Infectious Diseases, Including Infestations and Parasitic Diseases
This chapter will consider the histopathologic findings of a wide variety of infectious diseases and infestations, including those disorders caused by bacteria, fungi, viruses, arthropods and other parasites, and protozoa. Dermatopathology can play an important role in the diagnosis of these disorders in several ways. First, it may be possible to identify the infectious agent in tissue sections, using either routine staining (hematoxylin and eosin [H&E]) or special stains. The sensitivity of histopathologic analysis for infection has improved over the years with the introduction of immunohistochemical staining for identification of organism proteins or genetic material (through in situ hybridization). These methods can be further enhanced by systematic search for specifically stained infectious agents using techniques such as focus-floating microscopy.1,2 However, it is not always possible to find organisms in tissue sections using current methods. Second, histopathology may help find indirect clues to infection. These include such changes as pseudoepitheliomatous hyperplasia, abscess formation, suppurative granulomas, thromboembolic changes, or unexplained necrosis and connective tissue degeneration. These and other findings can trigger tissue culture studies, serologic evaluations, or other methods that may prove to be more successful. Still, it is impressive how often skin biopsy can yield a specific diagnosis of an infectious disease, even in the absence of supporting culture studies. This chapter is not by any means designed to be a complete review of all infectious disease, but it does focus on those infections that have relatively characteristic biopsy findings and are likely to be either actually encountered in clinical practice, or to become serious considerations in the histopathologic differential diagnosis.
Bacterial Diseases
Clinical Features: Impetigo is a superficial skin infection produced most often by staphylococci (particularly Staphylococcus aureus) but also by streptococci or a combination of the two. It is most common in children but also occurs in adults. In some instances, it may develop as a complication of allergic contact dermatitis. Erythematous macules evolve into thin-walled vesicles and bullae. These rupture and drain, producing a characteristic “honey-colored” crust. Infection with group A β-hemolytic streptococci can lead to acute glomerulonephritis in approximately 1% of cases.3 Streptococcal impetigo has a significant incidence in patients with atopic dermatitis.4 A variant called Bockhart impetigo presents as a superficial pustular folliculitis in hair-bearing areas; it may be complicated by superficial trauma or occlusion in the setting of S. aureus colonization.5
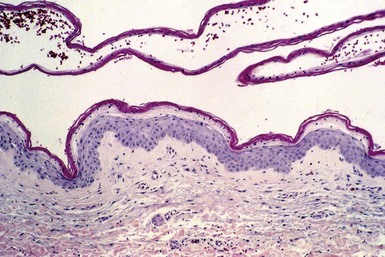
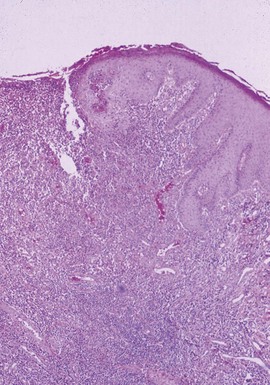
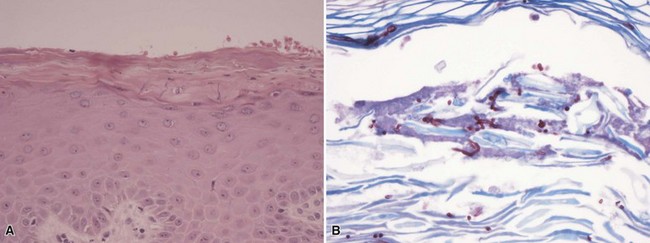
Microscopic Findings: A vesicle develops in the vicinity of the granular cell layer of the epidermis. When it is fully developed, it often appears in a subcorneal location. Neutrophils accumulate within the vesicle, forming a vesicopustule (Fig. 17-1). A minor degree of acantholysis can be identified at the base of the pustule, resulting from the effect of neutrophilic proteolytic enzymes, and evidence of degenerated keratinocytes has been found on ultrastructural examination.6 An upper dermal infiltrate often consists of a mixture of neutrophils and lymphocytes. Following rupture of the bulla, a surface crust is formed that is composed of transudated serum and degenerated neutrophils. Gram-positive cocci can sometimes be found within the vesicopustule.7 Bockhart impetigo shows a subcorneal vesicopustule at the follicular orifice.
Differential Diagnosis: Subcorneal pustule formation can also be identified in subcorneal pustular dermatosis and pemphigus foliaceus. However, the finding of bacteria in the vesicopustule points to a diagnosis of impetigo. Pemphigus foliaceus usually shows fewer neutrophils and a greater degree of acantholysis, often with evidence of dyskeratosis. Pustular psoriasiform dermatoses, including Reiter syndrome, candidiasis, and geographic tongue, in addition to psoriasis, ideally show spongiform postulation in the superficial portions of the spinous layer—a feature not typically seen in impetigo. Candidal organisms are of course expected in the pustular lesions of candidiasis. Immunoglobulin A (IgA) pemphigus of the subcorneal pustular dermatosis type uniquely shows intercellular IgA deposition on direct immunofluorescence examination. Acute generalized exanthematous pustulosis tends to feature smaller superficial pustules and may have the additional changes of apoptosis, underlying leukocytoclastic vasculitis, or a dermal infiltrate that includes eosinophils.
Staphylococcal Scalded Skin Syndrome
Clinical Features: This generalized exfoliative erythroderma is seen most often (by far) in young children but also encountered in immunosuppressed adults.8,9 It typically begins with fever and erythema in intertriginous sites, with sheetlike exfoliation following rapidly thereafter. The eruption is due to an exfoliative toxin produced by group 2 S. aureus, especially phage types 55 and 71.10,11 Antimicrobial therapy (typically with penicillins that are penicillinase-resistant) is usually successful, and most cases in children have a good prognosis.
Microscopic Findings: There is a separation below or within the granular cell layer of the epidermis; the exfoliating portion of epidermis has a necrotic appearance (Fig. 17-2). Inflammation is minimal. Bacteria are generally not identified, since the changes result from the effects of the circulating toxin. The actual focus of infection most often involves mucosal surfaces, including oral or nasal cavities.
Differential Diagnosis: The lesions must be differentiated from toxic epidermal necrolysis, in which there is full-thickness epidermal necrosis. This differentiation can be made rapidly using frozen sections or rapid processing of exfoliated skin, because staphylococcal scalded skin syndrome shows only superficial epidermal necrosis. Similarly, bullous impetigo can often be distinguished from early staphylococcal scalded skin syndrome, because neutrophils are frequently present, organized as subcorneal pustules. The separation that occurs in this syndrome is at the same level of the epidermis as pemphigus foliaceus; the exfoliative toxins act on desmoglein 1, the antigenic target of that form of pemphigus.12 However, the relative lack of inflammation and characteristic clinical setting of staphylococcal scalded skin syndrome allow distinction in most cases. In more difficult circumstances, direct immunofluorescence is decisive, because only pemphigus foliaceus shows the characteristic intercellular fluorescence.
Ecthyma
Clinical Features: Ecthyma is sometimes considered a “deeper” variant of impetigo. It is produced by streptococci or staphylococci, and it presents as a shallow ulcer with a thick overlying scale-crust. The lower legs and feet are particularly common sites of involvement. Treatment is by cleansing and use of topical or systemic antimicrobial agents.13,14
Microscopic Findings: Biopsy confirms the presence of a shallow ulcer with overlying neutrophilic scale-crust (Fig. 17-3). Neutrophils are identified in the underlying dermal infiltrate. Bacteria can sometimes be identified with special staining.
Differential Diagnosis: These lesions closely resemble the excoriations with shallow ulcers that can be seen in a variety of pruritic conditions, including prurigo simplex.15
Necrotizing Fasciitis
Clinical Features: This is a “deep” form of cellulitis with necrosis. A penetrating injury to the skin often initiates the process. Affected individuals may be immunosuppressed to some degree. The process begins with erythema and edema, followed by blistering and necrosis and associated with systemic symptoms. The skin takes on a dusky, purpuric appearance. Mortality is significant, ranging from 20% to 34% in some series.16 A variety of organisms have been responsible, including both aerobic and anaerobic bacteria, but group A streptococci are particularly linked to this disorder.17 Streptococcal toxic shock syndrome accompanies some of these cases. Surgical débridement and antimicrobial therapy are the mainstays of management.
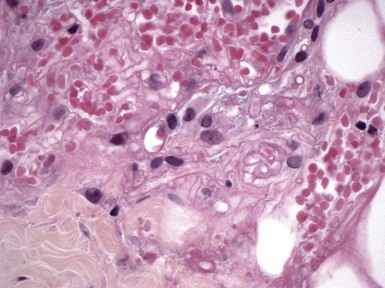
Microscopic Findings: Changes include edema, neutrophilic infiltration, necrosis of epithelial structures (including hyaline necrosis of eccrine sweat glands), and connective tissue degeneration18 (Fig. 17-4A and B). Mixed septal and lobular panniculitis and fasciitis have been described.19 Thrombosis of vessels is seen at all levels of the dermis and subcutis and is associated with neutrophilic infiltration, thereby having the appearance of a septic vasculitis18,20 (Fig. 17-5). Clotting abnormalities may play a role in some cases, and in fact protein S deficiency has been reported on several occasions.21,22 A histopathologic classification scheme has been developed that focuses on the intensity of neutrophilic infiltration and the frequency of finding bacteria on Gram staining; stage 1 features neutrophilic infiltration and an absence of detectable bacteria, whereas stage 3 features few or no neutrophils in the face of positive Gram staining.23
Differential Diagnosis: The depth of involvement, as well as the degree of connective tissue degeneration and necrosis, are much greater in necrotizing fasciitis than in erysipelas or traditional forms of cellulitis. Pyoderma gangrenosum shows neutrophilic infiltration and necrosis, but thrombosis with changes of septic vasculitis are not seen in that disorder, except perhaps for “secondary vasculitis” seen in the immediate vicinity of the ulceration. Necrosis and connective tissue degeneration are also seen in forms of ischemia, including calciphylaxis and bullae related to coma or barbiturate intoxication, but these conditions lack the neutrophilic infiltration of necrotizing fasciitis, and Gram stains and tissue culture studies are typically negative.
Infectious Folliculitis
Clinical Features: Folliculitis due to bacterial infection is relatively common. It can be seen in otherwise healthy individuals, as a complication of atopic dermatitis, or as a manifestation of immunosuppressed states, such as acquired immunodeficiency syndrome (AIDS).
The furuncle, commonly known as a boil, presents as a tender, erythematous nodule that often forms a central pustule. Incision and drainage can be used to remove purulent and necrotic material.
The carbuncle consists of several adjacent follicles involved with the same type of process. S. aureus is the responsible organism in most cases. Treatments include warm compresses, incision and drainage when lesions become well demarcated and fluctuant, and appropriate antimicrobial agents.
Pseudomonas folliculitis is often contracted in contaminated swimming pools or hot tubs—hence the alternative term “hot tub folliculitis.” Papules, pustules, and nodules develop over the trunk and extremities, particularly in areas covered by a bathing suit. Patients are typically well in other respects. The causative organism is Pseudomonas aeruginosa. Cleaning of the contaminated facilities, with proper filtration and chlorination or bromination, should prevent outbreaks of this disorder. The lesions typically involute without treatment in 7 to 10 days, but antimicrobial therapy can also be used in selected circumstances. In one set of cases of Pseudomonas folliculitis occurring in immunosuppressed patients, the lesions evolved into ecthyma gangrenosum (see later discussion). The organism, P. aeruginosa serotype O-11, was found in the hospital water system where the infections occurred.24
Microscopic Findings: In furunculosis, a neutrophilic abscess forms around and within one or more hair follicles; this extends into the surrounding dermis and into the subcutis (Fig. 17-6). Follicular destruction ensues.25 An experimental study in mice inoculated with S. aureus has shown that these organisms attach to keratinocytes, where they proliferate around and then invade the hair follicle, extending in single-file fashion between the inner and outer root sheaths.26 In Pseudomonas folliculitis, there is an acute suppurative folliculitis with intraluminal abscess formation and associated perifolliculitis.27,28 Organisms are difficult to identify in tissue sections but can be readily cultured from the lesions.29
Differential Diagnosis: Bacterial folliculitis can resemble other forms of acute suppurative folliculitis, and differentiation requires knowledge of the clinical presentation as well as Gram staining and/or culture studies. Examples include acneiform folliculitis, fungal folliculitis (Majocchi granuloma), and the early neutrophilic folliculitis of pyoderma gangrenosum.
Toxic Shock Syndrome
Clinical Features: Two disorders have received this designation: staphylococcal toxic shock syndrome and streptococcal toxic shock syndrome. Both are multisystem diseases. Staphylococcal toxic shock syndrome results from infection with strains of S. aureus that produce a particular toxin, termed toxic shock syndrome toxin-1 (TSST-1). Initial cases occurred in women using highly absorbent tampons; despite changes in tampon manufacturing, “menstrual” toxic shock syndrome still accounts for 50% of cases.30 Other sources of infection include surgical procedures, nasal packing, burns, and various cutaneous pyodermas. Features include fever, erythema, desquamation of palms and soles, hypotension, and involvement of multiple organ systems. Management includes the use of antibiotics, sometimes with corticosteroids or intravenous immunoglobulin. Streptococcal toxic shock syndrome accompanies disruption of the epidermal barrier with infection, is seen with destructive soft tissue infections such as necrotizing fasciitis, and is usually accompanied by bacteremia.31 The streptococci are usually M-types 1 and 3 with production of streptococcal pyrogenic exotoxins A to C (particularly A). Local pain, swelling, necrosis, or bulla formation is followed by fever, chills, myalgia, diarrhea, and central nervous system symptoms. Desquamation of palms and soles also occurs in some patients. Shock and multiorgan failure can ensue over a short period of time (48 to 72 hours). Both types of toxic shock syndrome are due to the superantigen properties of the exotoxins, resulting in profound T-cell activation and release of large quantities of cytokines.32 Treatment involves the use of antimicrobials that also inhibit bacterial toxins, along with intravenous immunoglobulin, surgical management of accompanying soft tissue infections, and supportive therapy.
Microscopic Findings: In staphylococcal toxic shock syndrome, there may be focal spongiosis with neutrophilic exocytosis, clusters of apoptotic keratinocytes, variable papillary dermal edema, and a perivascular and interstitial infiltrate that includes neutrophils, lymphocytes, and occasional eosinophils.33
In streptococcal toxic shock syndrome, the superficial changes may be similar to those of staphylococcal toxic shock syndrome, but there may be more extensive epidermal necrosis with subepidermal separation, hemorrhage, thrombosis,34 and suggestive vasculitic changes. Deeper thrombosis and inflammation are encountered when the findings are superimposed on necrotizing fasciitis.
Differential Diagnosis: Some of the superficial changes, such as edema and neutrophilic infiltration, are also seen in cellulitis and erysipelas. Significant epidermal necrosis or thrombosis, with or without vasculitic changes, suggests that the more severe toxic shock syndromes may be present, particularly streptococcal toxic shock syndrome, when associated with necrotizing fasciitis or disseminated intravascular coagulation.
Erysipelas and Cellulitis
Clinical Features: These are similar processes.35 In erysipelas, well-demarcated, warm, erythematous, indurated plaques are found in a variety of locations, but particularly the face and legs. It is caused by group A beta-hemolytic streptococcal infection, although other streptococcal groups are responsible in different clinical circumstances. Various types of wounds may serve as the source of infection, with contributing factors that include chronic venous stasis and lymphedema.36,37 Cellulitis involves the deep dermis and subcutis and is most often due to group A streptococci or S. aureus, although Haemophilus influenzae has been a cause in children, and gram-negative aerobic and anaerobic bacteria can play a role in people with diabetes. Again, breaks in the skin, such as wounds or tinea pedis infection, may be responsible in many cases. Lesions consist of spreading erythema, sometimes with development of lymphangitic streaks and bullae. Fever and malaise may accompany the process. Both are treated with appropriate antimicrobial agents.38
Microscopic Findings: Erysipelas shows pronounced dermal edema, lymphatic dilatation, and a diffuse but often sparse infiltrate composed of neutrophils (Fig. 17-7). Perivascular accentuation of these cells is only slight. Although bacteria can be found within the involved tissues and sometimes in lymphatic spaces using Gram stain or other methods,39 in the author’s experience this is a rare event, and even tissue culture studies yield a high false negative rate. Immunofluorescent staining, if available, is a more sensitive method of detecting the causative organisms. The findings are similar in cellulitis, but the changes may be found deeper in the dermis or in the subcutis (Fig. 17-8).
Differential Diagnosis: Histopathologic evaluation is not always carried out in cases of erysipelas and cellulitis, and when it is used it generally provides only supportive rather than diagnostic information. The finding of neutrophils in the dermis, in the absence of an obvious source, such as ulceration, ruptured follicles or cysts, or leukocytoclastic vasculitis, should always raise the possibility of infection and prompt cultures or other laboratory studies. The neutrophilic infiltrate in Sweet syndrome is much heavier than in erysipelas or cellulitis, tends to be concentrated in the mid-dermis (although subcutaneous infiltration can definitely occur in that disease), and is associated with leukocytoclasis. Rheumatoid disease can be accompanied by cutaneous neutrophilic infiltrates, two examples being rheumatoid neutrophilic dermatosis and interstitial granulomatous dermatitis (the latter is not restricted to rheumatoid disease and can be seen in a wide variety of connective tissue, inflammatory, and infectious processes). The density of neutrophils in rheumatoid neutrophilic dermatosis and the presence of interstitial granulomas in interstitial granulomatous dermatitis would be distinctive. Alpha-1 antitrypsin deficiency panniculitis can be preceded or accompanied by deep dermal neutrophils that “splay” the collagen bundles, but there are characteristic degenerative and necrotizing changes in the subcutis that set that process apart.
Cutaneous Manifestations of Septicemia
Clinical Features: Characteristic cutaneous features are seen in several septicemic processes, largely due to the vasculopathy that accompanies these lesions.
Meningococcemia is due to infection with Neisseria meningitidis, a gram-negative diplococcus. In acute meningococcemia, there are fever, chills, hypotension and meningeal signs and symptoms, accompanied by an eruption of petechial to ecchymotic lesions. The latter are often angulated and display a “gun metal gray” necrotic appearance. This is a fulminant lethal disease if not treated promptly with antimicrobial therapy. Chronic meningococcemia is a more indolent although relapsing manifestation of the infection in which papular or nodular, purpuric skin lesions are accompanied by episodes of fever and arthralgias. Whereas acute meningococcemia is associated with endothelial injury and bacterial endotoxin–induced clotting (disseminated intravascular coagulation),40 chronic meningococcemia represents an immune complex vasculitis,41 most likely triggered by release of bacterial antigens. C7 deficiency has also been reported in association with chronic meningococcemia.42
Gonococcemia features fever, arthralgias, and a hemorrhagic pustular eruption. Typically, there are relatively few of these necrotizing pustules, and they are located on distal portions of extremities, particularly over large joints. This manifestation of gonococcal sepsis, sometimes termed the gonococcal dermatitis-arthritis syndrome, is uncommon, but tends to be seen in women with genital infections,43 following menses, or in those with deficiencies in late complement components. Endothelial damage with subsequent thrombosis is thought to be responsible, but circulating immune complexes can also be detected.44
Ecthyma gangrenosum is a manifestation of Pseudomonas sepsis. The lesions initially present as wheals, papules, and papulopustules that rapidly become hemorrhagic, violaceous, and necrotic. They quite often develop over the buttocks, inguinal region, or extremities. Patients with ecthyma gangrenosum are often significantly debilitated, with underlying conditions that include pancytopenia, leukemia, severe burns, or malignancy.
Subacute bacterial endocarditis, most often associated with infection by S. aureus, is associated with several classic peripheral signs that include Roth spots (retinal hemorrhages with white or pale centers), subungual hemorrhages, Osler nodes, and Janeway lesions. Osler nodes are tender erythematous papules that occur on ventral surfaces of the fingertips and toes, whereas Janeway lesions are similar-appearing but nontender macules, papules, or nodules that develop on the palms and soles. The current view is that, in subacute bacterial endocarditis, these two lesions are manifestations of immune complex vasculitis—most likely a response to bacterial antigens—rather than embolic phenomena arising from endocardial vegetations.45 However, in acute bacterial endocarditis, there is evidence that septic emboli are responsible.46,47
Microscopic Findings: In acute meningococcemia, there is severe vessel injury, consisting of thrombosis, necrosis of endothelial cells, smooth muscle cells and pericytes, and relatively sparse infiltrates of neutrophils with minimal leukocytoclasis. Numerous meningococci can be observed within endothelial cells and neutrophils. Immunoglobulins and complement can be identified in vessel walls.48 In chronic meningococcemia, the changes are those of leukocytoclastic vasculitis49 (Fig. 17-9). Organisms cannot be demonstrated with the usual histopathologic methods, including direct immunofluorescence.50 However, N. meningitides–specific polymerase chain reaction (PCR) technology applied to skin biopsy specimens has recently been used successfully to diagnose chronic meningococcemia.51

