Other T-Cell and Natural Killer Cell Lymphomas Involving the Skin
Blastic Plasmacytoid Dendritic Cell Neoplasm
Clinical Features: This tumor has undergone several name changes over recent years; the best known include blastic NK-cell lymphoma and, later, CD4+/CD56+ hematodermic neoplasm. It is now believed that the neoplastic cell of BPDCN is related to a precursor of the plasmacytoid dendritic cell, a cell that is known to produce large amounts of α-interferon.463 It most commonly arises in middle-aged to elderly individuals. Cutaneous nodules often constitute the presenting finding.464,465 Skin lesions are sometimes described as “rash” or exanthema,466,467 and in one example the lesions were said to resemble angiosarcoma.465 Disseminated disease is often present, with lymphadenopathy, anemia, cytopenia, liver, spleen, and/or bone marrow involvement.467–470 This is an aggressive lymphoma, with deaths usually occurring several months to 3 years following diagnosis.469,471
Microscopic Findings: There are dermal infiltrates of medium-sized cells with fine chromatin and lymphoblastic features.464,467 Extension into the subcutis may be seen. Other described features include a perivascular or periadnexal configuration of the infiltrate and admixtures with cells having elongated or distorted features472 (Fig. 27-42A and B). Although there is some variability in the reported immunophenotype, neoplastic cells are regularly CD56+, usually CD4+ and CD43+, and negative for surface CD3. They typically express the plasmacytoid dendritic cell antigen CD123 and T-cell leukemia/lymphoma 1,473,474 and they can also stain with antibodies to MxA, a surrogate marker for interferon-α production.475 Tumor cells can also stain for TdT.476 T-cell receptor genes are germline.465,471,477 In contrast to NK/T-cell lymphoma, nasal type, these cases are negative for EBV.465,467,477

Figure 27-42 Blastic plasmacytoid dendritic cell neoplasm. A, There is a dense, nodular infiltrate extending into the deep dermis and subcutis. The infiltrate surrounds a portion of a hair follicle to the lower right of the figure. B, The neoplastic cells have fine chromatin and lymphoblastic features.
Differential Diagnosis: There can be a morphologic resemblance to other lymphomas and leukemias. In terms of immunohistochemical staining, expression of CD56 can also be seen in extranodal NK/T-cell lymphoma, nasal type, and cutaneous γδ T-cell lymphoma, and TdT expression is characteristic of precursor T-lymphoblastic lymphoma (PTLL). Distinction can be made by the expression of plasmacytoid dendritic antigens in BPDCN and the usual negativity for EBV antigens or EBV-encoded small nuclear RNA, in contrast to the positivity for EBV associated with NK/T-cell lymphoma, nasal type. A recent paper described cutaneous lesions composed of plasmacytoid dendritic cells associated with acute myeloid leukemia. As is the case for the neoplastic cells in BPDCN, the cells in the latter condition express CD123, CD4, and T-cell leukemia/lymphoma 1. Among the microscopic differences, the plasmacytoid dendritic cells associated with myeloid leukemia form aggregates that are demarcated from the leukemic cells; they are small- to medium-sized cells with rounded to oval nuclei and fine chromatin. The cells of BPDCN are morphologically similar but are medium- to large-sized and not associated with leukemic cells. In terms of immunohistochemical differences, the cells of BPDCN are regularly CD56+ and sometimes express TdT, whereas those in lesions associated with myeloid leukemia are CD56− and TdT−.478
Precursor T-Lymphoblastic Lymphoma
Clinical Features: This neoplasm is composed of T-lineage lymphoblasts. It is essentially the same disorder as precursor T-lymphoblastic leukemia, the only difference being that the lymphoma presents as a mass lesion with less than 25% lymphoblasts in the marrow.479 PTLL primarily occurs in adolescent males. It often presents as a rapidly growing mediastinal mass with pleural effusion. Extranodal sites include the liver, spleen, Waldeyer ring, and the central nervous system as well as the skin. A unique feature of this lymphoma is that, although it represents the vast majority of all lymphoblastic lymphomas, it apparently involves the skin less often than does precursor B-lymphoblastic lymphoma.480,481 Cutaneous involvement most often consists of one or more tumor nodules, with a propensity to occur in the head and neck regions.480,482 Responses to therapy are comparable to those of precursor B-lymphoblastic lymphoma.
