induced an increase in the viscosity, luminosity, and whiteness values of the almond milk. High probiotic survivals were also observed in the fermented almond milk after submitting the product to in vitro digestion, enhancing the product value as a probiotic.
Processed meat products can be a valuable vehicle in delivery of functional ingredients (Olmedilla‐Alonso et al. 2006). Such an approach can decrease the unhealthy attributes of meat products, e.g. saturated fats and sodium. While there is currently no application using almonds or their derivatives, walnuts were incorporated into restructured beef steak to enhance sensory and healthy attributes (Jiménez Colmenero et al. 2003). Thus, the incorporation of nuts in meat products can be a means to confer their potential heart‐healthy benefits to generally unhealthy but popular products. Further research is warranted to enhance understanding of the interactions between constituents in added nuts and meat products with the concerns of food safety and texture change being taken into account (Fernández‐Ginés et al. 2005).
Functional foods are one of the growing segments of the food industry with the potential to improve health and help to slow the increase in healthcare costs. New approaches to formulating functional ingredients or bioactives in functional foods are being undertaken by the academic and private sectors. Most importantly, the route to success in development of a functional food must start from selection of functional ingredients or bioactives and appropriate vehicles and then determine consumer acceptability and stability of functional nutrients (Betoret et al. 2011). Further, studies must be undertaken to elucidate any changes in absorption, disposition, metabolism, and excretion of functional nutrients in the new food matrixes, as well as bioefficacy. Finally, in the era of personal medicine, nutrigenomics must be taken into consideration when a functional food is developed for general or specific populations (Hasler 2002).
14.5 Conclusion
Among many recent innovations in the food industry, functional foods are recognized as one of the most interesting areas with great growth potential. Development of new functional foods appears to follow a market trend, which begins with an influx of new research data showing health benefits of foods or nutrients beyond their standard nutrition value. Further, the growing interest of consumers in functional foods for the promotion of overall wellbeing and reduction in risk of chronic diseases drives the development of these foods and their commercialization. Almonds are one type of nut whose consumption was associated with a reduced risk for mortality in the Physicians’ Health Study (Hshieh et al. 2015). While almonds are rich in calories, they have become recognized as a food with multiple health attributes due to their nutrition profile which is rich in oleic acid, fiber, α‐tocopherol, magnesium, riboflavin, and polyphenols. The growing body of clinical evidence has shown that their consumption is linked with reduction in blood cholesterol, improvement in glucose regulation and antiinflammation, and amelioration of oxidative stress in those who are healthy or have chronic diseases.
With their health benefits and taste, texture, and flavor characteristics, almonds are a great ingredient to be formulated in functional foods. They can be incorporated into functional foods in diverse forms, for example as whole almonds (slices, flour, and paste), skins, milk, and oil. Even though almonds are traditionally used in bakery and confectionery products or consumed as a snack, almonds or their derivatives can be formulated into functional foods, e.g. cereal products, processed meats, and can replace unfavorable ingredients, such as refined wheat flour or oil.
References
- Abazarfard, Z., Salehi, M. & Keshavarzi, S. (2014) The effect of almonds on anthropometric measurements and lipid profile in overweight and obese females in a weight reduction program: a randomized controlled clinical trial. Journal of Research in Medical Sciences 19(5), 457–464.
- Abbey, M., Noakes, M., Belling, G. B., et al. (1994) Partial replacement of saturated fatty acids with almonds or walnuts lowers total plasma cholesterol and low‐density‐lipoprotein cholesterol. American Journal of Clinical Nutrition 59(5), 995–999.
- Ahrens, S., Venkatachalam, M., Mistry, A. M., et al. (2005) Almond (Prunus dulcis L.) protein quality. Plant Foods for Human Nutrition 60(3), 123–128.
- Appel, L. J., Sacks, F. M., Carey, V. J., et al. (2005) Effects of protein, monounsaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA 294(19), 2455–2464.