Figure 17-9 Chronic meningococcemia. The findings are those of leukocytoclastic vasculitis involving a medium-sized, deep dermal vessel.
In gonococcemia, findings are similar to those in meningococcal septicemia and include a combination of vasculitis and thrombosis (Fig. 17-10). Superficial and deep dermal vessels are involved, including arterioles, and the infiltrating cells include lymphocytes as well as neutrophils. Erythrocyte extravasation is also prominent. Gonococci are uncommonly identified in blood vessel walls with routine methods (i.e., Gram staining),52 but they can be found within the contents of pustular lesions and can also be detected using direct immunofluorescence. The likelihood of finding organisms in tissue sections may depend on the degree of bacteremia and the age of the sampled lesion.52 Culture studies from skin lesions are frequently negative, probably indicating that organisms present in these lesions are frequently nonviable. However, cultures from cervix, blood, or other sites can be used to confirm the diagnosis.
In ecthyma gangrenosum, necrotizing changes of dermal vessels are present, often with limited or no appreciable inflammation.53,54 Numerous Pseudomonas organisms can be identified in the walls of vessels, generally sparing the lumen and intima (Fig. 17-11). Necrosis of epidermis and adnexal structures and degenerative changes in connective tissues are frequently observed.55 The changes in Osler nodes and Janeway lesions in bacterial endocarditis may vary, depending on whether the condition is acute or subacute.

Figure 17-11 Ecthyma gangrenosum. Numerous bacillary organisms, representing Pseudomonas, surround thrombosed vessels with necrotizing changes.
Lesions of subacute bacterial endocarditis show leukocytoclastic vasculitis56 (Fig. 17-12), while those of acute bacterial endocarditis are more apt to show emboli and dermal microabscess formation.46
Differential Diagnosis: The changes of “septic vasculitis” (vascular injury with both inflammation and fibrosis) are features of acute meningococcemia, gonococcemia, ecthyma gangrenosum (although inflammation is often slight in the latter), and acute bacterial endocarditis. Similar findings could be seen in septicemia due to other microorganisms, and therefore special stains and culture studies are warranted as part of the workup of these cases, with the realization that both may be negative when performed on the skin lesions. For that reason, cultures of blood or other tissues or special methods such as PCR may be necessary. Vasculitis with thrombosis can also be seen in noninfectious disorders—particularly, in the author’s experience, cryoglobulinemia. The infiltrate-poor vascular changes in ecthyma gangrenosum can also be seen in Vibrio vulnificus septicemia. This infection can develop following consumption of raw seafood or through wounds contracted along coastal waters. Indurated plaques and bullae show cutaneous necrosis and connective tissue degeneration, with myriads of organisms (which, like Pseudomonas, are gram-negative bacilli) in the walls of vessels. The changes in chronic meningococcemia and subacute bacterial endocarditis resemble those of leukocytoclastic vasculitis due to other causes, and therefore other clinical and laboratory data are necessary to establish the correct diagnosis in such cases.
Botryomycosis
Clinical Features: Botryomycosis is an uncommon form of bacterial infection characterized microscopically by the presence of granules that resemble those of actinomycosis and mycetoma (see subsequent discussions). The lesions consist of nodules that can become crusted or drain purulent material containing granules similar to the sulfur granules of actinomycosis. Patients with botryomycosis lesions are often immunosuppressed, demonstrating deficient cell-mediated immunity in the face of a normal or hyperactive humoral immune system. Association with AIDS has been reported.57 A wide variety of gram-positive and gram-negative organisms can produce these lesions, with S. aureus being a frequent agent. Experimental reproduction of these lesions has been difficult to accomplish.58,59 Cultures of lesions are frequently negative, and even mouse inoculation may fail to demonstrate evidence for infection. Furthermore, the lesions may be resistant to systemic antimicrobial therapy. These findings may be related to the relatively “protected” environment created by the peculiar host interaction with the organism.
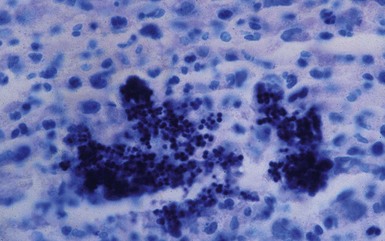
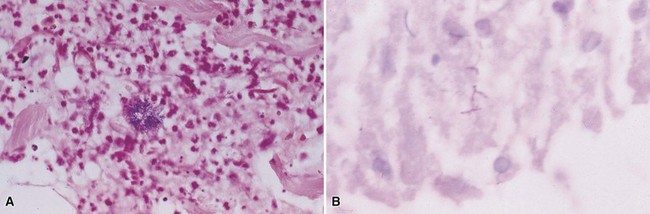
Microscopic Findings: Skin biopsies show varying degrees of hyperkeratosis and acanthosis. There is a perivascular infiltrate composed of lymphocytes, macrophages, and plasma cells within the superficial and deep dermis. Dermal neutrophilic abscesses contain granules that consist of an eosinophilic matrix (Splendore-Hoeppli phenomenon) and central collections of organisms (Fig. 17-13). In the case of S. aureus, these are composed of basophilic, gram-positive cocci, arranged in pairs and tetrads, which stain particularly well with the Giemsa method (Fig. 17-14). IgG and C3 deposition within the granules has been found with both immunofluorescent and immunohistochemical methods57,60 and reflects a host immune response to bacterial antigens.
Differential Diagnosis: The grains of botryomycosis must be distinguished from those associated with other classes of organisms, including higher bacteria, such as actinomycetes and Nocardia, and true fungi. The morphology of these organisms is quite different from the agents of botryomycosis and can be evaluated through the use of periodic acid–Schiff (PAS) and Grocott methenamine silver (GMS) as well as Gram stains (for further information, see “Nocardiosis,” “Actinomycosis,” and “Mycetoma”).
Malakoplakia
Clinical Features: Malakoplakia (alternative spelling, malacoplakia) was first described in 1902, when Michaelis and Gutmann described three examples of a tumor of the urinary bladder with unique intracellular inclusion bodies.61,62 In 1903, von Hansemann described aggregates of short bacilli attached to similar-appearing cells. It was later learned that these cells, now called von Hansemann cells, are macrophages. He named the disorder “malacoplakia vesicae urinariae” (soft plaque of the urinary bladder).61,63 Although the genitourinary tract is involved most often, the process has been identified in skin, either through extension from an underlying focus of disease or as a primary cutaneous lesion.64,65 A number of the cutaneous cases have occurred in the perianal region.63 Lesions have presented as ulcers, indurated areas with draining sinuses, fluctuant masses, or perianal polyps.61 Escherichia coli is implicated in the majority of cases, but other organisms have been described, including Rhodococcus equi in patients with AIDS.66 The occurrence of malakoplakia has been linked to immunosuppression, associated with sarcoidosis or corticosteroid therapy in some cases.61 The pathogenesis of the disease may be related to macrophages with defective bactericidal capability. Although the mechanism is unclear, one study indicated decreased cyclic guanosine monophosphate in monocytes, associated with defective release of lysosomal β-glucuronidase.67 Treatment includes antimicrobial therapy, excision for localized lesions, and treatment of possible underlying immunosuppressive disorders.
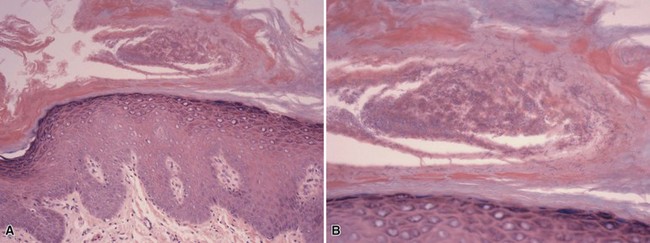
Microscopic Findings: There are sheets of large macrophages with granular cytoplasm (von Hansemann cells) within the dermis and subcutis (Fig. 17-15). The key microscopic features are the Michaelis-Gutmann bodies, found within macrophages. These targetoid inclusion bodies, somewhat difficult to identify with H&E stain, are thought to represent accumulations of nonexocytosed phagolysosomes that have calcified. They possess a dense calcium hydroxyapatite core and variable amounts of iron. Therefore, they are best demonstrated with PAS, von Kossa, Perls, and Giemsa methods68 (Fig. 17-16A and B). Bacteria, usually gram-negative bacilli (E. coli), can sometimes be identified within macrophages.69 Polyclonal anti–bacille Calmette Guérin (anti–Mycobacterium bovis) has also been used successfully to identify organisms and is particularly useful when only a few microorganisms are present in tissue sections.70
Differential Diagnosis: Macrophages with granular cytoplasm could bear some resemblance to the cells of granular cell tumor. The cells of granular cell tumor are polyhedral, have small central hyperchromatic nuclei, and occasionally contain large granules termed pustule-ovoid bodies of Milian. The classic examples of this tumor are thought to be composed of modified Schwann cells and are S-100 positive, a result not seen in malakoplakia. Granular cell changes in other tumors (e.g., dermatofibroma, basal cell carcinoma) have other configurational features more in keeping with ordinary examples of those tumors, as well as characteristic immunohistochemical findings. The rare examples of the primitive nonneural granular cell tumor are negative for S-100 but stain for other markers such as NKI/C3 and PGP9.5.71 At low magnification, the granularity of von Hansemann cells can suggest xanthoma, but close inspection of cell detail shows that the cytoplasm of these cells is granular rather than finely vacuolated. Sometimes, changes in healing wounds include accumulations of macrophages with granular cytoplasm. However, in none of these potentially confounding lesions are there structures with the morphologic and staining characteristics of Michaelis-Gutmann bodies.
Corynebacterial Infections
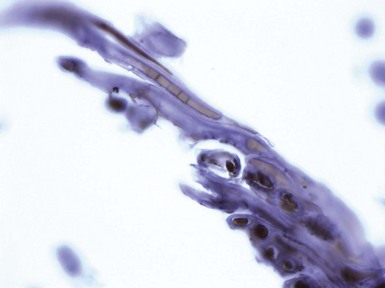
Clinical Features: Two particular cutaneous infections caused by corynebacteria are erythrasma and pitted keratolysis. Another cutaneous infection, trichomycosis axillaris/pubis, is caused by Corynebacterium tenuis and occasionally other Corynebacterium species. It is characterized by yellowish, red, or black nodular deposits along hairs and is diagnosed by gross or microscopic inspection of the hair shafts; the latter shows numerous bacteria embedded in the material encasing the involved hairs.
Erythrasma consists of well-demarcated, brownish red, scaly patches in intertriginous locations, especially the groin, toe webs, axillae, and inframammary areas. Itching, or a burning sensation, is common in the groin area. The causative organism is Corynebacterium minutissimum, a small gram-positive bacillus. The lesions show a distinctive coral red fluorescence with Wood light, which emits light mainly at a wavelength of 360 nm; the fluorescence is due to a porphyrin produced by the organism. This porphyrin production has actually been exploited in a treatment option using photodynamic therapy.72 Most often, however, topical or systemic antimicrobial agents are used in management, including erythromycin. Pitted keratolysis causes the formation of shallow, rounded pits in stratum corneum, especially in the soles of the feet. This is particularly prone to occur in hot, humid conditions when there is increased sweating. The author has also found this infection in a transgrediens variant of palmar hyperkeratosis. The cause is the keratolytic action of certain bacteria, including Corynebacterium species; however, other organisms can be responsible for this condition, including the gram-positive coccoid organism Kytococcus (formerly Micrococcus) sedentarius and Dermatophilus congolensis, a gram-positive facultative anaerobe that produces thin filaments and coccoid forms. Coalescent pits can form linear depressions or shallow “fissures.” Topical erythromycin and clindamycin have been used successfully to treat these lesions.73
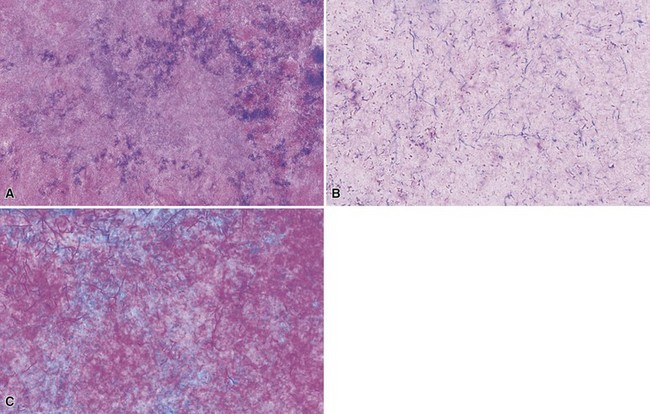
Microscopic Findings: In erythrasma, the biopsy findings are often minimal, sometimes consisting of only slight spongiosis. The organisms, small coccobacilli, can be found in the stratum corneum but most often require special staining for confirmation74,75 (Fig. 17-17A and B). Those that can be used effectively include Gram, PAS, or GMS stains.76 Pitted keratolysis shows irregularities of the surface stratum corneum with formation of pits that extend to various depths. Within the base of the pits are coccoid to filamentous organisms that again can be recognized with Gram (gram-positive), PAS, or GMS methods73,77,78 (Fig. 17-18A and B).
Differential Diagnosis: The microscopic changes of erythrasma and pitted keratolysis can be quite subtle (this is particularly the case in erythrasma), and as a result these conditions qualify as “invisible dermatoses.”79 In the absence of a supportive clinical history, a high index of suspicion needs to be maintained, and part of the evaluation of a biopsy with minimal findings includes the performance of special stains for organisms (Gram, PAS, or GMS). Once the organisms have been found, they must be distinguished from Pityrosporum organisms, dermatophytes, or Candida species. However, the hyphae, pseudohyphae, or spores in those conditions tend to be much larger than the small coccobacillary forms and filaments of corynebacteria, despite overlapping results with special stains.
Anthrax
Clinical Features: Anthrax has gained notoriety among professionals and the public due to its potential use in terrorist attacks. In the United States, the usual source of infection is the handling of infected animal hides or wool (“wool sorters disease”). Aerosolized spores of the infectious agent, Bacillus anthracis, sent through the mails in the form of a powder, were used as a form of bioterrorism in 2001.80,81 Inhalation results in a necrotizing mediastinal infection, with consequent bacteremia and hemorrhagic meningitis. Similarly, ingestion of spores causes intestinal ulceration with hemorrhage. Most cases in the United States consist of cutaneous lesions, which begin as papules that enlarge and evolve into hemorrhagic pustules, followed by formation of a black eschar surrounded by erythema. Surprisingly, pain is minimal. Prompt antimicrobial treatment is essential.
Microscopic Findings: Fully developed lesions are necrotic, with an ulcerated surface. The adjacent epidermis shows spongiosis with acantholysis and microvesicle formation. The dermis is markedly edematous. Vasodilatation and erythrocyte extravasation are pronounced. The intensity of inflammation is variable, but the infiltrate consists mainly of neutrophils, which are found within both the dermis (sometimes with abscess formation) and viable portions of the epidermis.82 A mixture with other inflammatory cell types, including lymphocytes and macrophages, is also described.83 There may be thrombosis and/or fibrin deposition around vessel walls.83 The numbers of organisms vary from case to case. They can occasionally be identified in routinely stained sections but often require special staining. With Gram staining, they appear as relatively large gram-positive bacilli measuring approximately 1 × 9 microns. Other methods that are more sensitive include silver stains (the Steiner and Steiner method) and immunohistochemical staining using antibody to B. anthracis antigen.82
Differential Diagnosis: The combination of necrosis, ulceration, and pronounced dermal edema with neutrophils certainly suggests other types of infectious disease, such as necrotizing fasciitis, although in the latter example the clinical findings are quite different. Other noninfectious dermatoses may be considered, particularly those that feature combinations of dermal edema and neutrophilic infiltration, such as pyoderma gangrenosum and Sweet syndrome. Pronounced dermal edema occurs in virally induced, orf, or milker nodules. In those infections, there are marked elongation of the rete ridges and vacuolated keratinocytes with intranuclear eosinophilic inclusion bodies. However, in the acute weeping stage of those lesions, the epidermis is necrotic and inclusions are not identifiable, so that there may be greater overlap with the findings in anthrax. The key to the diagnosis of anthrax is a high index of suspicion and the obtaining of a Gram stain, which has a high likelihood of showing the characteristic organisms. The infection can be confirmed by culture studies. There are several potential caveats. First, with age these organisms can show variable staining or become gram-negative. Second, if laboratories are not alerted to the possibility of anthrax, it is possible that cultures indicating the presence of Bacillus species may not be further analyzed due to the likelihood of a common contaminant organism, Bacillus cereus.80
Chancroid
Clinical Features: Chancroid is a sexually transmitted disease caused by Haemophilus ducreyi, a gram-negative coccobacillus. Regional outbreaks of the disease are reported from time to time (e.g., in Orange County, California,84 and in the New Orleans area),85 and it is encountered in human immunodeficiency virus (HIV)–positive individuals.86 Lesions begin in the genitalia as macules or pustules and evolve to form tender ulcers with minimal induration. Autoinoculation results in the formation of “kissing lesions.” Suppurative lymphadenitis occurs in some cases. Diagnosis is made by a combination of clinical features, smears, and cultures. However, coexistence with other sexually transmitted diseases, including syphilis, is definitely possible,87 and other evaluations, including serologic testing, should be part of the workup in cases of possible chancroid infection. Treatment includes antimicrobial therapy, including macrolide antibiotics or ciprofloxacin.
Microscopic Findings: The microscopic features of chancroid are fairly characteristic, if not pathognomonic, but require a well-oriented biopsy of sufficient depth to assess superficial and deep dermal changes. There is a “triple zone” arrangement beneath the ulcer, each horizontally oriented zone showing different microscopic changes. At the ulcer base, there is a zone of fibrin deposition with intermingled necrotic tissue, neutrophils, and erythrocytes. Beneath this is a zone composed of proliferative vessels, some with endothelial proliferation, degenerative change, and thrombus formation. Deep to this focus is a zone composed mainly of lymphocytes and plasma cells.88 Although this zonation can indeed be observed, it is not always as neatly laid out as implied by the description (Fig. 17-19A and B). Organisms are difficult to identify in tissue sections, even when stained with Gram or Giemsa methods. Smears from the undermined ulcer edge are more likely to show the organisms, which have a tendency to line up in a “school of fish” arrangement. Cultures require the use of special procedures and media, as have been detailed in a recent study.89
Differential Diagnosis: Despite the zonation observed in examples of chancroid, this arrangement may not be clearly observable in all cases. This may be due in part to biopsy technique; however, ulcerated lesions due to a number of different causes tend to show fibrin, proliferative vessels, and admixed acute and chronic inflammation. This makes specific diagnosis difficult, particularly when organisms are so difficult to identify in tissue sections. Two sexually transmitted diseases that bear a clinical resemblance to (and may even coexist with) chancroid are herpesvirus infection and syphilis. Therefore, histopathologic evaluation may also require careful search for viropathic changes or immunohistochemical evaluation for herpes simplex or treponemal antigens. Ulcerative diseases that are noninfectious, such as aphthosis or Behçet disease, also show similar morphologic features in biopsy specimens.
Granuloma Inguinale
Clinical Features: Granuloma inguinale, also known as donovanosis, is a sexually transmitted disease produced by the intracellular gram-negative bacillus Klebsiella granulomatis. This organism had been previously designated Calymmatobacterium granulomatis, but gene sequencing has shown a high level of identity with species of Klebsiella that are human pathogens.90 After a variable incubation period, a papule or nodule appears and subsequently ulcerates, forming a highly vascular, expanding lesion with a distinctly “beefy” appearance. “Pseudobuboes” represent inguinal swellings associated with the ulcers; they have received this name because they do not represent lymphadenopathy but subcutaneous granulation tissue that may subsequently break down and ulcerate. Lesions may be particularly extensive, but the level of pain associated with them tends to be disproportionately low. Diagnosis often depends on smears obtained from tissue scrapings or as touch preparations of skin biopsies; culture methods are being worked out but are not routinely available. The infectious agent has been detected using a procedure termed genital ulcer disease multiplex PCR, with amplification products detected by an enzyme-linked amplicon hybridization assay.91
Microscopic Findings: In fully developed lesions, there is an ulcer surrounded by marked acanthosis, which may achieve pseudoepitheliomatous proportions (Fig. 17-20). Exuberant granulation tissue is present near the ulcerated surface. The underlying dermis shows a dense, mixed infiltrate composed of macrophages, plasma cells, a few lymphocytes, and scattered neutrophils that sometimes form microabscesses (Fig. 17-21). The organisms, known as Donovan bodies, are found within macrophages but are easier to find in smears and tissue imprints. They are approximately 1 to 2 mm in diameter and best seen with Giemsa stain, showing bipolar staining with a resemblance to a “closed safety pin.”92,93 Wright-Giemsa is an effective staining method when examining smears. On semithin sections obtained from Epon-embedded specimens, the organisms appear to be surrounded by a vacuolated structure that represents a phagolysosome.94 However, ultrastructural study has also shown a layer of homogeneous material surrounding the organisms, suggesting that there may be a surrounding capsule.95
Differential Diagnosis: The tissue morphology of the granulomatous lesions, in the appropriate clinical context, is suggestive of granuloma inguinale but not entirely diagnostic, because many of the changes can be seen in ulcerative diseases due to a number of causes. Therefore, diagnosis depends on finding the organisms. Their intracellular location raises consideration of the other major “parasitized histiocyte” diseases: rhinoscleroma, histoplasmosis, and leishmaniasis. The ability to distinguish among them obviously is greatly enhanced by knowledge of the clinical presentation. However, the organisms of granuloma inguinale are slightly smaller (about 1 to 2 µm) than those in the other three conditions, whose organisms are all in the range of 2 to 4 µm. The organisms of rhinoscleroma (also representing a species of Klebsiella) lack a surrounding capsule-like space. Histoplasma organisms are surrounded by a clear space that does not represent a true capsule, and they stain well with the traditional methods for fungi, PAS, and GMS. Leishmania are nonencapsulated organisms that feature both a nucleus and a kinetoplast, best demonstrated with a Giemsa stain. Toxoplasma organisms can sometimes be found in macrophages but may be organized as pseudocysts and are also present in keratinocytes of epidermis and sweat ducts and within arrector pili muscle.96 The organisms are weakly positive for PAS but are negative for the GMS stain.96 They can also be stained with antibodies specific for Toxoplasma.
Rhinoscleroma
Clinical Features: Rhinoscleroma is a chronic inflammatory disease of upper respiratory structures caused by a gram-negative bacillus, Klebsiella rhinoscleromatis. This condition is encountered in Central and South America and parts of Asia, and it is occasionally reported in the United States. Rhinoscleroma particularly affects the nasal vestibule, but other parts of the nose and pharynx can also be involved. Nasal discharge is followed by nodular and sclerosing lesions that sometimes ulcerate. Nasal obstruction and mutilating changes of the central portions of the face ensue. Treatment is often prolonged and includes fluoroquinolones and other agents as well as surgical intervention when necessary.97,98
Microscopic Findings: The lesions are composed of numerous plasma cells, many of which form Russell bodies and vacuolated macrophages termed Mikulicz cells.98,99 Russell bodies are eosinophilic, somewhat refractile structures that are either anucleate or show a pyknotic, compressed nucleus at one edge. The appearance of these bodies is due to accumulation of immunoglobulin within plasma cell cytoplasm. Mikulicz cells, some of which are quite large (>100 µm in diameter), contain the Klebsiella organisms; these stain with Gram, PAS, or silver stains such as the Warthin-Starry stain. They can be seen in tissue sections, where they are somewhat easier to identify than the organisms of granuloma inguinale, but as in the latter infection, they can also be demonstrated in tissue smears or imprints.100
Differential Diagnosis: Differentiation from other infectious diseases with “parasitized histiocytes” is discussed earlier (see “Granuloma Inguinale”), with particular reference to the morphology of these microorganisms in tissues. In rhinoscleroma, the vacuolated Mikulicz cells are particularly distinctive, much more so than is the case in granuloma inguinale, which in turn is said to have dermal neutrophilic microabscesses as one of its distinctive features. Pseudo-Mikulicz cells have recently been reported adjacent to a superficially invasive squamous cell carcinoma; no organisms were found in this case, and progressive disease was not documented.101 Plasma cells can be encountered in any of these disorders, and in addition can be numerous in other situations; examples include secondary syphilis (where they are arranged around vessels in a “coat-sleeved” configuration), plasmacytoma, and as a host response to malignant epithelial tumors such as basal cell carcinoma and squamous cell carcinoma. Russell bodies are occasionally prominent in plasma cell infiltrates due to a variety of causes, but among the “parasitized histiocyte” diseases, they are most characteristic of rhinoscleroma. In the author’s experience, they are usually difficult to identify in neoplastic plasmacellular proliferations such as plasmacytoma or cutaneous involvement in multiple myeloma.
Cat Scratch Disease and Bacillary Angiomatosis
Clinical Features: Cat scratch disease in most cases is caused by Bartonella (Rochalimaea) henselae, an aerobic, gram-negative bacillus. Within 2 to 5 days following a scratch or bite from an infected cat, a skin lesion may develop, consisting of one or more papules resembling insect bites. Within several weeks of the primary skin lesion, tender, swollen lymph nodes develop in the drainage site. Fever, headache, backache, or abdominal pain may develop. Suppuration develops at the site of the involved lymph nodes in up to 50% of cases. A widespread exanthem and erythema nodosum are additional cutaneous findings that are observed in some cases. When the conjunctiva is the primary site of the lesion, granulomas can develop at the site associated with preauricular adenopathy; this is known as Parinaud oculoglandular syndrome. The adenopathy resolves over several months. Systemic complications include acute encephalopathy and splenomegaly, and involvement of liver and lung has been reported. Material obtained by skin biopsy or swab techniques can be cultured in human embryonic lung fibroblasts, but more rapid detection of the organism can be obtained by real-time reverse transcriptase–PCR using the same biopsy material.102
In bacillary angiomatosis, lesions develop that closely resemble pyogenic granulomas, although subcutaneous nodules or ulcers also occur. This condition most often develops in the setting of immunosuppression, particularly in AIDS, where the cause is also B. henselae, the same organism associated with cat scratch disease. The same types of lesions are encountered in homeless individuals who do not have AIDS; in these instances the causative organism is Bartonella quintana. Similar lesions also occur in individuals who live in the valleys of Peru and neighboring countries. Called verruga peruana, the lesions represent a stage of Oroya fever, or Carrion disease, and are caused by Bartonella bacilliformis. Systemic involvement can occur in bacillary angiomatosis, with lesions in the bones, liver, and spleen. Erythromycin, doxycycline, and other antimicrobial agents are effective in this disease.103–106
Microscopic Findings: In cutaneous lesions of cat scratch disease, the epidermis shows varying degrees of parakeratosis, edema, and exocytosis; an ulcer sometimes occurs. Within the dermis there are foci of necrobiosis, sometimes containing lymphocytes, surrounded by palisaded macrophages. Lymphocytes surround the palisaded macrophages, and eosinophils can also be identified. Additional findings include fragmented nuclei and perivascular infiltrates composed of lymphocytes, macrophages, and plasma cells107 (Fig. 17-22A). Bacillary organisms can be found in tissue sections, staining best with silver methods such as the Warthin-Starry stain (see Fig. 17-22B), but they can also be found with tissue Gram stains (Brown and Brenn).108 The organism, B. henselae, can also be detected by PCR methodology, and it can be found in archival tissues.109 Lymph node biopsies show similar but often better developed findings, including the characteristic “stellate” abscess, consisting of a central zone of necrosis containing numerous neutrophils, surrounded by palisades of macrophages, which in turn are surrounded by a zone of lymphocytes.107