Microscopic Findings: There is usually a dense, diffuse infiltrate composed of medium-sized lymphoid cells with high nuclear-to-cytoplasmic ratios. These cells display rounded to convoluted nuclei with dispersed to condensed chromatin and inconspicuous nucleoli479,480 (Fig. 27-43). Mitotic figures are readily identified. Scattered macrophages can produce a “starry sky” appearance. Several authors have emphasized that there are few, if any, morphologic differences between PTLL and its B-cell counterpart.481 Immunohistochemistry confirms the T-cell nature of the process, because tumor cells commonly express CD2, CD3, CD4, CD5, CD7, and/or CD8. Blastic cells may coexpress CD4 and CD8.479 As is characteristic of lymphoblastic lymphoma, cells are positive for TdT. Other antigens that are sometimes expressed are CD10, CD79a, and the myeloid markers CD13 and/or CD33.479,480 Other B-cell markers are negative.480 Clonal rearrangement of T-cell receptor genes can be detected. Translocations involve T-cell receptor loci and an assortment of partner genes, including several transcription factors.483
Differential Diagnosis: The “starry sky” appearance of some examples of PTLL can mimic Burkitt lymphoma or variants of mantle cell lymphoma, both uncommon in skin. However, nuclear TdT positivity confirms the diagnosis of lymphoblastic lymphoma. In contrast to blastic plasmacytoid dendritic cell neoplasm, which can also show TdT positivity, the neoplastic cells of PTLL lack the characteristic markers of plasmacytoid dendritic cells, such as CD123. Differential B-cell and T-cell staining can be used to determine the precise lineage of the neoplastic cells.
Hodgkin Disease (Hodgkin Lymphoma)
Hodgkin disease is a lymphoma composed of a particular type of atypical cell—the Reed-Sternberg cell (and its variants, such as the lacunar cell)—together with a mixed inflammatory cell infiltrate and, sometimes, stromal changes. This disorder has a bimodal age of incidence, with one peak occurring in early adult life and another in later life. Typically, Hodgkin disease presents with lymphadenopathy, particularly cervical, mediastinal, axillary, para-aortic, or inguinal-femoral.484,485 Intermittent fever, night sweats, weight loss, and anemia can accompany the lymphadenopathy. Treatment involves radiation and chemotherapy. Over the years, there has been progressive improvement in the prognosis, and now cures are possible in many cases. Stage of the disease and associated symptoms are better predictors of outcome than histopathologic type.343,484,485 The neoplastic cells of Hodgkin disease are believed to most often arise from a germinal center B cell,486 but T-cell derivation is also possible.306,487 EBV is detected in a number of cases and may play a pathogenetic role, particularly in patients who are immunosuppressed.485
Cutaneous manifestations are found in about 25% of all patients with Hodgkin disease. Nonspecific skin lesions are most common and may precede the diagnosis by months or years. Among the most common are pruritus, Addisonian hyperpigmentation, acquired ichthyosis, infectious complications such as herpes zoster, and erythroderma.484 Specific cutaneous infiltrates of Hodgkin disease are uncommon, with reported incidences ranging from 0.5% to 7.5%.485,488,489 They consist of papules, plaques, nodules, or larger tumors that sometimes ulcerate.490 Rarely, exfoliative dermatitis accompanying Hodgkin disease shows specific histopathologic features. Bullous lesions have also been described,491 and rare examples of granulomatous slack skin have been reported in Hodgkin disease.488,490 Most often, the mechanism of skin involvement is via retrograde lymphatic spread distal to involved lymph nodes.489,492 Other, less common mechanisms include direct extension from an underlying nodal focus and hematogenous dissemination. In some cases, specific skin lesions are noted at the time of first presentation of the disease, with node involvement being detected either simultaneously or shortly thereafter.489,492,493 Hodgkin disease has been preceded by lymphomatoid papulosis306,494 and accompanied or followed by mycosis fungoides.306,495 Usually, the finding of specific cutaneous lesions indicates stage IV disease and carries an adverse prognosis.489,496
However, there may also be rare instances of primary cutaneous Hodgkin disease.489 Lesions have most often been isolated cutaneous or subcutaneous nodules,289,497,498 but there have also been papules or nodules that have undergone spontaneous involution.318 Diagnosis of primary cutaneous Hodgkin disease requires identification of typical histopathologic and immunohistochemical features together with a negative workup for systemic disease.318,497 Primary cutaneous Hodgkin disease may have a more favorable prognosis than does secondary cutaneous involvement arising in established disease. In fact, some examples of primary cutaneous disease persist in the skin for years without systemic findings.318 Nevertheless, typical nodal involvement may eventually occur, at intervals ranging from several months to 6 years following the diagnosis of cutaneous disease.318,497,498
Microscopic Findings
As in lymph nodes and other tissues, a diagnosis of cutaneous involvement by Hodgkin disease rests on the finding of atypical cells with the characteristics of Reed-Sternberg cells. This often occurs in the setting of a polymorphous infiltrate that may include lymphocytes, macrophages, neutrophils, and eosinophils.318,490,493 The Hodgkin cell features lightly basophilic cytoplasm and a large rounded nucleus with a single prominent acidophilic nucleolus. The Reed-Sternberg cell possesses a bilobed nucleus, each lobe containing a prominent acidophilic nucleolus485 (Fig. 27-44A and B). A lacunar cell has also been described in cutaneous lesions; this is a Hodgkin or Reed-Sternberg cell with retraction of the cytoplasmic membrane, giving the impression that the cell resides in a space or lacuna.485,490 The lesional configurations in skin can mimic those of the established nodal subtypes of the disease, and accordingly, nodular sclerosis, mixed cellularity, and lymphocyte-depleted forms have been observed in skin.489,490,492 The cutaneous pattern frequently reflects that in the involved lymph nodes,490 and in one study, the cutaneous pattern was always in the same or a prognostically worse histopathologic category when compared to that displayed in the proximal nodal focus of disease.489

Figure 27-44 Hodgkin disease. A, Low-power view of a cutaneous nodule that showed a mixed cellularity configuration. This was the first detected lesion of Hodgkin disease in this patient; a proximal nodal focus of disease was found subsequently. B, A typical Reed-Sternberg cell is shown.