- Asegaonkar, S. B., Marathe, A., Tekade, M. L., et al. (2011) High‐sensitivity C‐reactive protein: a novel cardiovascular risk predictor in type 2 diabetics with normal lipid profile. Journal of Diabetes and its Complications 25(6), 368–370.
- Atwater, W. O. & Bryant, A. P. (1900) The Availability and Fuel Value of Food Materials. Agriculture Experiment Station 12th Annual Report. Washington, DC: US Government Printing Office, pp 73–110.
- Bernat, N., Cháfer, M., Chiralt, A., et al. (2015) Development of a non‐dairy probiotic fermented product based on almond milk and inulin. Food Science and Technology International 21(6), 440–453.
- Berry, S. E., Tydeman, E. A., Lewis, H. B., et al. (2008) Manipulation of lipid bioaccessibility of almond seeds influences postprandial lipemia in healthy human subjects. American Journal of Clinical Nutrition 88(4), 922–999.
- Berryman, C. E., Preston, A. G., Karmally, W., et al. (2011) Effects of almond consumption on the reduction of LDL‐cholesterol: a discussion of potential mechanisms and future research directions. Nutrition Reviews 69(4), 171–185.
- Berryman, C. E., West, S. G., Fleming, J. A., et al. (2015) Effects of daily almond consumption on cardiometabolic risk and abdominal adiposity in healthy adults with elevated LDL‐cholesterol: a randomized controlled trial. Journal of the American Heart Association 4(1), 1–11.
- Bes‐Rastrollo, M., Wedick N. M., Martínez‐González, M. A., et al. (2009) Prospective study of nut consumption, long‐term weight change, and obesity risk in women. American Journal of Clinical Nutrition 89(6), 1913–1919.
- Betoret, E., Betoret, N., Vidal, D., et al. (2011) Functional foods development: trends and technologies. Trends in Food Science and Technology 22(9), 498–508.
- Bigliardi, B. & Galati, F. (2013) Innovation trends in the food industry: the case of functional foods. Trends in Food Science and Technology 31(2), 118–129.
- Calder, P. C., Albers, R., Antoine, J. M., et al. (2009) Inflammatory disease processes and interactions with nutrition. British Journal of Nutrition 101, S1–45.
- California Almonds (2014) Adding almonds to your food products. Available at: www.almonds.com/food‐professionals/manufacturers (accessed 1 July 2016).
- Casas‐Agustench, P., Bullo, M. & Salas‐Salvado, J. (2010) Nuts, inflammation and insulin resistance. Asia Pacific Journal of Clinical Nutrition 19(1), 124–130.
- Cassady, B. A., Hollis, J. H., Fulford, A. D., et al. (2009) Mastication of almonds: effects of lipid bioaccessibility, appetite, and hormone response. American Journal of Clinical Nutrition 89(3), 794–800.
- Chen, C., Milbury, P. E., Lapsley, K., et al. (2005) Flavonoids from almond skins are bioavailable and act synergistically with vitamins C and E to enhance hamster and human LDL resistance to oxidation. Journal of Nutrition 135(6), 1366–1373.
- Chen, C. Y., Lapsley, K. & Blumberg, J. (2006) A nutrition and health perspective on almonds. Journal of the Science of Food and Agriculture 86(14), 2245–2250.
- Choudhury, K., Clark, J. & Griffi, H. R. (2014) An almond‐enriched diet increases plasma α‐tocopherol and improves vascular function but does not affect oxidative stress markers or lipid levels. Free Radical Research 48(5), 599–606.
- Cohen, A. E. & Johnston, C. S. (2011) Almond ingestion at mealtime reduces postprandial glycemia and chronic ingestion reduces hemoglobin A(1c) in individuals with well‐controlled type 2 diabetes mellitus. Metabolism 60(9), 1312–1317.
- Damasceno N. R., Perez‐Heras, A., Serra, M., et al. (2011) Crossover study of diets enriched with virgin olive oil, walnuts or almonds. Effects on lipids and other cardiovascular risk markers. Nutrition, Metabolism and Cardiovascular Diseases 21(S1), S14–20.