Figure 17-22 Cat-scratch disease. A, This lesion was tangentially sectioned, accounting for the appearance of the epidermis. Within cross-sectional profiles of dermis, there are lymphocytes, macrophages, and plasma cells with some nuclear fragmentation and erythrocyte extravasation. B, Bacillary organisms can be identified with the Warthin-Starry stain.
In bacillary angiomatosis, there are dermal collections of blood vessels with plump endothelial cells that sometimes display significant cytologic atypia110 (Fig. 17-23). Occasional ulceration and a surrounding epidermal collarette contribute to an image resembling pyogenic granuloma. Neutrophils, with leukocytoclasis, are also identified (Fig. 17-24). The organisms appear as gray, granular material on H&E-stained sections. As in cat scratch disease, they are best demonstrated with silver stains, particularly Warthin-Starry and GMS (Fig. 17-25), but they can also be identified with Giemsa, PAS, or Alcian blue methods.110,111 Direct immunofluorescence or PCR can also be used to confirm the presence of organisms in tissues.

Figure 17-23 Bacillary angiomatosis. Low-power view shows an exuberant proliferation of vessels with accompanying inflammatory cells and erythrocyte extravasation.
Differential Diagnosis: The skin lesions are characteristic but not pathognomonic for cat scratch disease, and the changes could be mimicked by other conditions that feature palisaded necrobiotic granulomas, including tuberculosis, granuloma annulare, lupus miliaris, and the short-lived primary cutaneous lesions of lymphogranuloma venereum. The “stellate abscess” found in lymph nodes is also typical for this disease but can be found in a number of other conditions, including tularemia, lymphogranuloma venereum, and sporotrichosis. The author has also seen similar abscesses within lymph nodes draining the site of vaccination therapy for the treatment of melanoma. Therefore, detection of the organism using silver stains or PCR methods is key to making a specific diagnosis.
Regarding bacillary angiomatosis, these lesions should be distinguished from pyogenic granuloma, which shares a number of the low-power features, including (sometimes) ulceration, an epidermal collarette, and proliferative vessels in a variably edematous stroma. However, the endothelial cells in bacillary angiomatosis are larger and demonstrate greater atypia than seen in pyogenic granuloma. Neutrophils represent a significant finding in bacillary angiomatosis and are found in lesions even in the absence of ulceration. Furthermore, in contrast to bacillary angiomatosis, pyogenic granuloma represents a form of lobular capillary hemangioma, and, although grouping of vessels in lobulated fashion is not always obvious, most examples of pyogenic granuloma show this feature. The key to diagnosis is the detection of bacillary organisms, which as mentioned present as aggregates of gray material surrounded by neutrophils on H&E sections and can be more clearly demonstrated with silver methods. Kaposi sarcoma might also be considered in the differential diagnosis of lesions of bacillary angiomatosis, but irregular vascular channels, spindled cells, and hyaline globules are not found in bacillary angiomatosis,110 and staining for human herpesvirus-8 is also negative in this condition.112 Regarding endothelial markers, the cells in bacillary angiomatosis are regularly positive for factor VIII–related antigen and Ulex europaeus lectin110; in Kaposi sarcoma, the cells of well-formed vessels stain positively for factor VIII–related antigen and with Ulex europaeus, but the spindle cell component does not reliably stain for these markers.113
Mycobacterial Infections
Clinical Features: Cutaneous tuberculosis has become uncommon in the United States but is still seen in travelers, immigrants, and immunosuppressed individuals. Among those forms of the disease that are uncommonly encountered here are primary cutaneous tuberculosis, miliary tuberculosis, and tuberculosis cutis orificialis. Primary cutaneous tuberculosis arises from direct inoculation of the skin in an individual who has not been previously infected by Mycobacterium tuberculosis. It manifests as an ulcerated lesion that is followed by enlarged, tender regional lymph nodes—a similar scenario to the Ghon complex that develops in the lungs. Miliary tuberculosis consists of widespread papules and pustules on the skin due to hematogenous dissemination, usually in the setting of advanced disease. In tuberculosis cutis orificialis, shallow ulcers develop in mucocutaneous surfaces, again the result of advanced disease.
Other manifestations of the disease that are occasionally seen include lupus vulgaris, tuberculosis verrucosa cutis, and scrofuloderma. Lupus vulgaris is a form of secondary or reactivated tuberculosis that develops in previously infected individuals. It presents typically in the head and neck region as reddish brown papules or nodules. When examined using diascopy (pressing the lesions with a glass slide), the lesions have a distinctive “apple-jelly” appearance. Ulcerations and scars develop, and new lesions can develop in older, atrophic areas. Considerable facial deformity can occur. Squamous cell carcinoma can develop in old lesions. Tuberculosis verrucosa cutis arises from inoculation of the skin of an individual who has been previously infected and has substantial immunity to the organism. A classic example of this phenomenon is the “prosector’s wart” due to inoculation during performance of an autopsy. These verrucous papules and plaques most commonly arise in acral sites that are readily subjected to trauma. They may heal with scarring. In scrofuloderma, subcutaneous nodules form as the result of cutaneous infection from an underlying focus of disease, typically from cervical lymph nodes but also from other structures or via surgical procedures. These nodules suppurate and form draining sinuses, healing with linear, cordlike scars.
Tuberculids are cutaneous lesions that develop secondary to a distant focus of disease. They tend to reflect strong host immunity and have been considered autosensitization phenomena as is the case in “ids” of other types. Although mycobacteria traditionally cannot be identified in these lesions, M. tuberculosis DNA has been found in some of these lesions using PCR methods. Three lesions have been confirmed in their association with tuberculosis: erythema induratum (see Chapter 7),114 papulonecrotic tuberculid,115 and lichen scrofulosorum. The latter has not to date shown demonstrable mycobacterial DNA in skin lesions by PCR methods, although mycobacterial DNA has been found in lichen scrofulosorum lesions associated with Mycobacterium szulgai infection (an atypical mycobacterium in group II of the Runyon classification). Regarding the clinical appearance of these lesions, erythema induratum presents as subcutaneous nodules, sometimes ulcerated, particularly on the calves of the lower legs; papulonecrotic tuberculid as bilaterally symmetrical crops of necrotic to pustular lesions on the face and extremities; and lichen scrofulosorum as tiny discrete follicular papules on the trunk.
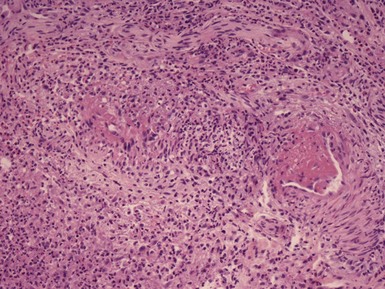
Microscopic Findings: Lupus vulgaris shows tuberculoid granulomas that are often concentrated in the upper dermis, particularly around follicular units, although they can also extend into the subcutis (Fig. 17-26A). Tuberculoid granulomas contain epithelioid macrophages, including multinucleated giant cells (some of the Langhans type with horseshoe arrangements of nuclei), surrounded by lymphocytes and plasma cells. Caseation is frequently absent, but when observed tends to be slight (see Fig. 17-26B). Zones of fibrosis are identified in long-standing lesions. Organisms are difficult to identify, even with traditional special stains such as the Ziehl-Neelsen method. Better results may be obtained by specific immunohistochemical staining or, particularly, with PCR methods.116 Tuberculosis verrucosa cutis shows hyperkeratosis, papillomatosis, and acanthosis (Fig. 17-27A). The inflammatory infiltrate can vary, from a mixture of neutrophils (including intraepidermal or dermal abscess formation), lymphocytes, and granulomatous elements to a more tuberculoid granulomatous infiltrate, uncommonly with caseous necrosis117,118 (see Fig. 17-27B). Although organisms are said to be more numerous in these lesions, they are usually difficult to identify with special stains, and even culture results are frequently negative. Scrofuloderma features a zone of caseous necrosis, surrounded by epithelioid macrophages, multinucleated giant cells of Langhans type, and plasma cells (Fig. 17-28). Organisms are present in sufficient numbers to be readily identified with special stains.119