The neoplastic cells in Hodgkin’s disease are CD30+, CD15+ and CD45−.318,490,497,498 A substantial minority of the cells expresses CD20 with varying levels of intensity.485 However, T-cell antigens have been expressed on Reed-Sternberg cells in some cases.499 EBV-encoded latent membrane protein is sometimes detected.485 Overexpression of cytokines and their receptors by the neoplastic cells may account for the admixture of inflammatory cells so often observed in Hodgkin disease.485 Immunoglobulin gene rearrangements are found in most cases, but there are rare cases in which T-cell receptor gene rearrangements have been detected.487 Most often, gene rearrangements are detected in isolated Reed-Sternberg cells rather than in whole-tissue specimens.
Differential Diagnosis
Cutaneous lesions with heavy mixed infiltrates could be confused with a variety of inflammatory dermatoses, unless the typical cells of Hodgkin disease are identified. Of course, this is less likely to be a problem when there is a known history of nodal or systemic disease. The neoplastic cells of Hodgkin disease closely resemble the atypical cells of type A lymphomatoid papulosis. Lymphomatoid papulosis can precede the onset of Hodgkin disease, and rare cases of primary cutaneous Hodgkin disease present with self-healing papulonodular lesions that are close clinical mimics of lymphomatoid papulosis. Although the atypical cells of lymphomatoid papulosis are also CD30+, they are usually CD15− and CD45+, in contrast with Hodgkin and Reed-Sternberg cells. CD30+ ALCL can also resemble Hodgkin disease. However, the polymorphous inflammatory infiltrate of Hodgkin disease is distinctive.318 Also, in contrast to ALCL, the neoplastic cells of Hodgkin disease are positive for B-cell–specific activator protein and are negative for epithelial membrane antigen and the ALK protein.485,500 The latter two staining results help make a distinction from systemic ALCL. Although the neoplastic cells in primary cutaneous CD30+ large-cell lymphoma are also typically negative for epithelial membrane antigen and ALK, those cells are usually positive for CD4.209 Positivity for EBV latent membrane protein also favors Hodgkin disease in this context.485 A distinction from anaplastic large B-cell lymphoma may not always be possible, but this is not likely to be a major issue in cases of cutaneous Hodgkin disease, particularly when all the clinical and histopathologic features are taken into account. Recently, Cho and colleagues reported an example of cutaneous nodules occurring in a man with classic Hodgkin lymphoma. As in a lymph node biopsy, the Reed-Sternberg and Hodgkin cells showed weak PAX5 positivity and weak focal Oct-2 positivity and were negative for BOB.1. These results are consistent with Hodgkin lymphoma and argue against T-cell lymphoma (in which all three markers are absent) and other non-Hodgkin B-cell lymphomas (in which all three markers are strongly expressed).501–503
Leukemia Cutis
Clinical Features
Cutaneous involvement in the leukemias can be categorized as specific leukemic infiltrates and nonspecific manifestations. This section will focus on specific lesions of leukemia cutis. However, nonspecific lesions can be sources of clinical confusion with true leukemic infiltrates, complications related to leukemias (e.g., purpura due to thrombocytopenia, cutaneous infection due to immunosuppression), or closely linked reactive processes. Examples of the latter conditions include generalized pruritus, Sweet syndrome, pyoderma gangrenosum,504 and drug eruptions.505 More recently, there have been reports of arthropod bite–like reactions in patients with leukemias and lymphomas506 (Fig. 27-45).

Figure 27-45 Arthropod bite–like reaction in a patient with leukemia. Findings include spongiotic intraepidermal vesicles and a wedge-shaped dermal infiltrate containing numerous eosinophils. Leukemic cells were not identified in the sections.