- Danesh, J., Wheeler, J. G., Hirschfield, G. M., et al. (2004) C‐reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. New England Journal of Medicine 350(14), 1387–1397.
- Day, L., Seymour, R.B., Pitts, K. F., et al. (2009) Incorporation of functional ingredients into foods. Trends in Food Science and Technology 20(9), 388–395.
- Di Bella, J. M., Bao, Y., Gloor, G. B., et al. (2013) High throughput sequencing methods and analysis for microbiome research. Journal of Microbiological Methods 95(3), 401–414.
- Estruch, R., Martínez‐González, M. A., Corella, D., et al. (2006) Effects of a Mediterranean‐style diet on cardiovascular risk factorsa randomized trial. Annals of Internal Medicine 145(1), 1–11.
- Evans, J. A., Garlick, J. A., Johnson, E. J., et al. (2013) A pilot study of the photoprotective effect of almond phytochemicals in a 3D human skin equivalent. Journal of Photochemistry and Photobiology 126, 17–25.
- FDA (Food and Drug Administration) (2013) Guidance for Industry: A Food Labeling Guide (14. Appendix F: Calculate the Percent Daily Value for the Appropriate Nutrients). Available at: hwww.fda.gov/Food/GuidanceRegulation/GuidanceDocumentsRegulatoryInformation/LabelingNutrition/ucm2006828.htm (accessed 1 July 2016).
- FDA (Food and Drug Administration) (2014) Summary of Qualified Health Claims Subject to Enforcement Discretion. Available at: www.fda.gov/Food/IngredientsPackagingLabeling/LabelingNutrition/ucm073992.htm (accessed 1 July 2016).
- Fernández‐Ginés, J., Fernández‐López, J., Sayas‐Barberá, E., et al. (2005) Meat products as functional foods : a review. Journal of Food Science 70(2), 37–43.
- Festa, A., d'Agostino, R., Tracy, R. P., et al. (2002) Elevated levels of acute‐phase proteins and plasminogen activator inhibitor‐1 predict the development of type 2 diabetes: the insulin resistance atherosclerosis study. Diabetes 51(4), 1131–1137.
- Foster, G. D., Shantz, K. L., Vander Veur, S. S., et al. (2012) A randomized trial of the effects of an almond‐enriched, hypocaloric diet in the treatment of obesity. American Journal of Clinical Nutrition 96(2), 249–254.
- Garrido, I., Monagas, M., Gómez‐Cordovés, C., et al. (2008) Polyphenols and antioxidant properties of almond skins: influence of industrial processing. Journal of Food Science 73(2), 106–115.
- Gibson, G. R., Probert, H. M., van Loo, J. A. E., et al. (2004) Dietary modulation of the human colonic microbiota: updating the concept of prebiotics. Nutrition Research 17(2), 257–259.
- Gibson, G. R., Scott, K. P., Rastall, R. A., et al. (2010) Dietary prebiotics: current status and new definition. Food Science and Technology Bulletin: Functional Foods 7(1), 1–19.
- Griel, A. E. & Kris‐Etherton, P. M. (2007) Tree nuts and the lipid profile: a review of clinical studies. British Journal of Nutrition 96(S2), S68–78.
- Grundy, M. M. L., Grassby, T., Mandalari, G., et al. (2015) Effect of mastication on lipid bioaccessibility of almonds in a randomized human study and its implications for digestion kinetics, metabolizable energy, and postprandial lipemia. American Journal of Clinical Nutrition 101, 25–33.
- Hardy, G. (2000) Nutraceutical and functional foods: introduction and meaning. Nutrition 16(7‐8), 688–698.
- Hasler, C. M. (2002) Issues and opinions functional foods: benefits, concerns and challenges – a position paper from the American Council on Science and Health. Journal of Nutrition 132(12), 3772–3781.
- Hellwig, J. P., Otten, J. J. & Meyers, L. D., eds. (2006) Dietary Reference Intakes: The Essential Guide to Nutrient Requirements. Washington, DC: National Academies Press.