Figure 17-26 Lupus vulgaris. A, This manifestation of secondary (reactivated) tuberculosis shows granulomas concentrated in the upper dermis. B, The granulomas are composed of epithelioid macrophages, surrounded by lymphocytes and plasma cells. Caseation is frequently absent.
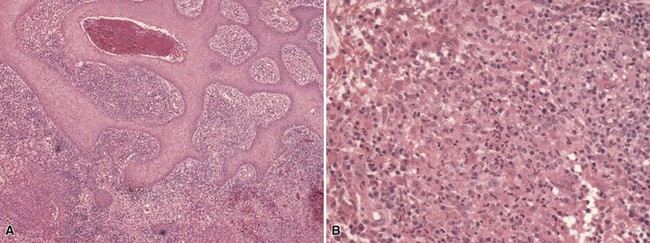
Figure 17-27 Tuberculosis verrucosa cutis. A, Findings include marked acanthosis (probably exaggerated here by tangential sectioning). B, The dermal infiltrate includes a mixture of neutrophils and epithelioid macrophages. Caseous necrosis was not identified in this lesion.

Figure 17-28 Scrofuloderma. Zones of caseous necrosis are surrounded by granulomatous elements, lymphocytes, and plasma cells.
Regarding the histopathology of tuberculids, the findings in erythema induratum are discussed in Chapter 7. Papulonecrotic tuberculid features vasculitis with thrombosis, wedge-shaped degenerative changes of the dermis or follicular necrosis, and poorly organized granulomatous inflammation.120–122 Organisms are not identified with routine methods but have been found with PCR technology. Lichen scrofulosorum lesions show noncaseating granulomas, typically surrounding hair follicles or sweat ducts; organisms are not identified.123
Differential Diagnosis: The rarity of tuberculosis in the United States (with the possible exception of immunosuppressed populations) increases the risk that tuberculosis may not be considered when evaluating granulomatous dermal infiltrates. It most often becomes a consideration when degrees of necrobiosis are seen in granulomatous infiltrates; therefore, conditions that may be confused include granuloma annulare, some examples of necrobiosis lipoidica, rheumatoid nodule, necrobiotic xanthogranuloma, and lupus miliaris (disseminatus faciei). Each of these has unique morphologic features that are not expected in cutaneous tuberculosis, including mucinous necrobiosis (granuloma annulare), horizontally layered fibrinous necrobiosis alternating with granulomatous foci (necrobiosis lipoidica), subcutaneous lesions with fibrinous necrobiosis, associated with vasculitic changes and a history of arthritis (rheumatoid nodules), and lipidization with foamy macrophages (necrobiotic xanthogranuloma). Lupus miliaris was once considered a tuberculid, shows necrobiosis that has a distinctly caseous appearance, and as in lichen scrofulosorum frequently involves hair follicles. However, these lesions are unassociated with other manifestations of tuberculosis and may have a closer relationship with acne rosacea or perioral/periorbital dermatitis. Mycobacteria are of course not identified in any of these conditions, but they may also be difficult to find in cutaneous manifestations of tuberculosis, requiring PCR or culture studies for confirmation.
However, forms of cutaneous tuberculosis often have little or no caseous necrosis; this is particularly the case in lupus vulgaris and tuberculosis verrucosa cutis. Therefore, granulomatous infiltrates of a variety of types need to be included in the differential diagnosis. These include other infectious diseases, such as atypical mycobacterioses, sarcoidosis, and granulomas of foreign body type. Tuberculids by definition do not show organisms with routine methods, and culture studies are negative. In these difficult circumstances, a diagnosis of tuberculosis requires a high index of suspicion; sometimes the use of PCR technology; and, most importantly, correlation with other clinical and laboratory findings together with an exposure history and possible link to immunosuppression.
Atypical Mycobacterial Infections
Clinical Features: Atypical mycobacteria are pathogenic organisms that are not causes of either tuberculosis or Hansen disease (leprosy). The traditional Runyon classification system for these organisms is based on certain growth characteristics:
• Group I, the photochromogens, produce a yellow color when exposed to light. This group includes two common causes of atypical mycobacterial infection: Mycobacterium marinum and Mycobacterium kansasii.
• Group II, the scotochromogens, also produce a yellow pigment in the dark. The principal organism is Mycobacterium scrofulaceum.
• Group III represents the nonchromogens and includes Mycobacterium avium-intracellulare as well as Mycobacterium haemophilum. Mycobacterium ulcerans is also included in this category.
• Group IV includes the rapid growers, producing colonies in 5 days. The organisms include Mycobacterium fortuitum, Mycobacterium chelonae, and Mycobacterium abscessus.
Among the atypical mycobacterial infections that involve the skin, three are discussed here: those due to M. marinum, M. chelonae, and M. ulcerans. M. marinum produces a lesion called “swimming pool granuloma,” which describes one potential source of this infection. The organism is found in fish and in both fresh and salt water, and many infections arise in connection with home aquariums. There is usually a history of previous or concomitant trauma to the skin, and acral surfaces, particularly the hands, are usually the sites of infection. Lesions consist of erythematous papules or nodules, which may have hyperkeratotic or verrucous surfaces. Involvement of underlying joints may occur. Sporotrichoid nodules extending up an extremity are sometimes seen, and more extensive disease occurs in immunosuppressed patients. Lesions tend to be persistent without therapy, although spontaneous resolution may occur over the course of a year or so. Treatments include antimicrobial agents such as minocycline and doxycycline.124,125
Infection due to rapid growers such as M. chelonae and M. abscessus follow various forms of trauma, including medical procedures, and have been associated with foot baths in nail salons. The author has observed this type of infection following corticosteroid injections, eventually traced to contaminated multiuse vials. The resulting lesions range from papules to abscesses. The lesions are resistant to the usual antituberculous therapy but generally respond to clarithromycin.126,127
M. ulcerans produces a lesion known as Buruli ulcer. It is actually a common infection worldwide and is endemic in tropical and subtropical countries. The disease typically begins as a subcutaneous nodule on a leg or arm that ulcerates, forming undermined borders, after a period of several months. The ulcers can spread and become quite large and destructive. Although antibiotics are used, treatment often involves local heat therapy or surgical excision.128,129
Microscopic Findings: The histopathologic image of M. marinum infection can vary considerably, ranging from a predominantly neutrophilic infiltrate with abscess formation to a patchy lymphocytic and histiocytic (macrophagic) infiltrate to a predominantly granulomatous process. The latter is often loosely organized, and although tuberculoid granulomas can be observed, caseation is generally not seen. Panniculitis can show granulomas mixed with neutrophils, and fat microcysts lined by neutrophils are identified130,131 (Fig. 17-29A-C). Varying degree of acanthosis and even pseudoepitheliomatous hyperplasia may accompany the dermal changes, and occasionally a blastomycosis-like tissue reaction pattern is observed. Organisms are often difficult to find, even with Ziehl-Neelsen or Fite stains (Fig. 17-30), but may be numerous in immunosuppressed patients (Fig. 17-31). M. marinum organisms are larger and more plump than M. tuberculosis organisms and may have a “beaded” configuration in tissue sections (Fig. 17-32). Immunohistochemical staining for mycobacterial antigen may enhance the likelihood of finding organisms, and PCR methods can also be used.132 Culture studies are useful, but it is essential that cultures be carried out at room temperature for this particular organism.

Figure 17-29 Mycobacterium marinum infection. There is a mixed septal and lobular panniculitis consisting of suppurative granulomatous inflammation (A). Focal granulomas (B) and fat microcysts lined by neutrophils (C) are observed.

Figure 17-30 Atypical mycobacterial infection. Organisms are often sparse but can be identified with acid-fast stains such as the Fite stain.

Figure 17-31 Atypical mycobacterial infection (due to Mycobacterium marinum). With Ziehl-Neelsen stain, numerous organisms are identified within a microcyst lined by neutrophils. These are longer and more plump than Mycobacterium tuberculosis.

Figure 17-32 Atypical mycobacterial infection. “Beading” of organisms is well demonstrated in this example.
M. chelonae infection shows dermal neutrophilic abscesses with granulomatous inflammation, consisting of epithelioid cells with some Langhans-type giant cells. A fistulous tract may extend into the epidermis. Central zones of caseous necrosis have been identified in some cases. Lymphocytes and plasma cells are also observed. Other findings include hemorrhage, fibrosis in later stage lesions, and secondary vasculitic changes. With special stains, organisms can be found in about 25% of cases.133
M. ulcerans infection evolves in three stages. The first consists of widespread tissue necrosis and connective tissue degeneration. The deeper portions of an ulcerated lesion display “ghost-like” outlines of the original tissue structure.134 At this stage, there is minimal inflammation, and organisms are readily identified. This is followed by an organizing stage, in which there are numerous macrophages, plasma cells, and lymphocytes at the margins of necrotic tissue, associated with proliferating capillaries and fibroblasts. Granulomatous elements phagocytose lipid and necrotic cell debris during the healing stage, and mycobacteria become difficult to identify.134,135
Differential Diagnosis: Suppuration with granuloma formation, as seen in M. marinum and M. chelonae infections, should always raise the possibility of infection, and special stains and culture studies are essential. Other bacterial and fungal infections must be considered along with atypical mycobacterial infection and diagnostic studies planned accordingly. In the case of M. marinum infection, a major differential diagnostic consideration is sporotrichosis, because there can be considerable clinical and histopathologic overlap of these infections. This is particularly the case when ascending sporotrichoid nodules are encountered. Because in both infections organisms may be difficult to identify with special staining, culture studies obviously gain in importance. The necrotizing and degenerative appearance of M. ulcerans infections must be distinguished from toxin-mediated lesions, such as spider bites, or from the degenerative changes produced by vascular occlusion (e.g., due to calciphylaxis or antiphospholipid antibodies) or ischemia (e.g., coma bullae). Spider bites, however, show accompanying inflammation, including eosinophils. Thrombosed and/or or calcified vessels provide a clue to other possible causes of tissue degeneration and necrosis, but it must also be pointed out that intimal thickening and occlusion of small arteries have been identified in the periphery of lesions due to M. ulcerans.135 Because numerous organisms can be identified in the latter at an early stage of the infection, special staining (e.g., with Fite stain) can be decisive, and therefore an index of suspicion for mycobacterial infection must be maintained in such cases.
Hansen Disease (Leprosy)
Clinical Features: The numbers of individuals who have leprosy worldwide have diminished substantially in recent years due to ongoing multidrug therapy programs. The areas of greatest prevalence are in Central and South America, Africa, and portions of Asia. This infection is encountered in the United States, especially in areas of Texas and Louisiana and among immigrant populations. The causative agent is Mycobacterium leprae, a small, curved acid-fast bacillus. It tends to be found in macrophages and Schwann cells. Leprosy involves the skin and mucous membranes but also peripheral nerves, bones, and some internal organs. The organisms grow best at temperatures less than 37° C and tend to cause infection in cooler areas of the body.
The classification of leprosy infections is based on the polar division of the various forms of the disease, depending on levels of cell-mediated immunity to the organism. The Ridley and Jopling system for leprosy recognizes the following: lepromatous (at the immunosuppressed end of the spectrum), borderline lepromatous, borderline-borderline (at the midpoint of the spectrum), borderline tuberculoid, and tuberculoid (at the immunocompetent end of the spectrum).136 Indeterminate leprosy may represent the initial presentation of the disease and is capable of resolving spontaneously or progressing to one of the other forms of the disease. The middle stages of the disease are unstable, and patients may move either toward the lepromatous or the tuberculoid pole, depending on the status of their immune systems and/or response to therapy. Lesion types vary according to this classification system. Therefore, indeterminate lesions are often hypopigmented macules, lepromatous lesions range from papules to nodules to diffuse cutaneous infiltration, and tuberculoid lesions feature infiltrated, hypopigmented, anesthetic plaques. There are also reactional states in leprosy. Type I reactions result from change in the immunologic status of the patient, often due to therapy, and can represent reversal reactions (a shift toward the tuberculoid end of the spectrum) or downgrading reactions (a shift toward the lepromatous end of the spectrum). They are associated with swelling of preexisting lesions and neuritis. Type II reactions result from humoral immune reactions in patients with lepromatous leprosy undergoing therapy. The findings include formation of immune complexes and development of small-vessel vasculitis (erythema nodosum leprosum) or, in patients with the diffuse form of lepromatous leprosy, thrombosis with small-vessel vasculitis (Lucio phenomenon). Lepra bacilli have a long doubling time and cannot be cultured, although they can be identified by special staining of tissue samples and by PCR technology.137
Treatments include multidrug antimicrobial therapy. Among the treatments for reactional states are prednisone (for type I reactions and the Lucio phenomenon) and thalidomide (with appropriate precautions) in type II reactions (erythema nodosum leprosum).
Microscopic Findings: The findings in indeterminate leprosy, as might be suspected, are nonspecific, including patchy perivascular and periappendageal infiltrates composed of lymphocytes and macrophages; organisms are usually not identified.138 In lepromatous lesions, numerous foamy macrophages are seen. Characteristically, larger aggregates are separated from the epidermis by a grenz zone of uninvolved collagen (Fig. 17-33). These macrophages, called Virchow cells, contain numerous organisms surrounded by lipid. Clumps of organisms called globi can also be identified. Lepra bacilli are gram-positive bacteria that also stain red with Ziehl-Neelsen and Fite stains139 (Fig. 17-34 A and B). The histoid variant of lepromatous leprosy consists of fibrotic papules and nodules resembling dermatofibromas; these lesions consist of spindled cells, which also contain numerous acid-fast bacilli that tend to line up along the long axis of the cells140 (Fig. 17-35). In tuberculoid leprosy, there are epithelioid cell granulomas that follow the course of dermal nerves undergoing necrosis; some of these granulomas appear to be elongated or “sausage shaped” when cut longitudinally (Fig. 17-36). Organisms are generally difficult to identify in these lesions but sometimes can be found on careful search. S-100 staining can be useful in identifying fragmented dermal nerve patterns in tuberculoid lesions.141 Varying mixtures of the lepromatous and tuberculoid patterns can be seen in borderline forms of leprosy (Fig. 17-37). Histopathologic shifts toward the tuberculoid or lepromatous ends of the disease spectrum can be seen, respectively, in reversal or downgrading reactions. Erythema nodosum leprosum shows leukocytoclastic vasculitis, sometimes with fragmented bacilli around vessels or within macrophages142 (Fig. 17-38), whereas the Lucio phenomenon shows either necrotizing vasculitis142 or endothelial proliferation and luminal occlusion, with ischemic changes, ulceration, and necrosis.143 Acid-fast organisms can be identified within endothelial cells.

Figure 17-33 Lepromatous Hansen disease. Numerous macrophages are present that contain gray, granular material representing lepra bacilli.

Figure 17-34 Lepromatous Hansen disease. A, With the Fite stain, numerous bacilli can be identified within macrophages and forming globi. B, Lepra bacilli are also positive for Gram stain.

Figure 17-35 Histoid Hansen disease. This spindle cell lesion bears a resemblance to dermatofibroma. Numerous lepra bacilli are identifiable within macrophages.