Specific cutaneous lesions have been reported in most of the leukemias. These include acute myeloid leukemias (M1–M3 in the French-American-British [FAB] classification), acute myelomonocytic leukemia (M4), acute monocytic leukemia (M5), acute erythroleukemia (M6), acute lymphoblastic leukemia, chronic myelogenous leukemia, chronic myelomonocytic leukemia, chronic lymphocytic leukemia, adult T-cell leukemia, T-cell prolymphocytic leukemia (formerly T-cell chronic lymphocytic leukemia), and hairy cell leukemia.507 The author has already discussed the lymphomatous counterparts of acute lymphoblastic leukemia (precursor B-lymphoblastic lymphoma), ATLL, and precursor T-lymphoblastic leukemia/lymphoma. Aggressive NK cell leukemia will be considered briefly; although it may represent the leukemic form of extranodal NK/T-cell lymphoma, nasal type, this has not been established with certainty.508
The frequency of specific lesions of leukemia cutis varies, depending on the type of leukemia. Cutaneous involvement is common in monocytic leukemia, occurring in about 10% to 50% of cases.509 It is also relatively frequent in chronic lymphocytic leukemia, particularly in T-cell subtypes, such as T-cell chronic lymphocytic leukemia507 and T-cell large granular lymphocytic leukemia.510,511 Specific skin lesions are less common in other varieties, such as acute lymphoblastic leukemia, myeloid leukemias, and hairy cell leukemia.507,512,513 Lesions of leukemia cutis are most often papules and nodules. Plaques, ulcers, and purpuric lesions also occur.509,514,515 Unusual manifestations include erythroderma507,516 and bullous lesions.515 Pigmentary changes have been associated with leukemia cutis; both hyperpigmented and vitiligo-like lesions have been described.517,518 Gingival hypertrophy is associated with monocytic and myelomonocytic leukemias. The chloroma (granulocytic sarcoma), a green to greenish-gray tumor, is a characteristic if uncommon lesion associated with acute myeloid leukemia. Its greenish color is due to the presence of high concentrations of myeloperoxidase in infiltrating myeloblasts.509 Lesions appearing at arterial and venous puncture sites have been reported in acute promyelocytic leukemia (M3).519
There is a strong association between specific lesions of leukemia cutis and involvement of other extramedullary sites.520,521 The appearance of cutaneous lesions of leukemia is associated with a poor prognosis.515,521 Aleukemic leukemia cutis refers to the occurrence of leukemic skin lesions prior to detection of peripheral blood or bone marrow involvement. Although uncommon, there are numerous reports of this phenomenon, most of which involve cases of acute monocytic or myelomonocytic leukemia,522–524 but also in acute lymphoblastic leukemia.525
Microscopic Findings
Most varieties of leukemia cutis feature dense dermal infiltrates of atypical cells with infiltration between collagen bundles.526 A grenz zone separating the infiltrate from the overlying epidermis is frequently described in the non–T-cell leukemias.507,527 The atypical cells often display a perivascular and/or periadnexal distribution.507,513 Infiltration and destruction of adnexal structures, vessels, nerves, and muscle are seen, particularly in myeloid, monocytic, and myelomonocytic leukemias507,526 (Fig. 27-46). Examples of “leukemic vasculitis” have been described, in which infiltration of vessels by atypical cells may be associated with destruction of vessels walls and fibrin deposition.528–530 A Kaposi sarcoma–like pattern has been reported in myeloid leukemias, in which myeloblasts appear to line slitlike, blood-filled spaces.527 The cells of chronic lymphocytic leukemia can also appear in other types of cutaneous lesions, where they can be recognized in varying sized clusters of monotonous, small- to medium-sized cells531 (Fig. 27-47A and B). Recognition of the cytologic characteristics of the cells can be important in refining the diagnosis. Formerly described T-cell variants of chronic lymphocytic leukemia were reported to show epidermotropism.507 However, a recent series of cases of T-cell prolymphocytic leukemia (the current term that encompasses T-cell chronic lymphocytic leukemia) showed that dermal infiltrates were angiocentric rather than epidermotropic, composed of cells with irregular nuclei, small nucleoli, and rims of eosinophilic cytoplasm531a (Fig. 27-48A and B). The myeloblasts of acute myeloid leukemias are large cells with round to oval nuclei, fine to coarse (when minimally differentiated) chromatin, and scant basophilic cytoplasm (Fig. 27-49). Chronic myeloid leukemia features a much more varied cell population, including atypical myelocytes, metamyelocytes, eosinophil precursors, and mature neutrophils. In monocytic leukemias, monocytoid forms have indented nuclei with fine to lacy chromatin and one or more distinct nucleoli. In acute lymphoblastic leukemia, the cells are medium to large with fine to reticulated chromatin and scant to moderate amounts of cytoplasm (Fig. 27-50); in comparison, the lymphocytes are smaller and relatively mature in appearance in chronic lymphocytic leukemia. In hairy cell leukemia, the characteristic cytoplasmic projections are not seen in routinely processed skin specimens; tissue imprints are required to see them.507 Furthermore, fresh specimens are necessary to demonstrate staining with tartrate-resistant acid phosphatase.532
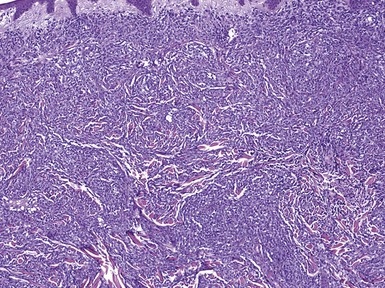
Figure 27-46 Myelomonocytic leukemia. There is a dense dermal infiltrate, separated from the epidermis by a grenz zone of uninvolved connective tissue. The leukemic cells insinuate between collagen bundles and also permeate vessels and adnexal epithelia.