- Hollis, J. & Mattes, R. (2007) Effect of chronic consumption of almonds on body weight in healthy humans. British Journal of Nutrition 98(3), 651–656.
- Hshieh, T. T., Petrone, A. B., Gaziano, J. M., et al. (2015) Nut consumption and risk of mortality in the Physicians’ Health Study. American Journal of Clinical Nutrition 101, 407–412.
- Hull, S., Re, R., Chambers, L., et al. (2015) A mid‐morning snack of almonds generates satiety and appropriate adjustment of subsequent food intake in healthy women. European Journal of Nutrition 54(5), 803–810.
- Hyson, D. A., Schneeman, B. O. & Davis, P. A. (2002) Almonds and almond oil have similar effects on plasma lipids and LDL oxidation in healthy men and women. Journal of Nutrition 132(4), 703–707.
- Jaceldo‐Siegl, K., Sabaté, J., Batech, M., et al. (2011) Influence of body mass index and serum lipids on the cholesterol‐lowering effects of almonds in free‐living individuals. Nutrition, Metabolism and Cardiovascular Diseases 21(S1), S7–13.
- Jambazian, P.R., Haddad, E., Rajaram, S., et al. (2005) Almonds in the diet simultaneously improve plasma [alpha]‐tocopherol concentrations and reduce plasma lipids. Journal of the American Dietetic Association 105(3), 449–454.
- Jenkins, D. J. A., Kendall, C. W. C., Marchie, A., et al. (2002) Dose response of almonds on coronary heart disease risk factors: blood lipids, oxidized low‐density lipoproteins, lipoprotein(a), homocysteine, and pulmonary nitric oxide: a randomized, controlled, crossover trial. Circulation 106(11), 1327–1332.
- Jenkins, D. J. A., Kendall, C. W. C., Marchie, A., et al. (2003) The effect of combining plant sterols, soy protein, viscous fibers, and almonds in treating hypercholesterolemia. Metabolism 52(11), 1478–1483.
- Jenkins, D. J. A., Kendall, C. W. C., Josse, A. R., et al. (2006) Almonds decrease postprandial glycemia, insulinemia, and oxidative damage in healthy individuals. Journal of Nutrition 136(12), 2987–2992.
- Jenkins, D. J. A., Kendall, C. W. C., Marchie, A., et al. (2008) Almonds reduce biomarkers of lipid peroxidation in older hyperlipidemic subjects. Journal of Nutrition 138(5), 908–913.
- Jiang, R., Jacobs, D. R. Jr., Mayer‐Davis, E., et al. (2006) Nut and seed consumption and inflammatory markers in the multi‐ethnic study of atherosclerosis. American Journal of Epidemiology 163(3), 222–231.
- Jiménez Colmenero, F., Serrano, A, Ayo, J., et al. (2003) Physicochemical and sensory characteristics of restructured beef steak with added walnuts. Meat Science 65(4), 1391–1397.
- Jones, P. J. & Jew, S. (2007) Functional food development: concept to reality. Trends in Food Science and Technology 18(7), 387–390.
- Josse, A. R., Kendall, C. W., Augustin, L. S., et al. (2007) Almonds and postprandial glycemia – a dose‐response study. Metabolism 56(3), 400–404.
- Kamil, A. & Chen, C. Y. O. (2012) Health benefits of almonds beyond cholesterol reduction. Journal of Agricultural and Food Chemistry 60(27), 6694–6702.
- Kendall, C. W. C., Josse, A. R., Esfahani, A. & Jenkins, D. J. (2010) Nuts, metabolic syndrome and diabetes. British Journal of Nutrition 104(4), 465–473.
- Kris‐Etherton, P. M., Karmally, W. & Ramakrishnan, R. (2009) Almonds lower LDL cholesterol. Journal of the American Dietetic Association 109, 1521–1522.
- Li, N., Jia, X., Chen, C. Y., et al. (2007) Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. Journal of Nutrition 137(12), 2717–2722.
- Li, S. C., Liu, Y. H., Liu, J. F., et al. (2011) Almond consumption improved glycemic control and lipid profiles in patients with type 2 diabetes mellitus. Metabolism 60(4), 474–479.