Figure 17-36 Tuberculoid Hansen disease. Granulomas often show an elongated or “sausage-shaped” configuration, following the course of dermal nerves. Organisms are difficult to identify even with special stains.
Differential Diagnosis: Indeterminate leprosy lesions show nonspecific changes that can resemble a variety of inflammatory dermatoses. The clinical context of the lesions may raise suspicion, and the diagnosis is achieved either by the finding of organisms with special staining (an uncommon event) or by sampling of more infiltrative lesions. Lepromatous lesions must be distinguished from xanthomas. The grenz zone separating large aggregates of foamy macrophages from the epidermis is a typical feature of lepromatous leprosy. The Virchow cells of lepromatous leprosy contain neutral fat and phospholipid and not cholesterol; therefore, the lipid is not doubly refractile when examined with formalin fixed, frozen sections.144 However, this requires a certain degree of prescience to be useful diagnostically. The key to diagnosis is recognizing the organisms, which produce grayish, somewhat filamentous aggregates that can be seen on H&E-stained sections and allow definitive diagnosis with acid-fast staining. The granulomas of tuberculoid leprosy can be mistaken for those of sarcoidosis. The finding that granulomas surround degenerated nerve elements (aided with S-100 staining) is a helpful clue to the diagnosis. A reticulin stain may also be helpful144a in that sarcoidal granulomas contain a delicate reticulin meshwork, whereas this finding is absent in the granulomas of tuberculoid leprosy. Organisms can occasionally be identified in tuberculoid leprosy, but their detection requires careful search and sometimes multiple levels. Fite stain is recommended for this purpose, because lepra bacilli decolorize readily with the usual Ziehl-Neelsen method for acid-fast bacilli. Type II lepra reactions (erythema nodosum leprosum and the Lucio phenomenon) must be distinguished from other causes of thrombosis or leukocytoclastic vasculitis. This may particularly be a problem when vasculitis coexists with lipidization, as occurs in papular neutrophilic xanthoma or later stages of erythema elevatum diutinum. Again, in these cases, detection of organisms with special stains is essential.
Rickettsiosis (Rocky Mountain Spotted Fever)
Clinical Features: Among the rickettsial diseases, the one that is generally of greatest concern in the United States is Rocky Mountain spotted fever. This condition, caused by the organism Rickettsia rickettsii, is transmitted by the bite of a tick (usually Dermacentor variabilis in the East but also Amblyomma americanum [the “lone star tick”] and Dermacentor andersoni in the West). Several weeks after a tick bite, fever, chills, headache, and weakness develop, and there is an eruption of small erythematous macules to papules that classically begins acrally (wrists, ankles) and then spreads centrally to the trunk. The lesions subsequently become petechial to purpuric. However, skin lesions do not always develop, and when they occur they do not always evolve in the classic sequential manner. Occasionally, there may be an eschar at the site of a tick bite, but this is often not the case. Symptoms of meningitis and multiorgan failure can develop in severe disease. Serologic testing can confirm the diagnosis, but because of delays in obtaining results, it is generally recommended that treatment be initiated empirically or based on clinical suspicion, particularly if the patient has been in an endemic region. Doxycycline is often the treatment of choice, with chloramphenicol the alternative agent.145
Microscopic Findings: The cutaneous lesions show a lymphocytic vasculitis involving small vessels of the dermis and subcutis. This is associated with endothelial cell injury, focal thrombosis, and erythrocyte extravasation (Fig. 17-39A and B). The organisms are quite small—about 1 µm in diameter—and are found within endothelial cells. As a result, they are difficult to identify even with special stains such as Giemsa. A somewhat more effective method is the Pinkerton stain, whose main ingredients are methylene blue, basic fuchsin, and citric acid. With this stain, rickettsia stain bright red, and the nuclei blue.146 When available, specific immunoperoxidase or immunofluorescent methods are best for identifying rickettsial organisms in tissue sections147 (Fig. 17-40).
Differential Diagnosis: The microscopic differential diagnosis includes other forms of lymphocytic vasculitis. Examples are pityriasis lichenoides, pigmented purpuric eruptions, and perniosis. The clinical presentations of these conditions are quite different from Rocky Mountain spotted fever, and other associated microscopic findings are different as well (substantial epidermal changes in pityriasis lichenoides, inflammation confined to superficial dermal vessels in forms of pigmented purpura, “fluffy edema” around vessel walls in perniosis). Of greater difficulty are biopsies of later stages of leukocytoclastic vasculitis due to other causes, in which perivascular lymphocytes become more prominent. Correlation with the clinical presentation should point to Rocky Mountain spotted fever as a better diagnostic choice, and that impression could be supported by immunostaining if available.
Actinomycosis
Clinical Features: The classic presentation of actinomycosis consists of nodules, with induration and draining sinuses, that tend to form in the cervicofacial region, especially in the mandibular area (“lumpy jaw”). Similar lesions develop on the chest wall, associated with pulmonary involvement, or the abdominal wall, due to underlying intestinal disease. Hematogenous spread can also result in multiple cutaneous lesions.148 The organism, usually Actinomyces israelii, is an anaerobic, gram-positive filamentous organism that can be found in tonsils, carious teeth, or even lachrymal ducts. Actinomyces filaments aggregate into hard yellow bodies, called grains, that are discharged in the purulent drainage associated with the skin lesions. Penicillins and other antimicrobials are used to treat these lesions.149
Microscopic Findings: Within the dermis (often the deep dermis) or subcutis is a zone of granulation tissue, including plasma cells, macrophages, and fibroblasts, surrounding a neutrophilic abscess. This abscess may extend to the epidermal surface. Within the abscess are lobulated bodies measuring roughly 300 µm in diameter; these are the “sulfur granules.” They are usually described as being basophilic, but with eosinophilic “clubs” at the periphery. The filamentous organisms can be seen with Gram stain, PAS, or silver methods; they are not acid-fast. They form tangled aggregates but extend outward in radiating fashion, surrounded by “clubs” of eosinophilic material.150 This material represents host protein, an immune response to the organism. This phenomenon, the Splendore-Hoeppli phenomenon, is the same reaction seen in botryomycosis and forms of mycetoma produced by “higher” bacteria (actinomycetoma) and true fungi (eumycetoma) (Fig. 17-41A-C).
Differential Diagnosis: Microscopically, actinomycosis should be distinguished from other infections that produce grains in tissues. Botryomycosis is associated with conventional, nonfilamentous bacteria, both gram-positive and gram-negative (frequently, S. aureus). These can be well demonstrated with Giemsa as well as Gram stains. Nocardia can form grains and are also filamentous, but the filaments tend to be shorter, and unlike Actinomyces, Nocardia organisms are acid-fast (however, see “Nocardiosis”). The filaments of other actinomycetomas (including those caused by Actinomadura or Streptomyces species) can have a close resemblance to Actinomyces, but the organisms of eumycetomas (e.g., Madurella spp. and Phialophora spp.) form segmented hyphae that are much broader (2 to 5 µm in diameter) than those of Actinomyces (about 1 µm). Grossly, inspecting the color of the grains in lesional drainage can provide clues to the diagnosis, which can then be supported by microscopic and culture studies. Thus, the “sulfur granules” of actinomycosis are yellow, whereas most other actinomycetoma organisms are associated with light colored grains (Actinomadura pelletieri is an exception, producing red grains), and pigmented eumycetoma organisms produce black grains.
Nocardiosis
Clinical Features: Nocardiosis frequently develops in immunosuppressed individuals. The two species of interest are Nocardia asteroides and Nocardia brasiliensis. These organisms are aerobic, gram-positive filamentous bacteria that tend to fragment into smaller forms.151 Hematogenous dissemination occurs most often with N. asteroides, which can result in cutaneous abscesses, pustules, or ulcers. N. brasiliensis is prone to form localized cutaneous infection following injury to the skin. A primary ulcer with ascending sporotrichoid nodules can sometimes occur (“lymphocutaneous nocardiosis”).152 Both organisms are capable of producing mycetoma, with grain formation, but this is more commonly the case with N. brasiliensis.153 Antimicrobial therapies include trimethoprim-sulfamethoxazole.
Microscopic Findings: Abscesses form in the deep dermis or subcutis.154 Several examples of panniculitis caused by Nocardia also showed evidence for vasculitis involving medium-sized vessels.155 Organisms appear as fragmented, branching, narrow filaments (about 1 µm in diameter) that are gram-positive and acid-fast (Fig. 17-42A and B). Acid-fast staining with the traditional Ziehl-Neelsen stain tends to be weak, but Fite staining is more sensitive and is therefore the preferred method. Organisms are not organized in grains except in the case of mycetomas associated with Nocardia infection.
Differential Diagnosis: The differential diagnosis with regard to eumycetoma due to Nocardia is similar to that for actinomycosis. Nocardia infection unassociated with grain formation should be differentiated from other infections due to filamentous bacteria. With regard to Actinomyces, as discussed previously, Nocardia organisms are shorter and have a greater tendency to fragment, sometimes even forming bacillary structures. Although it is generally thought that Nocardia species are acid-fast and Actinomyces species are not, the latter can be acid-fast under certain conditions, notably with the Putt modification of the Ziehl-Neelsen stain,156 whereas at the same time, as mentioned, Nocardia is usually only weakly acid-fast with the traditional Ziehl-Neelsen method. It is also doubtful that methenamine silver stains are truly helpful in distinguishing small bacillary fragments of Nocardia from other acid-fast bacilli, as has been suggested in the past, because both M. tuberculosis and M. leprae are stainable with methenamine silver stains under varying conditions.157–159 Therefore, specific in situ hybridization methods160 or culture studies may be necessary in some cases.
Selected Spirochetal Diseases
Clinical Features: Syphilis is caused by the spirochete Treponema pallidum, a motile, gram-negative organism that cannot be cultured in vitro but can be identified in tissue with special stains—traditionally, silver methods such as Warthin-Starry or modifications thereof (van Orden or Steiner and Steiner stains) and more recently with an immunoperoxidase stain that can be used in formalin-fixed, paraffin-embedded tissue. Diagnosis is generally carried out using serologic methods (rapid plasma reagin [RPR], fluorescent treponemal antibody-absorbed [FTA-abs]).
In addition to congenital syphilis (acquired in utero from a mother who has early syphilis), acquired syphilis occurs in three stages.
• Primary syphilis presents as a chancre, a crusted erosion that eventually forms an indurated border. There may be one or more of these lesions, accompanied by regional lymphadenopathy that develops within several weeks after onset of the primary lesion(s).
• Secondary syphilis in the majority of cases is associated with an outbreak of generalized maculopapular lesions that can involve the palms and soles. Other special types of lesions developing during this stage include annular skin lesions, mucous patches or “split papules” at the oral commissures, syphilitic “moth-eaten” alopecia, and hypertrophic lesions of the anogenital region called condylomata lata. Lymphadenopathy is frequent, and there may be involvement of internal organs, including kidneys, liver, nervous system, eyes, and joints. Secondary lesions involute (although there can be relapses), followed by a latent period.
• Tertiary syphilis may develop 3 to 5 years after infection and can be accompanied by late manifestations such as cardiovascular disease, osseous involvement, and neurosyphilis. A small minority of patients can develop localized and sometimes destructive skin lesions in this stage, including nodular, sometimes annular lesions and gummas, the latter consisting of necrotic, ulcerative lesions.161 Specific treatment protocols are available for the various stages of the disease, with penicillin still the agent of choice, and other alternatives in the case of penicillin allergy.162
Microscopic Findings: In primary syphilis, lesions typically show epidermal attenuation or shallow ulceration with adjacent acanthosis. Within the dermis, there is a prominent infiltrate composed of lymphocytes and plasma cells (Fig. 17-43). At the periphery of the lesion, the infiltrate is mainly perivascular in distribution, with associated endothelial swelling and some permeation of vessel walls by the inflammatory infiltrate. Using silver stains such as the Steiner and Steiner method, or with specific immunostaining, spirochetes can be found in the overlying epidermis or in vessel walls. With these methods, the spiral configuration of the organisms is apparent.

Figure 17-43 Primary syphilis. There is an ulcer with a dense dermal infiltrate composed of lymphocytes and plasma cells.
In secondary syphilis, the lesional configuration can vary considerably, in concert with the clinical appearance of the lesions. Thus, the epidermis may appear to be essentially normal or show distinctly psoriasiform or lichen planus–like changes (Fig. 17-44A and B). However, this is often associated with significant exocytosis of inflammatory cells, sometimes including neutrophils.163 Ulcers can be identified in so-called lues maligna, whereas acanthosis can be marked in lesions of condyloma lata. Within the dermis, inflammatory cells form tight aggregates around the vessels, or “coat-sleeved” perivascular infiltrates. These are composed of lymphocytes and plasma cells in varying combinations. Plasma cells, considered a hallmark of syphilitic lesions, can be sparse and occasionally are absent164 (Fig. 17-45). Granulomas can be seen in secondary lesions and may become more prominent in late secondary disease (Fig. 17-46). Spirochetes can be identified in lesions of secondary syphilis, particularly in those with marked exocytosis. Although these have been found both in the epidermis and in dermal vessels, the author has most often found the organisms near the epidermal surface of exudative lesions or in the vicinity of the dermal-epidermal junction. Again, silver stains are commonly used for this purpose but are frustratingly negative in a number of cases; sensitivity can be enhanced by specific immunostains (Fig. 17-47).
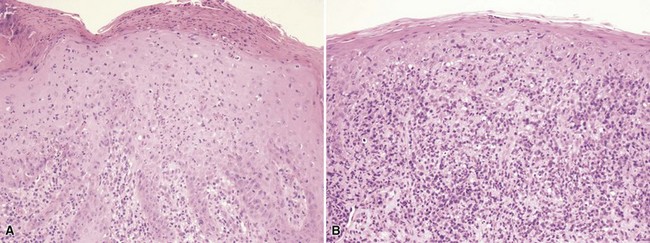
Figure 17-44 Secondary syphilis. A, Psoriasiform lesion, with surface neutrophils and slight spongiform pustulation. B, Lichenoid lesion, with considerable exocytosis. The infiltrate contains lymphocytes and plasma cells.
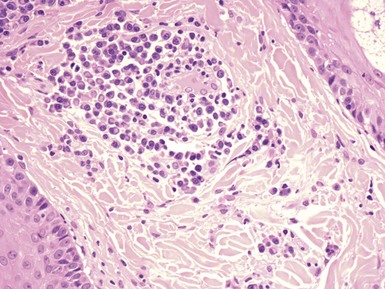
Figure 17-45 Secondary syphilis. A “coat-sleeved” perivascular infiltrate composed mainly of plasma cells.