Figure 27-47 Chronic lymphocytic leukemia. Aggregates of small, monotonous-appearing lymphoid cells are found adjacent to islands of basal cell carcinoma (A). Higher power view (B) shows the cytologic detail of these cells. The cells of chronic lymphocytic leukemia frequently stain positively for CD20 (C) and CD43 (D).

Figure 27-48 T-cell prolymphocytic leukemia. A, Dermal cellular aggregates are angiocentric rather than epidermotropic. B, The cells have irregularly contoured nuclei, small nucleoli, and rims of eosinophilic cytoplasm.

Figure 27-50 Acute lymphoblastic leukemia. In this particular preparation, the cells show fine chromatin and generally scant amounts of cytoplasm.
Histochemistry and immunohistochemistry can be of great help in reaching a specific diagnosis of the type of leukemia cutis. Among the most useful stains in formalin-fixed, paraffin-embedded tissues are chloroacetate esterase (Leder stain; CAE) and immunohistochemical stains for myeloperoxidase (MPO), lysozyme, and CD20, CD30, CD43, CD45, CD45RO, and CD68. The Leder stain (CAE) typically decorates only myelocytes and mature granulocytes (mast cells also stain with this method), and therefore earlier myeloid precursors require recognition through other means. Several detailed schemes have been provided for the use of immunohistochemical stains in the workup of atypical dermal infiltrates for possible leukemia cutis.507,533 Cells of myeloid leukemias express lysozyme and (within the limits specified above) CAE. MPO positivity is found in myeloid and myelomonocytic leukemias. Monocytic and myelomonocytic leukemias are CD43+ and CD68+.534–536 In the algorithm proposed by Cronin and associates, atypical CD3− and CD20− infiltrates can be stained for CD43, MPO, CD68, CD56, and CD117 as a means of separating myeloid leukemia cutis from other entities such as BPDCN, mast cell sarcoma, and other lymphoproliferative disorders.533 This will be discussed further (see “Differential Diagnosis”). In acute lymphoblastic leukemia, the usual profile is CD19+, CD20+ (variable), CD79a+, CD10+, and HLA-DR+. As a practical matter in formalin-fixed skin specimens, the most noteworthy immunohistochemical finding is that neoplastic cells are weakly positive or negative for CD20 and, among the other common lymphocyte markers, may only express CD45.507 In paraffin-embedded tissues, the cells of chronic lymphocytic leukemia frequently express CD20 and CD43 (see Fig. 27-47C and D).