- Liu, J. F., Liu, Y. H., Chen, C. M., et al. (2013) The effect of almonds on inflammation and oxidative stress in Chinese patients with type 2 diabetes mellitus: a randomized crossover controlled feeding trial. European Journal of Nutrition 52(3), 927–935.
- Liu, Z., Lin, X., Huang, G., et al. (2014) Prebiotic effects of almonds and almond skins on intestinal microbiota in healthy adult humans. Anaerobe 26, 1–6.
- Lo Piparo, E., Scheib, H., Frei, N., et al. (2008) Flavonoids for controlling starch digestion: structural requirements for inhibiting human alpha‐amylase. Journal of Medicinal Chemistry 51(12), 3555–6351.
- Lovejoy, J. C., Most, M. M., Lefevre, M., et al. (2002) Effect of diets enriched in almonds on insulin action and serum lipids in adults with normal glucose tolerance or type 2 diabetes. American Journal of Clinical Nutrition 76(5), 1000–1006.
- Luc, G., Bard, J. M., Juhan‐Vague, I., et al. (2003) C‐reactive protein, interleukin‐6, and fibrinogen as predictors of coronary heart disease: the PRIME Study. Arteriosclerosis, Thrombosis and Vascular Biology 23(7), 1255–1261.
- Lucotti, P., Monti, L., Setola, E., et al. (2009) Oral L‐arginine supplementation improves endothelial function and ameliorates insulin sensitivity and inflammation in cardiopathic nondiabetic patients after an aortocoronary bypass. Metabolism 58(9), 1270–1276.
- Mandalari, G. (2012) Potential health benefits of almond skin. Journal of Bioprocessing and Biotechniques 2, 5.
- Mandalari, G., Nueno‐Palop, C., Bisignano, G., et al. (2008) Potential prebiotic properties of almond (Amygdalus communis L.) seeds. Applied and Environmental Microbiology 74(14), 4264–4270.
- Mandalari, G., Tomaino, A., Arcoraci, T., et al. (2010a) Characterization of polyphenols, lipids and dietary fibre from almond skins (Amygdalus communis L.). Journal of Food Composition and Analysis 23(2), 166–174.
- Mandalari, G., Faulks, R. M., Bisignano, C., et al. (2010b) In vitro evaluation of the prebiotic properties of almond skins (Amygdalus communis L.). FEMS Microbiology Letters 304(2), 116–122.
- Milbury, P. E., Chen, C. Y., Dolnikowski, G. G., et al. (2006) Determination of flavonoids and phenolics and their distribution in almonds. Journal of Agricultural and Food Chemistry 54(14), 5027–5033.
- Mirrahimi, A., Srichaikul, K., Esfahani, A., et al. (2011) Almond (Prunus dulcis) seeds and oxidative stress. In: V. Preedy, R. Ross Watson & V. B. Patel, eds. Nuts and Seeds in Health and Disease Prevention. San Diego: Academic Press, pp 161–166.
- Monagas, M., Garrido, I., Lebrón‐Aguilar, R., et al. (2007) Almond (Prunus dulcis (Mill.) DA Webb) skins as a potential source of bioactive polyphenols. Journal of Agricultural and Food Chemistry 55(21), 8498–8507.
- Mori, A. M., Considine, R. V. & Mattes, R. D. (2011) Acute and second‐meal effects of almond form in impaired glucose tolerant adults: a randomized crossover trial. Nutrition and Metabolism 8(6), 1–8.
- Mozumdar, A. & Liguori, G. (2011) Persistent increase of prevalence of metabolic syndrome among U.S. adults: NHANES III to NHANES 1999–2006. Diabetes Care 34(1), 1–4.
- Nishi, S., Kendall, C. W., Gascoyne, A. M., et al. (2014) Effect of almond consumption on the serum fatty acid profile: a dose‐response study. British Journal of Nutrition 112(7), 1137–1146.