Figure 17-47 Secondary syphilis. With a spirochete immunostain, numerous organisms are identified in the vicinity of the dermal-epidermal junction.
In tertiary syphilis, nodular lesions are small granulomas amid lymphocytes and plasma cells, with little or no caseous necrosis, whereas gummas show more extensive granulomatous inflammation in the dermis and subcutis with significant, centrally located caseous necrosis (Fig. 17-48). Endothelial swelling is a prominent feature,165 particularly in gummatous lesions. Spirochetes cannot generally be found by routine methods but have been found in tertiary lesions using indirect immunofluorescent and PCR methods.166,167
Differential Diagnosis: Primary syphilis could mimic ulcers due to a variety of other causes, including those due to other sexually transmitted diseases. The predominance of lymphocytes and plasma cells, endothelial swelling, and features resembling “lymphocytic vasculitis” should suggest the diagnosis of syphilis and prompt the use of special stains for spirochetes. The various forms of secondary syphilis can be confused with other disorders showing patchy perivascular dermal infiltrates, especially those with “coat-sleeved” infiltrates around vessels—in particular, erythema annulare centrifugum. Plasma cells in the perivascular infiltrates argue for secondary syphilis, which may be further supported by clinical features, special stains, and serologic studies. At times, syphilitic lesions can resemble psoriasis, lichen planus, or pityriasis lichenoides. Suggestive evidence of secondary syphilis is provided by the degree of exocytosis of mononucleated cells (more prominent than in psoriasis or lichen planus), the composition of the inflammatory infiltrate (plasma cells are more common in syphilis), and the degree of vasculopathic change. Finding spirochetes on special staining is obviously definitive. However, it must be realized that they cannot always be found in lesions of secondary syphilis, particularly with silver stains; a negative result does not exclude the diagnosis of syphilis. For that reason, serologic studies are advised, and the possibility of a false negative RPR due to a prozone phenomenon (secondary to antigen excess) must also be kept in mind. Silver stains also detect melanin, and positively stained melanocytic dendrites should not be confused with intraepidermal spirochetes (staining of melanin within dendrites is beaded in type and lacks the spiral configuration of T. pallidum). Small epithelioid granulomas (e.g., in late secondary syphilis) can resemble sarcoidosis, whereas the granulomas of nodular tertiary syphilis can closely resemble those of lupus vulgaris, and gummatous lesions can be confused with forms of cutaneous tuberculosis showing caseation, especially scrofuloderma or the tuberculid, erythema induratum. In most situations, the vasculopathy of syphilis is distinctive, with the possible exception of erythema induratum, but other clinical and laboratory data may be necessary to differentiate those other disorders. This is particularly true because spirochetes cannot be readily identified in tertiary lesions, requiring PCR technology in many, if not most, cases.
Borreliosis and Lyme Disease
Clinical Features: Borrelia species are gram-negative spirochetes that are difficult to identify in tissues with conventional methods but can sometimes be found with silver stains such as Warthin-Starry, with specific antibodies using immunoperoxidase, or through PCR technology. Borrelia burgdorferi causes Lyme disease in most parts of the United States, and another organism, Borrelia lonestari, causes a similar tick-borne disease in the southern United States.168 In Europe and Japan, Borrelia garinii is the agent responsible for Lyme disease, whereas Borrelia afzelii has been linked to acrodermatitis chronica atrophicans,169 morphea, and lichen sclerosus.170 The disease is transmitted through the bite of a tick, usually of the Ixodes species (as well as Amblyomma americanum in the southern United States).
Lyme disease begins with a small erythematous papule at the site of the tick bite, followed by an expansile erythematous lesion that may be annular. Multiple secondary annular lesions then develop in up to 50% of patients. The annular lesions are designated erythema (chronicum) migrans, which is discussed with other annular erythemas (see Chapter 2). Early symptoms tend to be mild and include headache, fever, stiff neck, and arthralgia. A relatively small group of patients later develops chronic arthritis, cardiovascular complications that include heart block, and central nervous system symptoms and signs that include headache, meningitis, and neuropathy. Serologic studies are used for diagnosis, and treatment includes antimicrobials, particularly doxycycline.171 Acrodermatitis chronica atrophicans occurs mainly in Europe; is prone to develop on the extremities; and manifests as erythema or edema, followed by atrophy with wrinkling of the skin. Fibrous bands develop in the ulnar or tibial regions, and subcutaneous nodules can form over the joints. Treatments are similar to those for Lyme disease.
Microscopic Findings: The lesions of erythema chronicum migrans show a superficial and deep perivascular and interstitial infiltrate composed of lymphocytes and plasma cells. Eosinophils are sometimes present, particularly when biopsies are obtained from the centers of lesions near the bite of a tick172 (see Figures 2-7 and 2-8). Borrelia organisms can be identified using silver stains such as Warthin-Starry or, better still, with specific antibodies using the immunoperoxidase technique. They are most often found in the superficial dermis but are sometimes seen in the epidermis or follicular epithelia. Early lesions of acrodermatitis chronica atrophicans show nonspecific changes, mainly a perivascular dermal infiltrate composed of lymphocytes and various admixtures of plasma cells. Later, epidermal atrophy develops, and dermal atrophy is manifested by edema, degenerated collagen, and loss of elastic fibers.173 A mid-dermal, bandlike infiltrate is composed of lymphocytes, macrophages, and plasma cells. Eccrine sweat coils, spared until late in the process, appear to be located higher in the dermis than is normally the case, and hair follicles and sebaceous glands become atrophic. Lipoatrophy is also present. As expected, fibrous bands show sclerotic collagen, whereas subcutaneous nodules demonstrate hyalinized connective tissue that may have an “onion-skin” configuration.174 Plasma cells are seen in all of these manifestations of acrodermatitis chronica atrophicans, and Borrelia organisms can be identified with the methods described above or by culture on special media such as modified Kelly-Pettenkofer.175
Differential Diagnosis: Erythema chronicum migrans has overlapping features with other conditions characterized by superficial and deep perivascular infiltrates, including photodermatoses, syphilis, drug reactions, and other annular erythemas. In contrast to erythema chronicum migrans, erythema annulare centrifugum tends to show a tightly “coat-sleeved” perivascular infiltrate with minimal interstitial inflammation, and plasma cells are generally not a feature. Plasma cells, however, are regularly found in lesions of syphilis. Staining for Borrelia can be helpful if positive, but in the author’s experience, silver stains are often disappointing. There can be considerable background staining with these methods, and Borrelia organisms in tissue sections often have wavy rather than tightly coiled contours, and therefore they tend to be overlooked or misinterpreted. Better results can be obtained with specific immunoperoxidase methods if available, but often the pathologist can only suggest the diagnosis, which can then be confirmed by serologic methods. Acrodermatitis chronica atrophicans is rarely observed in the United States. Fully developed lesions are fairly characteristic. Edema with diminution and loss of elastic fibers can also be seen in anetoderma (macular atrophy), but epidermal atrophy and mid-dermal bandlike inflammation are not typical of that condition. Fibrous bands and subcutaneous nodules could be confused with morphea or scars. Special stains for Borrelia can be useful in recognizing acrodermatitis chronica atrophicans. A technique that improves the likelihood of finding Borrelia in tissue sections is called focus-floating microscopy. In this method, a polyclonal antibody to Borrelia is used, ideally with a red chromogen and absence of counterstaining. Microscopic sections are then examined in a systematic fashion, horizontally and vertically, using the fine-focus control. Such methods have achieved more than 60% positivity in study cases and 90% positivity in positive controls.176,177
Fungal Diseases
Clinical Features: Dermatophytosis is among the most common fungal diseases.178,179 It represents a superficial infection of skin, hair, and nails. Most often, transmission is human to human (anthropophilic), but some infections are transmitted either by animals (zoophilic) or via the soil (geophilic). The most important organisms are Trichophyton and Microsporum species. Epidermophyton is also responsible for infections of skin and nails. The term “tinea,” when combined with an anatomic site, provides a name for each clinical subset of disease. Thus, there are tinea capitis, tinea faciale or barbae, tinea cruris, tinea manus and pedis, tinea unguium, and tinea corporis.
In the United States, noninflammatory tinea capitis is most often caused by Trichophyton tonsurans. Microsporum audouini is rarely encountered, but in the past, it had been the most common cause of this form of tinea capitis. Clinical features include patches of hair loss with scaling or formation of black dots, the latter resulting from breakage of hair shafts at the scalp surface, seen particularly with T. tonsurans infection.180 Inflammatory tinea capitis is particularly associated with Microsporum canis but also with T. tonsurans, Trichophyton mentagrophytes, and Microsporum gypseum. In this form of tinea capitis, there may be swelling with development of inflammatory papules and pustules (so-called kerion),181 potentially leading to scarring alopecia. The causative organisms may differ in other geographic locations; for example, a survey of tinea capitis in Guinea showed that Trichophyton violaceum is the most common dermatophyte found in this setting.182 Favus is a rare form of tinea capitis in the United States; it is caused by Trichophyton schoenleinii and presents with yellowish crusts around each involved hair. The presentation of tinea barbae ranges from superficial folliculitis to nodular, kerion-type lesions of the beard area. Superficial scaly lesions of the face without beard involvement are termed tinea faciale. Boggy, infiltrative lesions are caused by T. mentagrophytes or Trichophyton verrucosum, and organisms causing tinea faciale include M. canis and T. tonsurans. T. mentagrophytes and Trichophyton rubrum can produce both tinea barbae and tinea faciale. Tinea cruris consists of scaly circinate patches on the upper inner thighs and is produced particularly by T. rubrum, T. mentagrophytes, and Epidermophyton floccosum. Majocchi granuloma is a term applied to fungal folliculitis, typically with deep follicular involvement, rupture, and an intense, mixed inflammatory infiltrate. Boggy lesions involving follicles of the scalp and beard area represent forms of Majocchi granuloma, but similar lesions can be found in other locations where there are hair follicles; thus, for example, these lesions may be found in the setting of tinea corporis or tinea cruris. Tinea manus and pedis sometimes manifest as erythematous, scaly lesions, forming at times a moccasin-like appearance when involving the sides and soles of the feet; T. rubrum is a common cause. T. mentagrophytes often causes a more inflammatory, vesiculobullous form of tinea manus and pedis. E. floccosum can also produce scaling and blistering of the soles. Tinea unguium (onychomycosis) can be produced by dermatophytes but sometimes also may be attributed to nondermatophytic molds. T. mentagrophytes is known for producing white, superficial onychomycosis, whereas T. rubrum produces subungual hyperkeratosis and yellowish discoloration of the nail plate. Tinea corporis involves other cutaneous sites and consists of erythematous, scaly patches with annular or arcuate borders, sometimes with microvesiculation. Causes include T. tonsurans, T. rubrum, T. mentagrophytes, and M. canis.
Microscopic Findings: The usual way to diagnose dermatophyte infections is through potassium hydroxide preparations obtained via scraping of lesional scale or plucking of hairs. However, biopsies are sometimes performed to rule out other conditions, and occasionally the detection of fungal organisms turns out to be a surprise to the contributing clinician. In most nonfolliculocentric forms of tinea, the overall lesional configuration is that of spongiotic dermatitis—ranging from acute and vesiculobullous in lesions due to T. mentagrophytes to subacute or chronic in infections produced by T. rubrum. Varying numbers of neutrophils can be seen in the stratum corneum in some cases. Fungal hyphae, seen longitudinally and in cross-sectional profile, can be identified in the stratum corneum. When numerous, these can be found even on routine staining with H&E (Fig. 17-49A), but often special stains are necessary to see them. The stains most often used are PAS, preferably with light green counterstaining to enhance visualization of the organisms (see Fig. 17-49B), or silver stains such as GMS. Both are valid methods, although dead fungi may not stain with PAS.183 Fungal hyphae are identified in the nail plate (or in fragments thereof) in examples of onychomycosis. In follicular fungal infections, organisms in the form of hyphae and/or arthrospores can be found within the hair shaft or follicular lumen, again best visualized with special stains but often identifiable on H&E staining. In boggy, inflammatory fungal folliculitis or Majocchi granuloma, there may be follicular rupture with mixed perifollicular dermal inflammation, including neutrophils, lymphocytes, and sometimes (but not always) granulomatous elements (Fig. 17-50A). Fungal organisms may be sparse, and PAS or GMS stains are often required to find them, often in or near hair shafts or the follicular lumen. Certain fungi are prone to invading hair shafts, termed endothrix, whereas others sometimes invade hair shafts but can also surround them without invading (ectothrix/endothrix) (see Fig. 17-50B). Organisms associated with endothrix infection include T. violaceum, T. tonsurans, Trichophyton sabouraudi, Trichophyton sulfureum (a variant of T. tonsurans), and T. schoenleinii, and ectothrix/endothrix organisms include M. audouini, M. canis, M. gypseum, T. mentagrophytes, T. rubrum, and T. verrucosum.

Figure 17-49 Dermatophytosis. A, The overall findings are those of spongiotic dermatitis. Slightly basophilic hyphal organisms are barely perceptible in the stratum corneum. B, The organisms are more readily identified with a PAS–light green stain.