A leukemia-associated surface antigen, JL1, appears to stain examples of both lymphoblastic and acute myelogenous leukemia and does not stain other cutaneous lesions or normal tissues. Despite this degree of specificity, JL1 appears to have limited sensitivity; it was positive in about two thirds of cases of acute lymphoblastic leukemia/lymphoma and one third of cases of acute myelogenous leukemia.537 CD99, representing the E21/MIC2 gene product, is regularly positive in skin lesions of acute lymphoblastic leukemia and in a high percentage of those of acute myeloid leukemia.538 In a case of aggressive NK cell leukemia involving the skin, the neoplastic cells were CD2+, CD8+, CD56+, TIA-1+, and granzyme B+; positive for EBV by in situ hybridization; and CD3−, CD4−, CD16−, and CD57−.539 In T-cell large granular lymphocytic leukemia, the infiltrating cells are CD3+, CD4−, and CD8+, and EBV infection may again be demonstrable.510
Differential Diagnosis
The presence of atypical cutaneous infiltrates with the morphologic changes outlined above should suggest the possibility of leukemia cutis. A more detailed clinical and laboratory investigation should then follow; specific diagnosis depends on findings in bone marrow and peripheral blood. In the case of aleukemia cutis, close clinical follow-up is indicated to detect early development of systemic disease. A distinction from cutaneous lymphoma can be a major challenge, but it should be recognized that a number of these disorders (e.g., ATLL, acute lymphoblastic leukemia/precursor B-lymphoblastic lymphoma) actually have both leukemic and nonleukemic phases. Diagnosis may also be challenging when there are only a few atypical cells obscured by a dense, reactive infiltrate, as has been described in some lesions resembling Sweet syndrome or pyoderma gangrenosum.527 Regarding immunohistochemical staining, myeloid leukemia cutis is a likely diagnosis when CD3−/CD20− infiltrates are found to be CD43+/MPO+ or CD43+/MPO−/ CD68+/CD56−/CD117−.533 Examples that are CD56+ can be distinguished from BPDCN by using stains for CD4 and CD123, which are positive in the latter disorder (CD4 may also be positive in myeloid leukemias with monocytic differentiation); those that prove to be CD117+ can be distinguished from mast cell sarcoma by staining for tryptase or microphthalmia transcription factor, each of which stains mast cells but not myeloid cells.533,540 S-100 positivity has been reported in rare examples of myeloid leukemia cutis, and in one recent case this was associated with cytophagic activity.541 Such a result could lead to an erroneous diagnosis of melanoma, Rosai-Dorfman disease, or phagocytic activity associated with a lymphoid neoplasm, thus emphasizing the need for use of immunohistochemical panels as well as clinicopathologic correlation.
Extramedullary Hematopoiesis
Clinical Features
Cutaneous hematopoiesis normally occurs in the 8-cm stage embryo and ceases by 34 to 38 weeks’ gestation.542 After this stage, cutaneous hematopoiesis is distinctly unusual, arising principally in two settings: (1) in neonates, associated with abnormalities that have begun in utero, and (2) in adults, usually associated with myelofibrosis and often following splenectomy.543
Neonatal hematopoiesis has been associated with congenital viral infections, particularly rubella, cytomegalovirus, and coxsackie virus B2, as well as in twin transfusion syndrome, Rh-hemolytic disease of the newborn, and hereditary spherocytosis.542,544–546 It has also been reported in diffuse hemangiomatosis in a newborn.547 In cases not associated with hemangiomas, the cutaneous eruption consists of petechiae, purpuric macules, papules, and plaques, and ecchymotic lesions; the more purpuric of these are often referred to as “blueberry muffin” lesions.542,548 The head, neck, and trunk are mainly involved.542 The prognosis depends on the underlying condition and its resulting complications. Skin lesions tend to fade within 3 to 6 weeks after birth.542
Most adults reported with cutaneous extramedullary hematopoiesis have had myelofibrosis,543,549,550 but sporadic cases have also been associated with agnogenic myeloid metaplasia, polycythemia vera, and chronic myelogenous leukemia.551 Lesions consist of erythematous to purpuric papulonodules.549,552,553 A case with bilateral leg ulcers has also been described.554 Head and neck, trunk, and extremities have all been involved. Again, prognosis depends on the course of the underlying disease. Responses have been reported to hydroxyurea543,555 and electron beam therapy.543
Microscopic Findings
An infiltrate of varying intensity is found in the dermis and subcutis, both in a perivascular and interstitial distribution. It includes varying combinations of myeloid and erythroid elements as well as megakaryocytes. Myeloid cells in different stages of maturation are present; in some reports, there has been an increase in cells of eosinophil lineage.549,552 Nucleated erythrocytes are often observed542 (Fig. 27-51). Megakaryocytes are large, atypical cells with pale eosinophilic cytoplasm and multiple or multilobated nuclei.552,555 The neonatal cases frequently display only erythrocyte precursors, and myeloid cells are only occasionally present; for this reason, the term cutaneous erythropoiesis is sometimes applied to lesions in newborns.542 All three hematopoietic elements are present in some adult cases,550,553 but some reports have indicated a lack of erythrocyte precursors.552 Fibroblasts often accompany the process. Reticulin can be shown to surround megakaryocytes with Wilder or other methods.552
Granulocytes stain red with chloroacetate esterase (Leder stain; CAE) and also stain positively for MAC387. Megakaryocytes and scattered fibroblast-like cells are positive for factor XIIIa, and megakaryocytes also express factor VIII–related antigen.552 Fine cytologic details of cells of erythroid and myeloid lineage can be viewed ultrastructurally.548
Recently, mutation of the Janus kinase-2 gene (JAK2 V617F) was identified within lesions of cutaneous myelofibrosis, demonstrating that such lesions represent metastases and not simply “reactive” extramedullary hematopoiesis.556 In another study, Kawakami and coworkers found that transforming growth factor-beta (TGF-β) was overexpressed in the hematopoietic cells and dermal fibroblasts of cutaneous extramedullary hematopoiesis in a patient with myelofibrosis who eventually succumbed to bone marrow failure. A high titer of TGF-β was also found in the patient’s plasma. It was postulated that this peptide might have played a role in the promotion of extramedullary hematopoiesis and the myelofibrosis that occurred in this patient.557
Differential Diagnosis
In neonates, the microscopic features of cutaneous extramedullary hematopoiesis provide a clue to an underlying systemic condition, particularly a congenital infection or hematologic abnormality. In adults, extramedullary hematopoiesis can be difficult to differentiate from chronic myelogenous leukemia. This is a particular problem in myelofibrosis-associated extramedullary hematopoiesis, which has a number of features in common with chronic myelogenous leukemia. However, myelofibrosis is not associated with the Philadelphia chromosome.552 Furthermore, erythroid precursors are of help in the recognition of extramedullary hematopoiesis, and the finding of megakaryocytes can also aid in the distinction between this disorder and chronic myelogenous leukemia.552,555
References
1. Norton, AJ. Classification of cutaneous lymphoma: a critical appraisal of recent proposals. Am J Dermatopathol. 1999;21(3):279–287.