- Novotny, J. A., Gebauer, S. K. & Baer, D. J. (2012) Discrepancy between the Atwater factor predicted and empirically measured energy values of almonds in human diets. American Journal of Clinical Nutrition 96(2), 296–301.
- Olmedilla‐Alonso, B., Granado‐Lorencio, F., Herrero‐Barbudo, C., et al. (2006) Nutritional approach for designing meat‐based functional food products with nuts. Critical Reviews in Food Science and Nutrition 46(7), 537–542.
- Paniagua, J. A., Gallego de la Sacristana, A., Romero, I., et al. (2007) Monounsaturated fat‐rich diet prevents central body fat distribution and decreases postprandial adiponectin expression induced by a carbohydrate‐rich diet in insulin‐resistant subjects. Diabetes Care 30(7), 1717–1723.
- Pineiro, M., Nils‐Georg, A., Reid, G., et al. (2008) FAO Technical Meeting on Prebiotics 2007. Journal Of Clinical Gastroenterology 42, S156–S159.
- Pradhan, A. D. & Ridker, P. M. (2002) Do atherosclerosis and type 2 diabetes share a common inflammatory basis? European Heart Journal 23, 831–834.
- Pradhan, A. D., Manson, J. E., Rifai, N., et al. (2001) C‐reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus. JAMA 286(3), 327–334.
- Rajaram, S. & Sabaté, J. (2006) Nuts, body weight and insulin resistance. British Journal of Nutrition 96(S2), S79–S86.
- Rajaram, S., Connell, K. M. & Sabaté, J. (2010) Effect of almond‐enriched high‐monounsaturated fat diet on selected markers of inflammation: a randomised, controlled, crossover study. British Journal of Nutrition 103(6), 907–912.
- Ros, E. (2009) Nuts and novel biomarkers of cardiovascular disease. American Journal of Clinical Nutrition 89(5), 1649S–1656S.
- Round, J. L. & Mazmanian, S. K. (2009) The gut microbiota shapes intestinal immune responses during health and disease. Nature Reviews, Immunology 9, 313–323.
- Sabaté, J. (2003) Nut consumption and body weight. American Journal of Clinical Nutrition 78(S3), 647S–650S.
- Sabaté, J., Haddad, E., Tanzman, J. S., et al. (2003) Serum lipid response to the graduated enrichment of a Step I diet with almonds: a randomized feeding trial. American Journal of Clinical Nutrition 77(6), 1379–1384.
- Sabaté, J., Oda, K. & Ros, E. (2010) Nut consumption and blood lipid levels: a pooled analysis of 25 intervention trials. Archives of Internal Medicine 170(9), 821–827.
- Salas‐Salvadó, J., Casas‐Agustench, P., Murphy, M. M., et al. (2008) The effect of nuts on inflammation. Asia Pacific Journal of Clinical Nutrition 17(S1), 333–336.
- Shahidi, F. (2009) Nutraceuticals and functional foods: whole versus processed foods. Trends in Food Science and Technology 20(9), 376–387.
- Siegrist, M., Stamfli, N. & Kastenholz, H. (2008) Consumers’ willingness to buy functional foods. The influence of carrier, benefit and trust. Appetite 51(3), 526–529.
- Singh, U., Devaraj, S. & Jialal, I. (2005) Vitamin E, oxidative stress, and inflammation. Annual Review of Nutrition 25, 151–174.
- Siró, I., Kápolna, E., Kápolna, B., et al. (2008) Functional food. Product development, marketing and consumer acceptance – a review. Appetite 51(3), 456–467.
- Sloan, A. E. (2000) The top ten functional food trends. Food Technology 54, 33–62.
- Sloan, A. E. (2002) The top 10 functional food trends. The next generation. Food Technology 56, 32–57.
- Sloan, A. E. (2004) The top ten functional food trends. Food Technology 58, 28–51.
- Sloan, A.E. (2014) The top ten functional food trends. Food Technology 68(4). Available at: http://www.ift.org/newsroom/news‐releases/2014/april/21/top‐ten‐functional‐food‐trends‐for‐2014.aspx (accessed 1 July 2016).