Figure 17-50 Majocchi granuloma. A, Associated with a ruptured follicle, there is an intense, mixed neutrophilic and granulomatous dermal infiltrate. A cross-sectional profile of a hair shaft can be identified on lower right center. B, The hair shaft is surrounded by fungal arthrospores, indicating ectothrix-type infection. In this case, the organisms can be readily identified in H&E-stained sections.
Differential Diagnosis: A diagnosis of dermatophytosis requires an index of suspicion, followed by careful search and, if necessary, performance of PAS or GMS stains. Fungal organisms are not seen in areas of parakeratosis, probably due to the rapid epithelial turnover in these areas. Although most commonly the background changes in dermatophytosis are those of spongiotic dermatitis, a wide variety of reaction patterns can be associated with dermatophyte infections. This was most vividly demonstrated by Graham in a remarkable study presented in the textbook Dermal Pathology.184 For example, reaction patterns associated with T. rubrum infection alone include erythema multiforme, erythema perstans, purpuric dermatitis, granuloma faciale, granuloma annulare, pustular dermatitis, and papulonecrotic dermatitis with allergic angiitis, in addition to forms of spongiotic dermatitis.184 Fungal folliculitis must be distinguished from other forms of inflammatory and/or scarring alopecia, and Majocchi granuloma can resemble folliculitis with follicular rupture due to a variety of causes, including primary acneiform folliculitis or bacterial folliculitis.
Infections Due to Pityrosporum/Malassezia: Tinea Versicolor and Pityrosporum Folliculitis
Clinical Features: Two infections are associated with the organism Malassezia furfur or its yeast phase, Pityrosporum orbiculare: tinea versicolor and Pityrosporum folliculitis. Tinea versicolor is a common eruption that concentrates on the trunk and upper arms, most notably in summer months. It occurs as scaly macules that are often hypopigmented but may also be hyperpigmented or have a light pink hue. Hypopigmentation has been attributed to a substance elaborated by the organism that may prevent normal epidermal melanization; on ultrastructural examination, incompletely melanized melanosomes that are abnormally transferred to keratinocytes are present.185 Hyperpigmentation results from large, heavily melanized melanosomes—the reason for this is unclear.186 Frequently, abundant scale is produced when lesions are scraped. Pityrosporum folliculitis consists of small, dome-shaped follicular papules, which are erythematous to flesh colored. They also develop over the upper trunk but can be seen on the face and scalp. It may coexist with tinea versicolor and is often seen in transplant patients.187 Steroid acne associated with topical or systemic corticosteroid therapy has a strong association with Pityrosporum folliculitis.188
Microscopic Findings: In the clinic, the usual method of diagnosing tinea versicolor is by performing a microscopic examination of skin scrapings; potassium hydroxide and (sometimes) India ink are added to the scrapings prior to examination. Usually, abundant short hyphae and clusters of spores (“spaghetti and meatballs”) are readily identified; organisms are frequently numerous. On biopsy, the overall microscopic image is that of normal skin. However, on inspection of the stratum corneum, organisms with the features described above can be readily identified; they are faintly basophilic on H&E staining but can also be recognized with PAS (pink-red) or GMS (black) methods (Fig. 17-51A and B). In Pityrosporum folliculitis, there is dilatation (ballooning) of the involved follicular lumina, associated with keratin plugging. Intrafollicular mucin deposits have been reported.189 Surrounding inflammation is frequently mild and lymphocytic, but follicular rupture can be associated with more profound inflammation and neutrophilic abscess formation.190 Round to oval budding yeast organisms, 2 to 4 µm in diameter, are found within the follicular lumen or occasionally in the surrounding dermis; these can frequently be seen with routine staining but are also identified with PAS or GMS staining (Fig. 17-52).
Differential Diagnosis: Because most biopsies of tinea versicolor show minimal or no morphologic abnormalities involving the epidermis or dermis, it qualifies as one of the “nothing” lesions—that is, a group of disparate disorders whose microscopic findings are minimal despite often distinctive clinical features. When faced with this dilemma, systematic evaluation of the biopsy specimen is indicated to look for subtle abnormalities, beginning with the stratum corneum, where in tinea versicolor the characteristic short hyphae and spores can be identified. This unique arrangement of organisms also helps separate tinea versicolor from dermatophytosis or candidiasis. Pityrosporum folliculitis is distinguishable from other types of folliculitis by the characteristic dilated or ballooned lumina of involved follicles and the finding of Pityrosporum yeast within follicular infundibula.
Tinea Nigra
Clinical Features: Tinea nigra is an infection involving the palms and soles191 seen mainly in tropical climates. It is seen along the Gulf coast of the United States. The cause is the organism Hortaea werneckii (formerly Cladosporium werneckii and Exophiala werneckii), a dematiaceous fungus found in the soil. Tinea nigra results from direct inoculation of the fungus through contact with contaminated vegetation. The lesions are brown to black and flat, and they can be removed easily by scraping because organisms are confined to the stratum corneum.
Microscopic Findings: Within the compact stratum corneum are brown hyphae that are readily identifiable on routine H&E staining. The brown color is due to melanin. Other microscopic changes are minimal, but a mild lymphocytic infiltrate can be seen in the dermis in some cases.192
Differential Diagnosis: Clinically, the brown to black lesions can mimic melanocytic nevi or even melanoma, but scraping or biopsy reveals the presence of pigmented hyphae, confirming the diagnosis of tinea nigra. The confinement of organisms to the stratum corneum distinguishes this disease from other forms of phaeohyphomycosis (see later discussion).
Candidiasis
Clinical Features: Candidiasis describes infection most often due to Candida albicans. However, other Candida species can produce disease, particularly in the setting of disseminated infection as seen in immunocompromised individuals; examples include Candida tropicalis, Candida krusei, and Candida guilliermondii.193 Candidiasis is often divided into three disease groupings: (1) acute mucocutaneous candidiasis, (2) chronic mucocutaneous candidiasis, and (3) disseminated candidiasis.194
C. albicans frequently inhabits the intestinal tract and is found on mucosal surfaces, including the mouth and genitalia. Local factors such as moisture, heat, and irritation tend to promote infection and create conditions for its development in other areas, such as paronychial tissues. Predisposing factors include occupations involving wet work, obesity, diabetes mellitus, iron deficiency anemia, antibiotic therapy, and drug-induced immunosuppression. Forms include intertrigo, with erythema and satellite pustules, Candida paronychia, presenting as erythema and edema of the proximal nail folds, Candida vulvovaginitis, congenital cutaneous candidiasis associated with premature rupture of membranes,195 and acquired neonatal candidiasis with involvement of oral mucosa and diaper area.196 Chronic mucocutaneous candidiasis is associated with widespread cutaneous involvement and linked to an assortment of lethal and nonlethal immunodeficiency disorders197 as well as thymoma.194 Disseminated candidiasis is particularly associated with lymphoma, leukemia, or HIV infection, and presents with fever, myalgias, and one or more erythematous or purpuric papulonodules, sometimes ulcerated, on the trunk or proximal extremities.198,199
Microscopic Findings: Acute candidiasis often shows changes of a mild, spongiotic dermatitis. Neutrophils are found within the epidermis, sometimes forming subcorneal pustules or spongiform pustules.200 The organisms can be seen in the stratum corneum with Gram, PAS, or GMS methods and consist of septate hyphae and sometimes spores (Fig. 17-53A and B). The findings are similar in most forms of chronic candidiasis, but in Candida granuloma, there may be hyperkeratosis, irregular acanthosis, and loosely organized granulomatous dermal inflammation. Organisms are found in the epidermis but occasionally within granulomatous foci in the dermis.201,202 In disseminated disease, there are varying degrees of inflammation, sometimes with neutrophilic abscesses or leukocytoclastic vasculitis. Panniculitis may also be found, frequently with a prominent neutrophilic component.155 Both hyphae and budding yeast forms can be seen in these areas, but they are often difficult to find without special stains, particularly the PAS method.203,204
Differential Diagnosis: On morphologic grounds, candidiasis with epidermal involvement can resemble lesions characterized by subcorneal pustule formation (e.g., impetigo). When spongiform postulation is present, the differential diagnosis includes psoriasis, skin lesions of Reiter syndrome, or geographic tongue. In such cases, the workup clearly indicates the use of special stains, such as PAS, to rule out Candida infection. In the author’s experience, Candida granulomas are very rare, and the recent literature on these lesions is sparse. However, loosely organized dermal granulomas without explanation should prompt a search for organisms, particularly if there is a history of immunosuppression or endocrinopathy. Lesions of disseminated candidiasis show dermal and subcutaneous changes that are strongly suggestive of septicemia, and accordingly the workup usually includes a battery of special stains, including fungal stains, as well as culture studies. Once organisms are identified in tissue sections, an additional task is to exclude other fungal infections, such as dermatophytosis in the case of superficial infections or hyalohyphomycosis in the case of deep-seated lesions that suggest septicemia. The finding of spores and pseudohyphae can help support the interpretation of candidiasis, but in many cases, the precise identity of the organisms requires culture studies.
Hyalohyphomycosis
Clinical Features: Hyalohyphomycosis refers to a loosely organized group of opportunistic, nondematiaceous saprophytic fungi that usually cause disease in immunosuppressed individuals. Organisms variably included in this group include Aspergillus flavus, Aspergillus fumigatus, and Aspergillus niger; Fusarium solani; and Pseudallescheria boydii. Aspergillosis often arises as a generalized infection, although primary cutaneous disease has been described.205 Cutaneous Fusarium infection often represents dissemination from a primary focus in the lungs. P. boydii is a cause of mycetoma (see “Mycetoma”) but can also involve the lower respiratory tract, with direct extension through the chest wall or hematogenous dissemination to the skin.206 Cutaneous lesions resulting from these infections vary in appearance, ranging from plaques and violaceous nodules to ulcerative lesions with necrotic eschar formation caused by vascular occlusion.
Microscopic Findings: Changes in hyalohyphomycosis vary, but there are often abscess formation and invasion of vessel walls by organisms, with infarction and associated changes of necrosis and connective tissue degeneration.155,207,208 Granulomas tend to occur in localized lesions of aspergillosis in patients who are immunocompetent or who have received antifungal therapy.209 Organisms are best seen with special stains, especially silver methods such as GMS. Aspergillus organisms ideally display uniform, regularly septate hyphae, 3 to 6 µm in width, with right-angle branches that are dichotomous (i.e., the size is similar to those of the originating hyphae)209 (Fig. 17-54A and B). However, degenerated forms can be seen that can make specific identification difficult without culture studies. Fusarium organisms may be slightly wider than Aspergillus, and the branches arise at right angles and are constricted at the point of connection to the hyphae of origin. Pseudallescheria organisms are somewhat thinner than Aspergillus organisms, with haphazard branching and formation of vesicles and ovoid conidia.
Differential Diagnosis: Dermal or subcutaneous changes suggestive of sepsis, including varying aggregates of neutrophils and evidence of vascular occlusion, should prompt special staining to search for organisms. This is particularly the case in immunocompromised patients. Nonpigmented hyphal forms should raise the possibility of hyalohyphomycosis, and inspection of detailed structural characteristics using GMS stains may help identify the causative organism. However, this is often quite difficult in tissue sections, due to variations in morphology and degenerative changes, so as a practical matter, determination of the specific infectious agent usually requires culture studies.
Zygomycosis
Clinical Features: The term zygomycosis encompasses two categories. The first, subcutaneous phycomycosis, or entomophthoromycosis, is associated with subcutaneous masses in perinasal or other tissues, and it is often due to Conidiobolus coronatus or Basidiobolus ranarum. This infection is seen mainly in Africa, Asia, or periequatorial America.210,211 Biopsies show a mixed inflammatory infiltrate, with the organisms surrounded by eosinophilic material—an example of the Splendore-Hoeppli phenomenon. The organisms are broad and show right-angle branching, but they are often fragmented, forming short segments, and septation can be observed.212
Mucormycosis is caused by molds found in soil or decomposing material; causal molds include the genera Mucor, Rhizopus, Absidia, Mortierella, and Cunninghamella. This acute infection particularly occurs in individuals with diabetic ketoacidosis, lymphoma, leukemia, or in those suffering from burns and malnutrition.213 Otherwise healthy individuals can also become infected, sometimes because of contaminated dressings or various forms of trauma. Organisms are particularly prone to invade vessels, resulting in infarction and the formation of black, necrotic eschars. Therapy includes combinations of surgery and antifungal therapy.214
Microscopic Findings: Lesions may be suppurative, with a prominent neutrophilic component, or they may show mainly invasion of arteries and veins by the organisms, with associated thrombosis and infarctive change. This is particularly the case in patients who are granulocytopenic. Granulomatous inflammation is seen in localized, chronic infections or in patients who have been treated with amphotericin B. The organisms are broad (20 µm) but have thin walls, and therefore they often appear folded or distorted in tissue sections. Although they are usually described as nonseptate, a few septa are sometimes present (Fig. 17-55). Branching is haphazard and at right angles. The organisms do not stain well with the GMS method and can actually be better seen in H&E-stained sections. Some hyphae form rounded, thick-walled terminal structures called chlamydoconidia, which can be confused with yeast forms or sporangia215; they stain more intensely with fungal stains than the hyphae from which they arise.
Differential Diagnosis: In lightly stained sections with sparse numbers of organisms, zygomycetes can be mistaken for artifact or missed altogether. They must be distinguished from other organisms, particularly swollen, degenerated hyphae of Aspergillus. Cross-sectional profiles of zygomycete hyphae could be confused with empty spherules of Coccidioides immitis (see subsequent discussion), but unlike in Coccidioides, endosporulation is not seen, and other portions of the specimen are likely to show identifiable hyphal forms.
Phaeohyphomycosis
Clinical Features: This term is applied to a group of fungal infections caused by dematiaceous (darkly pigmented) fungi found in soil and vegetation. Among the numerous species producing this disease are Bipolaris spicifera, Exophiala jeanselmei, Phialophora parasitica, and Alternaria alternata.216 Widespread disease typically occurs in immunocompromised individuals, but individuals with apparently intact immunity can develop localized disease due to direct inoculation (e.g., due to a wood splinter), and this is also the case in some patients with central nervous system disease.217 Skin lesions vary in appearance, and have been described as crusted plaques, grouped pustules, cysts, or abscess-like lesions.218 Phaeomycotic cysts often arise from a splinter injury and present as fluctuant subcutaneous abscesses generally in exposed body surfaces.219 Systemic forms often result from respiratory tract infection due to inhalation of the infectious agent. Treatment is surgical excision for localized disease and antifungal chemotherapy for systemic infections.
Microscopic Findings: The typical findings are described in phaeomycotic cysts, located in the deep dermis/subcutis and consist of a central suppurative zone (containing neutrophils and necrotic material) surrounded by granulomatous inflammation (epithelioid macrophages and multinucleated giant cells) and an outer “capsule” of dense collagenous tissue.220 Similar changes can be seen in more superficial infections, although the inflammatory elements are often more loosely organized.218 The brown-staining organisms can be found within suppurative foci, surrounding giant cells, and also frequently in wood splinters that may still be present in tissue sections. These are septate, thick-walled hyphae, constricted at their septations, and sometimes show terminal swellings or yeastlike cells in chains. They are usually readily identified by their natural pigmentation (due to melanin), but structural details can be more precisely evaluated with special stains such as PAS or GMS (Fig. 17-56).
Differential Diagnosis: More lightly stained organisms could be confused with other opportunistic fungi, such as the organisms of hyalohyphomycosis, but the latter have much thinner walls, and close inspection using the high dry or oil objectives usually shows some degree of pigment in organisms of phaeohyphomycosis. Although there is a resemblance to the pigmented fungal organisms causing mycetoma, grain formation is not a characteristic of phaeohyphomycosis. The other brown-staining fungal organisms are those causing chromomycosis, but in tissue sections these consist of spherical bodies that reproduce by internal septation, and they are often found in the setting of a blastomycosis-like tissue reaction (see later discussion), with pseudoepitheliomatous hyperplasia and transepidermal elimination of the fungal elements.221 However, the author has seen a blastomycosis-like tissue reaction pattern in a case of phaeohyphomycosis due to Cladophialophora bantiana.222
Chromomycosis
Clinical Features: Chromomycosis (the term chromoblastomycosis is also still in use) is an infection of skin and subcutaneous tissue also caused by implantation of a dematiaceous fungal organism. However, as noted, in tissue the causative organisms in this disorder consist of rounded pigmented bodies (called Medlar bodies, sometimes referred to as “copper pennies”) that divide by internal septation. Among the most common species involved in chromomycosis are Fonsecaea pedrosoi (especially in the United States), Cladophialophora carrionii, E. jeanselmei, Fonsecaea compacta, and Phialophora verrucosa. Following trauma to the skin, verrucous nodules develop, which enlarge and show varying degrees of atrophy and scarring. Lesions can spread following trauma (e.g., scratching), and although lymphadenitis does occur, it is probably the result of secondary bacterial infection.223,224 Treatment is difficult, usually involving surgical excision for smaller lesions, and antifungal therapy, limb perfusion, or heat therapy in other cases.225
Microscopic Findings: The findings include pseudoepitheliomatous hyperplasia and a dermal infiltrate that contains neutrophils and granulomatous elements (Fig. 17-57A). Granulomas sometimes surround aggregates of neutrophils. Lymphocytes, plasma cells, Langerhans cells, and eosinophils are also present.226 In one study, eosinophils were particularly prominent.227 The characteristic brown-staining organisms (Medlar bodies) are typically found in microabscesses within the dermis228 (see Fig. 17-57B), although as noted transepidermal elimination of these bodies also occurs.221 These can also be demonstrated with the usual fungal stains (PAS and GMS). Internal septations are frequently observed. Short, pigmented hyphal forms can sometimes be recognized in tissue sections.
Differential Diagnosis: The histopathologic changes in tissue sections can resemble those in North American blastomycosis: pseudoepitheliomatous hyperplasia, a mixed dermal infiltrate including neutrophils and granulomas, and sometimes intraepidermal neutrophilic abscesses. North American blastomycosis is considered the prototype for this tissue reaction pattern, which can also be seen in South American blastomycosis, some examples of coccidioidomycosis, cryptococcosis in individuals with intact host immunity, sporotrichosis, some mycobacterial infections, and protothecosis. Diagnosis rests on finding the characteristic rounded pigmented bodies of chromomycosis. Other pigmented fungi, as seen in mycetoma and phaeohyphomycosis, primarily consist of hyphal forms, some with distinctive characteristics. Even though hyphae can occasionally be found in chromomycosis, they are usually inconspicuous and overshadowed by the more typical Medlar bodies.
North American Blastomycosis
Clinical Features: North American blastomycosis is an infection caused by a dimorphic fungus (mycelial at room temperature, yeast phase at 37° C), Blastomyces dermatitidis. This organism is present in the soil and prevalent along the basin of the Ohio River. Primary cutaneous infection is rare, having resulted from autopsy or laboratory accidents. Most commonly, skin involvement follows a primary infection in the lungs. The lesions consist of markedly verrucous plaques, usually on exposed surfaces. Other sites of involvement can include bones, liver, and the central nervous system.229 Treatments include the antifungals itraconazole and amphotericin B.
Microscopic Findings: The typical microscopic findings are pseudoepitheliomatous hyperplasia with intraepidermal neutrophilic microabscesses and varying degrees of overlying neutrophilic scale-crust. The dermis shows an infiltrate containing neutrophils, sometimes organized into microabscesses, and granulomas with multinucleated giant cells. The organisms are found in abscesses or in multinucleated giant cells and consist of thick-walled yeast forms approximately 10 µm in diameter. The walls are double contoured, and broad-based buds may be identified. These organisms can be seen in H&E-stained sections, but they are more readily identified with PAS or GMS methods230 (Fig. 17-58A-C).