2. Sander, CA, Flaig, MJ, Kaudewitz, P, et al. The revised European-American Classification of Lymphoid Neoplasms (REAL): a preferred approach for the classification of cutaneous lymphomas. Am J Dermatopathol. 1999;21(3):274–278.
3. Swerdlow, SH, Campo, E, Harris, NL, et al. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008.
4. Burg, G, Kempf, W, Cozzio, A, et al. WHO/EORTC classification of cutaneous lymphomas 2005: histological and molecular aspects. J Cutan Pathol. 2005;32(10):647–674.
5. Willemze, R, Meijer, CJ. EORTC classification for primary cutaneous lymphomas: the best guide to good clinical management. European Organization for Research and Treatment of Cancer. Am J Dermatopathol. 1999;21(3):265–273.
6. Jubert, C, Cosnes, A, Wechsler, J, et al. Anetoderma may reveal cutaneous plasmacytoma and benign cutaneous lymphoid hyperplasia. Arch Dermatol. 1995;131(3):365–366.
7. Bafverstedt, B. Lymphadenosis benigna cutis. Acta Derm Venereol. 1968;48(1):1–6.
8. Albrecht, S, Hofstadter, S, Artsob, H, et al. Lymphadenosis benigna cutis resulting from Borrelia infection (Borrelia lymphocytoma). J Am Acad Dermatol. 1991;24(4):621–625.
9. Hovmark, A, Asbrink, E, Olsson, I. The spirochetal etiology of lymphadenosis benigna cutis solitaria. Acta Derm Venereol. 1986;66(6):479–484.
10. Roo, E, Villegas, C, Lopez-Bran, E, et al. Postzoster cutaneous pseudolymphoma. Arch Dermatol. 1994;130(5):661–663.
11. Bachelez, H, Hadida, F, Parizot, C, et al. Oligoclonal expansion of HIV-specific cytotoxic CD8 T lymphocytes in the skin of HIV-1-infected patients with cutaneous pseudolymphoma. J Clin Invest. 1998;101(11):2506–2516.
12. Lanzafame, S, Micali, G. [Cutaneous lymphoid hyperplasia (pseudolymphoma) secondary to vaccination]. Pathologica. 1993;85(1099):555–561.
13. Braddock, SW, Harrington, D, Vose, J. Generalized nodular cutaneous pseudolymphoma associated with phenytoin therapy. Use of T-cell receptor gene rearrangement in diagnosis and clinical review of cutaneous reactions to phenytoin. J Am Acad Dermatol. 1992;27(2 Pt 2):337–340.
14. Crowson, AN, Magro, CM. Antidepressant therapy. A possible cause of atypical cutaneous lymphoid hyperplasia. Arch Dermatol. 1995;131(8):925–929.
15. Magro, CM, Crowson, AN. Drugs with antihistaminic properties as a cause of atypical cutaneous lymphoid hyperplasia. J Am Acad Dermatol. 1995;32(3):419–428.
16. Del Boz Gonzalez, J, Sanz, A, Martin, T, et al. Cutaneous pseudolymphoma associated with molluscum contagiosum: a case report. Int J Dermatol. 2008;47(5):502–504.
17. Flaig, MJ, Rupec, RA. Cutaneous pseudolymphoma in association with Leishmania donovani. Br J Dermatol. 2007;157(5):1042–1043.
18. Mitani, N, Nagatani, T, Ikezawa, Z, et al. A case of cutaneous T cell pseudolymphoma in a patient with Helicobacter pylori infection. Dermatology. 2006;213(2):156–158.