- Soinio, M., Marniemi, J., Laakso, M., et al. (2006) High‐sensitivity C‐reactive protein and coronary heart disease mortality in patients with type 2 diabetes: a 7‐year follow‐up study. Diabetes Care 29(2), 329–333.
- Spiller, G. A., Jenkins, D. A. J., Bosello, O., et al. (1998) Nuts and plasma lipids: an almond‐based diet lowers LDL‐C while preserving HDL‐C. Journal of the American College of Nutrition 17(3), 285–290.
- Spiller, G. A., Miller, A., Olivera, K., et al. (2003) Effects of plant‐based diets high in raw or roasted almonds, or roasted almond butter on serum lipoproteins in humans. Journal of the American College of Nutrition 22(3), 195–200.
- Sweazea, K. L., Johnston, C. S., Ricklefs, K. D., et al. (2014) Almond supplementation in the absence of dietary advice significantly reduces C‐reactive protein in subjects with type 2 diabetes. Journal of Functional Foods 10, 252–259.
- Tamizifar, B., Rismankarzadeh, M., Vosoughi, A., et al. (2005) A low‐dose almond‐based diet decreases LDL‐C while preserving HDL‐C. Archives of Iranian Medicine 8(1), 45–51.
- Tan, S. Y. & Mattes, R. D. (2013) Appetitive, dietary and health effects of almonds consumed with meals or as snacks: a randomized, controlled trial. European Journal of Clinical Nutrition 67, 1205–1214.
- Thomas, R. & Gebhardt, S. E. (2010) Sunflower seed butter and almond butter as nutrient‐rich alternatives to peanut butter. Journal of the American Dietetic Association 10(9), A52.
- Ukhanova, M., Wang, X., Baer, D. J., et al. (2014) Effects of almond and pistachio consumption on gut microbiota composition in a randomised cross‐over human feeding study. British Journal of Nutrition 111(12), 2146–2152.
- Urpi‐Sarda, M., Garrido, I., Monagas, M., et al. (2009) Profile of plasma and urine metabolites after the intake of almond [Prunus dulcis (Mill.) D.A. Webb] polyphenols in humans. Journal of Agricultural and Food Chemistry 57(21), 10134–10142.
- USDA (Department of Agriculture) (2011) Commercial Item Description: Nut, Butters and Nut Spreads. Available at: www.ams.usda.gov/sites/default/files/media/CID%20Nut%20Butters%20and%20Nut%20Spreads.pdf (accessed 1 July 2016).
- USDA (Department of Agriculture) (2014) USDA National Nutrient Database for Standard Reference, Release 27. Available at: www.ars.usda.gov/Services/docs.htm?docid=24912 (accessed 1 July 2016).
- Wells, B. J., Mainous, A. G. & Everett, C. J. (2005) Association between dietary arginine and C‐reactive protein. Nutrition 21(2), 125–130.
- WHO (World Health Organization) (2014) Obesity and Overweight. Available at: www.who.int/mediacentre/factsheets/fs311/en/ (accessed 1 July 2016).
- Wien, M. A., Sabaté, J. M., Iklé, D. N., et al. (2003) Almonds vs complex carbohydrates in a weight reduction program. International Journal of Obesity and Related Metabolic Disorders 27(11), 1365–1372.
- Yada, S., Lapsley, K. & Huang, G. (2011) A review of composition studies of cultivated almonds: macronutrients and micronutrients. Journal of Food Composition and Analysis 24(4‐5), 469–480.
- Yada, S., Huang, G. & Lapsley, K. (2013) Natural variability in the nutrient composition of california‐grown almonds. Journal of Food Composition and Analysis 30(2), 80–85.
- Yoon, J. H., Thompson, L. U. & Jenkins, D. (1983) The effect of phytic acid on in vitro rate of starch digestibility and blood glucose response. American Journal of Clinical Nutrition 38(6), 835–842.
- Zhang, H., Park, Y., Wu, J., et al. (2009) Role of TNF‐alpha in vascular dysfunction. Clinical Science 116(3), 219–230.