Figure 17-58 North American blastomycosis. A, A typical lesion, featuring pseudoepitheliomatous hyperplasia, intraepidermal neutrophilic abscess formation, and a mixed acute and granulomatous dermal infiltrate. B, Within the dermal infiltrate, there are thick-walled yeast forms with doubly contoured walls, showing broad-based budding. C, Higher power view, showing detail of a Blastomyces dermatitidis organism.
Differential Diagnosis: As noted previously, the tissue reaction pattern of North American blastomycosis can be seen in a number of other “deep” fungal infections as well as chromomycosis, protothecosis, and some mycobacterial infections (including M. marinum infection and tuberculosis verrucosa cutis). There can be a clinical resemblance to halogenodermas, including bromoderma and iododerma, and the latter lesions also have microscopic features of pseudoepitheliomatous hyperplasia and neutrophilic microabscess formation. However, halogenodermas usually do not have significant granulomatous elements, and of course organisms are lacking. Blastomyces in tissue sections can be distinguished from other similar fungal infections with yeast forms by their size, thick walls, broad-based buds, lack of endospores, and lack of a capsule.
South American Blastomycosis
Clinical Features: South American blastomycosis, also known as paracoccidioidomycosis, is caused by another dimorphic fungus, Paracoccidioides brasiliensis. It is endemic in most continental Latin American countries. Infection begins in the lungs, and the mucocutaneous lesions generally represent disseminated disease. Lesions begin as papules and ulcers, the latter becoming quite extensive. Verruciform lesions are also seen. Locations include the gingiva, tongue, lips and nose. Regional lymphadenopathy also occurs.231 The disease may pursue a rapid course or be chronic and progressive. There is a tendency for quiescent disease to reactivate, sometimes many years after the initial infection.232 The clinical course is severe in patients with AIDS.233 Treatments include itraconazole, ketoconazole, and amphotericin B.
Microscopic Findings: Pseudoepitheliomatous hyperplasia, abscess formation, and granulomatous inflammation are frequent findings, but ulcers are also common. Organisms are found within abscesses or in multinucleated giant cells. They appear as thin-walled, rounded cells (particularly on H&E staining) measuring approximately 30 µm in diameter, but they can be seen to better advantage with GMS stain, with which they can be shown to have multiple buds creating the appearance of a marine pilot’s wheel. However, it may require careful search to find these classic forms,234 because in many cases what are found are smaller, nonbudding or singly budding structures.
Differential Diagnosis: The pseudoepitheliomatous hyperplasia–abscess–granulomatous inflammation image of course resembles that of a number of other “deep” fungal infections. Ulcerative lesions, however, are particularly common; this, plus the mucocutaneous location of the lesions and occurrence in an endemic area, provides a potential clue to the diagnosis of South American blastomycosis. Forms without multiple buds can mimic Histoplasma (when particularly small and intracellular), Blastomyces, or capsule-deficient cryptococci. In such circumstances, culture studies may be necessary for definitive diagnosis, although this can be supplemented by serologic testing. Organisms have be detected by PCR technology, and this has been carried out in paraffin-embedded tissues.235
Lobomycosis
Clinical Features: Lobomycosis, also called keloidal blastomycosis, is a disease caused by the fungus Lacazia loboi (formerly Loboa loboi). The organism currently cannot be cultured in vitro. It is believed to exist in soil and vegetation and is most commonly found in Central and South America. It causes disease in both humans and bottle-nosed dolphins.236 Nodular, keloidal lesions are most common, but skin lesions can vary, presenting as macules, papules, plaques, and ulcers. Verruciform lesions also occur.237 Surgery is the treatment of choice for isolated lesions; the organism tends to be resistant to antifungal therapy.236,238
Microscopic Findings: The epidermis is usually normal to atrophic. There is extensive granulomatous dermal inflammation, with admixtures of lymphocytes and plasma cells. Numerous yeastlike organisms are seen, forming clusters and chains (Fig. 17-59A and B). In the latter, the cells are connected by tubelike structures. Individual organisms are approximately 10 µm in diameter and have thick, doubly contoured walls. Portions of these organisms can also be found within macrophages and giant cells.239,240 Ironically, fibrosis is not usually a prominent feature in lesions of lobomycosis.240
Differential Diagnosis: Multiple buds can sometimes be seen around the cells of lobomycosis,241 which can then be confused with the organisms of South American blastomycosis (P. brasiliensis). However, the latter are more variable in size and have thinner walls. Chains of cells as seen in lobomycosis are not observed in North American blastomycosis, and L. loboi organisms are larger than either Sporothrix schenckii or Histoplasma capsulatum. Despite the alternative name for this infection (keloidal blastomycosis) and the keloid-like clinical appearance of some lesions, the microscopic features of keloid are not observed.
Coccidioidomycosis
Clinical Features: Coccidioidomycosis is caused by Coccidioides immitis, a dimorphic fungus that can show endosporulation in tissue sections. It is found in the soil in North and South America, and is particularly encountered in arid regions such as the San Joaquin valley of California. Disseminated disease is more common in African-Americans, Filipinos, and Vietnamese, and somewhat more common in Mexicans and Native Americans, than in Caucasians. As is true of many of the “deep” fungal infections, disease is also more extensive in immunosuppressed individuals. Although primary inoculation of the skin can occur, the usual mode of infection is via the respiratory tract, with the development of flulike symptoms. In a minority of patients, erythema nodosum ensues, which is usually a favorable finding that portends self-limited disease. Cutaneous involvement is seen in up to 20% of patients and manifests as papules, verrucous nodules, plaques, and ulcers. Special precautions must be exercised when attempting to culture this organism, because it is highly infectious and grows at room temperature.242 Treatments include fluconazole, itraconazole, and amphotericin B.243
Microscopic Findings: Verrucous lesions show a blastomycosis-like tissue reaction pattern, but other lesional configurations include subcutaneous abscess formation with necrosis and a vasoproliferative form with perivascular inflammation that may be suggestive of vasculitis.244 Granuloma formation is a common theme in most of the forms of cutaneous disease, but abscess formation, necrosis (including caseous necrosis), and development of tuberculoid granulomas are highly variable from case to case.245,246 Eosinophils may be prominent in some cases.244 Spores are seen extracellularly and in multinucleated giant cells. Large, rounded, thick-walled spherules, ranging up to 80 µm in diameter, are identified in tissue sections. Endospores (about 1–5 µm in diameter) are found within some of these spherules and can be released into the surrounding tissue (Fig. 17-60A-C). Suppuration tends to surround released endospores, whereas mature spherules are associated with a more granulomatous reaction pattern.247 PAS and GMS stains may be used to highlight the fungi, although the walls of mature spherules do not stain well with the usual fungal stains.248
Differential Diagnosis: Lesions showing pseudoepitheliomatous hyperplasia can raise a differential diagnosis that includes other infections with a blastomycosis-like tissue reaction pattern. Other types of lesions can show tuberculoid granulomas (granulomas, with or without central caseation, surrounded by lymphocytes and plasma cells) or granulomas surrounding a central abscess. Those types of lesions can mimic forms of tuberculosis, other “deep” fungal infections, or phaeomycotic cysts. The finding of the characteristic endosporulation of Coccidioides rule out other possible infectious diseases, but problems can arise when only scattered endospores are identified, or in older lesions when organisms may no longer be detectable. Under those circumstances, serologic or culture studies may be necessary to confirm the diagnosis. Coccidioides species have also been detected using PCR technology.249 There is a resemblance between spherules containing endospores and an essentially artifactual finding called myospherulosis, in which erythrocytes in an environment containing lipid form similar-appearing aggregates. However, the latter structures tend to be pigmented, do not stain with methods used to stain fungi, and do stain positively with antibody to blood group antigens.250
Cryptococcosis
Clinical Features: This infectious disease is caused by Cryptococcus neoformans, an encapsulated yeast that is found in soil, dust, and pigeon excreta. Usually, cryptococcosis begins as a lung infection and disseminates hematogenously in about 10% of cases, generally in immunocompromised individuals. The skin and central nervous system are the common sites for dissemination. Primary inoculation cryptococcosis is extremely rare. Cutaneous lesions in disseminated disease are highly variable in terms of morphology; they have a special propensity for involving the head and neck region.251 Lesions include verrucous nodules or plaques, subcutaneous abscesses with draining sinuses and ulcers, or pustular lesions that can mimic acne vulgaris. Molluscum contagiosum–like lesions have been described, especially in HIV-positive patients.252 Cryptococcal cellulitis may be the first indication of disseminated disease.253 Amphotericin B and/or fluconazole are among the treatments used for cryptococcosis.
Microscopic Findings: There are two basic histopathologic configurations in cutaneous cryptococcosis. In patients who are profoundly immunodeficient, innumerable organisms may be present, consisting of gray-appearing (in H&E-stained sections) yeast forms measuring 4 µm or so in diameter, surrounded by coalescing clear spaces that represent the mucoid capsules of the organisms. This gives the tissue an overall gelatinous appearance. These structures essentially efface the normal tissue architecture (Fig. 17-61A). In individuals who are otherwise immunocompetent, there is a mixed neutrophilic and granulomatous host reaction, sometimes with overlying pseudoepitheliomatous hyperplasia. Yeast forms are not as numerous, tend to be pleomorphic and capsule-deficient (2 to 4 µm in diameter), and are found within macrophages and giant cells but sometimes also free in tissue254 (see Fig. 17-61B). The findings in cryptococcal cellulitis may vary. The infiltrate has been described as primarily lymphohistiocytic but with acute panniculitis.255 However, acute inflammation with abscess formation has been seen in a patient post–renal transplant,256 whereas marked dermal edema with necrotizing subcutaneous granuloma has been reported in an immunocompetent individual.257 In each case, organisms could be readily identified in tissue sections. Cryptococci can be well demonstrated with mucicarmine, PAS, and GMS stains (see Fig. 17-61C); the latter is particularly helpful when organisms are sparse (e.g., in lesions from patients who are immunocompetent). When present, the acid mucopolysaccharide-containing capsule can be stained with mucicarmine (red) or with Alcian blue (blue). Combinations of a mucin stain (e.g., Alcian blue) with PAS can display both the yeast and its surrounding capsule. Cryptococci also contain a melanin-like substance in their cell walls, which can be demonstrated with stains for reducing substances, such as the Fontana-Masson stain.258 In the author’s experience, a fine black stain around a portion of the yeast wall is most often found with this method.
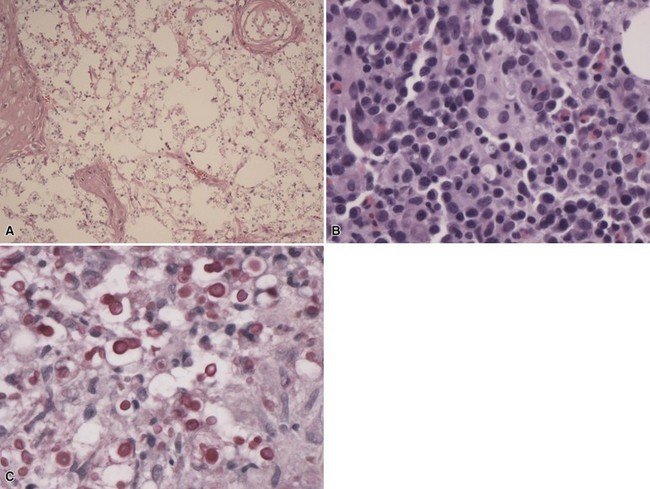
Figure 17-61 Cryptococcosis. A, In an immunodeficient patient, there are numerous organisms, whose capsules lend a gelatinous appearance to the lesion. B, In an immunocompetent individual, there are fewer organisms that can be found free in tissue as well as within macrophages and giant cells. C, Organisms are well demonstrated with a mucicarmine stain.
Differential Diagnosis: The “gelatinous” variant of cryptococcosis in patients who are immunodeficient has a characteristic microscopic appearance. Generally, in situations where some capsular material is present, mucin stains are positive, thereby identifying the unique feature of this organism. Capsule-deficient organisms in tissue sections from immunocompetent patients, which of course also show a significant host inflammatory reaction, can create significant diagnostic difficulties, because the organisms vary considerably in size. They can thereby potentially mimic H. capsulatum, microforms of B. dermatitidis, and less commonly Candida species or incompletely developed C. immitis spherules. In difficult situations, distinction can be made by direct immunofluorescence using specific C. neoformans antibodies, PCR methods, or electron microscopy (because even “capsule-deficient” cells can be shown to have attenuated capsules with this method). Fontana-Masson staining is also helpful (see earlier discussion), in that positive staining of the cell walls excludes Blastomyces and Histoplasma (however, note that S. schenckii and incompletely developed spherules of C. immitis can also stain with this method).259
Histoplasmosis
Clinical Features: Histoplasmosis is endemic in the central eastern portion of the United States, particularly in the vicinity of the Ohio and Mississippi river valleys. It is caused by the organism Histoplasma capsulatum, a dimorphous fungal organism, that is yeastlike in tissues and when cultured at 37° C, hyphal at room temperature. Although primary inoculation occurs, it is uncommon and self-limited, consisting of an ulcer with associated lymphangitis and lymphadenitis, changes similar to those of other primary inoculation infectious diseases. Most primary infections are pulmonary and are asymptomatic, although there may be flulike symptoms. Chronic pulmonary histoplasmosis is a disease that resembles pulmonary tuberculosis.260 Disseminated histoplasmosis occurs in infants and the elderly, as well as in severely immunosuppressed patients (e.g., those with lymphoma, leukemia, or AIDS).261 Oral mucosal lesions are common in disseminated disease, and they consist of granulomatous and ulcerated lesions.262 Skin involvement is quite uncommon, occurring in about 6% of patients with disseminated disease, and it consists of abscesses and pustules, nodules, plaques, and ulcers. Treatment for severe disease typically includes amphotericin B and/or itraconazole.
African histoplasmosis is caused by a variant of H. capsulatum, Histoplasma duboisii. In contrast to conventional histoplasmosis, this form of the disease commonly presents with lesions of skin, subcutaneous tissue, and bones. Skin lesions consist of nodules, plaques, and ulcers. They can be seen even in the “localized” form of the disease, which despite its chronic course apparently begins as a subtle pulmonary infection.
Microscopic Findings: Many lesions are ulcerated and include foci of necrosis and a chronic inflammatory infiltrate consisting of macrophages and some multinucleated giant cells.260 Although it is common in a number of “deep” fungal infections, a blastomycosis-like tissue reaction pattern is not found in histoplasmosis. H. capsulatum organisms are small, round to oval yeast forms, ranging from 2 to 4 µm in diameter, that are found within the cytoplasm of macrophages. Each organism is surrounded by a clear space; however, this does not represent a true capsule, but instead is a clear zone separating the cell wall from the surrounding cytoplasm of the macrophage it has “parasitized”261 (Fig. 17-62). These organisms can be seen in H&E-stained sections or with PAS and GMS stains, but they are also highlighted by Gram and Giemsa methods. In lesions of African histoplasmosis, there is typically a mixed infiltrate, including neutrophilic microabscesses and granulomatous elements. The organisms are larger in this disease variant, ranging from 8 to 15 µm, and can be seen free in tissue as well as within macrophages and giant cells.
Differential Diagnosis: Because of their small size and intracellular location, the organisms of histoplasmosis must be distinguished from other “parasitized histiocyte” diseases, including leishmaniasis, granuloma inguinale, rhinoscleroma, and toxoplasmosis. Leishmania organisms have a tendency to line up along the inside cell wall of the macrophage (the “marquee sign”), are not encapsulated, and have a small basophilic kinetoplast adjacent to the nucleus. K. granulomatis, the organism of granuloma inguinale, is slightly smaller than H. capsulatum and shows bipolar staining with the Giemsa method. A capsule appears to be present around these organisms, but it may not be identifiable in routine tissue sections and is best displayed in Epon-embedded thin sections. In rhinoscleroma, K. rhinoscleromatis organisms reside within particularly large, vacuolated macrophages (Mikulicz cells) and have a more prominent plasmacellular infiltrate. Unfortunately, PAS and silver stains are also positive in the latter disorder. Toxoplasma organisms can sometimes be found in macrophages but also appear as pseudocysts or as groupings within keratinocytes of the epidermis and sweat ducts and within arrector pili muscle.96 They are weakly positive for PAS but are negative with the GMS stain,96 and they can also be stained with antibodies specific to Toxoplasma. Other problematic infections include those due to Penicillium marneffei,263 Torulopsis glabrata,264 capsule-deficient cryptococci, or lesions containing microforms of B. dermatitidis. All of these can display small yeast forms that parasitize macrophages, but each has subtle differences from Histoplasma, such as lack of a pseudocapsule, different manner of reproduction, or presence of other structures such as hyphal forms (Penicillium) or more typical, larger, thick-walled spores with broad-based buds (Blastomyces). H. duboisii, the organism of African histoplasmosis, can mimic B. dermatitidis in tissue sections due to its similar size and thick wall, but only the former shows “double cell” forms (two organisms of similar size connected by a narrow bridge). In addition, unlike H. duboisii, B. dermatitidis organisms contain multiple nuclei.265 More specific diagnosis of histoplasmosis can be achieved using immunofluorescence staining, PCR methods, or serologic studies.
Sporotrichosis
Clinical Features: Sporotrichosis has a wide geographic distribution. It is produced by Sporothrix schenckii, another example of a dimorphic organism that is yeastlike in cultures obtained at 37° C but forms hyphae at room temperature. It occurs in three forms. By far the most common is lymphangitic, consisting of a crateriform nodule with ascending nodules along draining lymphatics. The next most frequent form is fixed cutaneous sporotrichosis, which consists of nodules, plaques, or a rosacea-like dermatosis involving the face. Disseminated disease typically develops in immunocompromised individuals and can involve the lungs, central nervous system, or gastrointestinal tract as well as other sites. Skin lesions in this form consist of suppurating nodules or ulcers.266,267 Saturated solution of potassium iodide is still used and considered effective for cutaneous forms of the disease,268 despite the lack of evidence for a direct effect of this agent on S. schenckii. Itraconazole is the agent most often used in disseminated infection.269
Microscopic Findings: Primary lesions of sporotrichosis initially show nonspecific changes (Fig. 17-63) but evolve into verruciform lesions in which there are acanthosis, sometimes reaching pseudoepitheliomatous proportions, intraepidermal neutrophilic microabscess formation, and a dermal infiltrate that includes abscesses and granulomas with multinucleated giant cells.270 Generally, it is difficult to find organisms in tissue sections from cases originating in the United States and in Europe, in contrast to cases from other geographic locations such as Japan.271 The organisms are round to oval spores measuring up to 6 µm in diameter. Single or multiple buds or elongated “cigar bodies” can also be identified (Fig. 17-64). Occasionally, asteroid bodies can be identified; again, these are uncommonly seen in U.S. material but are more frequent in other locations, such as South Africa. Asteroid bodies in this context consist of a central organism of S. schenckii surrounded by radiating eosinophilic material. They can be seen in H&E-stained sections, and the author once found an asteroid body coincidentally in a section stained with the Ziehl-Neelsen method! Asteroid bodies of this type appear to be specific for sporotrichosis.272 PAS and GMS stains are effective at demonstrating the organisms in tissue, but multiple sections may be necessary to find them. Fully developed lesions in lymphatic nodules show a central neutrophilic, or suppurative zone, surrounded by granulomatous inflammation, which, in turn, is surrounded by an infiltrate of lymphocytes and plasma cells.
Differential Diagnosis: The most common problem encountered in the diagnosis of sporotrichosis is the inability to find organisms in tissue sections, despite careful search. Lesions with similar clinical and microscopic appearances can also occur due to atypical mycobacterial infection, and those organisms can be similarly difficult to identify. Other organisms associated with a blastomycosis-like tissue reaction pattern can potentially be confused with S. schenckii, especially if found as isolated yeast forms. The changes in lymphatic nodules of sporotrichosis are similar to the “stellate” abscesses seen in lymphogranuloma venereum, tularemia, and cat scratch disease. Resolution of these issues requires culture study, direct immunofluorescence staining for S. schenckii (if available), or serologic testing. In one study of experimental sporotrichosis in mice, culture and antibody testing on blood samples were actually of greater diagnostic value than the nested PCR method.273
Mycetoma
Clinical Features: Mycetoma (also known as maduromycosis or Madura foot) is a chronic, deeply extending infection involving skin, subcutaneous tissue, and, sometimes, underlying bone. Mycetoma is caused by two types of organisms: “higher” bacteria with filamentous structures (e.g., A. israelii) and a group of true fungi. These infections are termed, respectively, actinomycetoma and eumycetoma. As in actinomycosis and botryomycosis, these infections are characterized by the formation of grains in tissue. Organisms causing actinomycetoma include Actinomadura madurae, Actinomadura pelletieri, N. asteroides and N. brasiliensis, and Streptomyces somaliensis. Among the best-known causes of eumycetoma are Neotestudina rosati, P. boydii, E. jeanselmei, Madurella grisea and Madurella mycetomatis, and Pyrenochaeta romeroi. Subcutaneous nodules develop, usually on exposed surfaces, evolving into abscesses with draining sinuses. The infection extends through tissue planes to involve muscle or even bone, resulting in osteomyelitis. Chronic inflammation and fibrosis result in marked deformity of the involved extremity.274–276 The grains found in material from draining sinuses vary in color depending on the organism, sometimes providing clues to the causative organism; grains can also be crushed and examined microscopically. Most grains from actinomycetomas are white, with the notable exceptions of A. pelletieri (producing red grains) and S. somaliensis (producing yellow grains). Eumycetoma grains are either white (e.g., N. rosati, P. boydii) or black (E. jeanselmei, M. grisea, M. mycetomatis, P. romeroi).277 Treatment of actinomycetoma mainly consists of antimicrobials such as penicillin or sulfonamides, depending on the causative organism. Surgery is the mainstay of treatment for eumycetoma, sometimes with the addition of antifungal agents such as itraconazole.
Microscopic Findings: Microscopic examination shows abscess formation with sinus tracts, containing neutrophils, necrotic material, and the characteristic grains. Surrounding the abscess are granulomas, admixed with lymphocytes and plasma cells. Fibrosis is extensive in chronic lesions. The grains are lobulated structures ranging from 0.25 to 2 mm in diameter. Actinomycetoma grains contain gram-positive filamentous organisms that also stain with PAS, whereas eumycetoma grains contain aggregates of hyphae that are positive for PAS and GMS. The surrounding lobulated material is eosinophilic, positive for PAS, and represents host protein—another example of the Splendore-Hoeppli phenomenon (Fig. 17-65A and B).
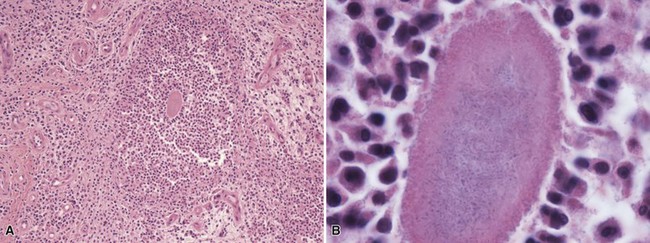
Figure 17-65 Mycetoma. A, Within a dermal abscess is a small grain with an eosinophilic outer border and a slightly basophilic center. B, Hyphal forms, sectioned longitudinally and in cross section, can be identified in the center of the grain on an H&E-stained section. This represents an example of a eumycetoma grain composed of true fungi.
Differential Diagnosis: The typical clinical presentation, combined with abscess formation, draining sinuses, and grains, leads to a diagnosis of mycetoma. Microscopic examination can help distinguish the grains of botryomycosis (which show conventional bacteria—usually gram-positive cocci) from those caused by filamentous bacteria and fungi. Similarly, morphology and differential staining can help separate actinomycetoma caused by filamentous, gram-positive bacteria from eumycetoma caused by fungi that form true hyphae.278,279 Precise identification of the species involved, of course, requires culture studies.
Rhinosporidiosis
Clinical Features: Rhinosporidiosis is endemic in India and Sri Lanka but has been occasionally seen in the United States and Europe.280 It is caused by Rhinosporidium seeberi, an organism that cannot be cultured in vitro. It produces papillomatous lesions that involve mucosa or mucocutaneous surfaces, especially the nasal cavity. Surgical excision is the appropriate treatment for these lesions.
Microscopic Findings: Within involved areas of the submucosa or dermis, there is a combination of chronic and granulomatous inflammation and granulation tissue. There are large sporangia, usually measuring between 100 and 200 µm, containing numerous smaller, rounded structures called sporangiospores281 (Fig. 17-66). When sporangia rupture, sporangiospores are released into the tissue and are believed to transform to trophocytes with dense eosinophilic walls and a single nucleus. These structures can be easily seen on H&E-stained sections but can also be demonstrated with PAS or GMS stains.282 Mucicarmine also stains the inner surfaces of sporangia as well as the sporangiospores.283
Differential Diagnosis: The clinical presentation and microscopic appearance of rhinosporidiosis are quite unique. The only commonly encountered organisms that could be confused would be the endospore-containing spherules of C. immitis. However, these are considerably smaller than the sporangia of Rhinosporidium and lack the mucicarmine positivity of the sporangiospores and inner sporangia wall.