19. Guis, S, Schiano de Colella, JM, Bonnet, N, et al. Cutaneous pseudolymphoma associated with a TNF-alpha inhibitor treatment: etanercept. Eur J Dermatol. 2008;18(4):474–476.
20. Stavrianeas, NG, Katoulis, AC, Bozi, E, et al. Cutaneous pseudolymphoma following administration of lornoxicam. Acta Derm Venereol. 2007;87(5):453–455.
21. Meyer, S, Vogt, T, Obermann, EC, et al. Cutaneous pseudolymphoma induced by Cimicifuga racemosa. Dermatology. 2007;214(1):94–96.
22. D’Incan, M, Souteyrand, P, Bignon, YJ, et al. Hydantoin-induced cutaneous pseudolymphoma with clinical, pathologic, and immunologic aspects of Sezary syndrome. Arch Dermatol. 1992;128(10):1371–1374.
23. Patterson, JW. Lymphomas. Dermatol Clin. 1992;10(1):235–251.
24. Caro, WA, Helwig, HB. Cutaneous lymphoid hyperplasia [lymphocytoma]. Cancer. 1969;24(3):487–502.
25. Duncan, SC, Evans, HL, Winkelmann, RK. Large cell lymphocytoma. Arch Dermatol. 1980;116(10):1142–1146.
26. LeBoit, PE, McNutt, NS, Reed, JA, et al. Primary cutaneous immunocytoma. A B-cell lymphoma that can easily be mistaken for cutaneous lymphoid hyperplasia. Am J Surg Pathol. 1994;18(10):969–978.
27. Medeiros, LJ, Picker, LJ, Abel, EA, et al. Cutaneous lymphoid hyperplasia. Immunologic characteristics and assessment of criteria recently proposed as diagnostic of malignant lymphoma [lymphocytoma]. J Am Acad Dermatol. 1989;21(5 Pt 1):929–942.
28. Fan, K, Kelly, R, Kendrick, V. Nonclonal lymphocytic proliferation in cutaneous lymphoid hyperplasia: a flow-cytometric and morphological analysis. Dermatology. 1992;185(2):113–119.
29. Burg, G, Braun-Falco, O, Hoffman-Fezer, O, et al. Differentiation between pseudolymphomas and malignant B cell lymphomas of the skin. In: Goos M, Christopher E, eds. Lymphoproliferative Diseases of the Skin. Berlin: Springer-Verlag; 1982:101.
30. Slater, DN. Recent developments in cutaneous lymphoproliferative disorders [lymphocytoma]. J Pathol. 1987;153(1):5–19.
31. Rijlaarsdam, JU, Meijer, CJ, Willemze, R. Differentiation between lymphadenosis benigna cutis and primary cutaneous follicular center cell lymphomas. A comparative clinicopathologic study of 57 patients. Cancer. 1990;65(10):2301–2306.
32. Shelley, WB, Wood, MG, Wilson, JF, et al. Premalignant lymphoid hyperplasia preceding and coexisting with malignant lymphoma in the skin [lymphocytoma]. Arch Dermatol. 1981;117(8):500–503.
33. Schmid, U, Eckert, F, Griesser, H, et al. Cutaneous follicular lymphoid hyperplasia with monotypic plasma cells. A clinicopathologic study of 18 patients. Am J Surg Pathol. 1995;19(1):12–20.
34. Spina, D, Miracco, C, Santopietro, R, et al. Distinction between diffuse cutaneous malignant follicular center cell lymphoma and lymphoid hyperplasia by computerized nuclear image analysis. Am J Dermatopathol. 1993;15(5):415–422.
35. Bergman, R. Pseudolymphoma and cutaneous lymphoma: facts and controversies. Clin Dermatol. 2010;28(5):568–574.
36. Toyota, N, Matsuo, S, Iizuka, H. Immunohistochemical differential diagnosis between lymphocytoma cutis and malignant lymphoma in paraffin-embedded sections. J Dermatol. 1991;18(10):586–591.
37. Miracco, C, Spina, D, Santopietro, R, et al. Apoptotic index: discriminant feature for the differentiation of cutaneous diffuse malignant follicular center cell lymphomas from lymphoid hyperplasia. J Invest Dermatol. 1993;100(5):699–704.
38. de Leval, L, Harris, NL, Longtine, J, et al. Cutaneous B-cell lymphomas of follicular and marginal zone types: use of Bcl-6, CD10, Bcl-2, and CD21 in differential diagnosis and classification. Am J Surg Pathol. 2001;25(6):732–741.
39. Baldassano, MF, Bailey, EM, Ferry, JA, et al. Cutaneous lymphoid hyperplasia and cutaneous marginal zone lymphoma: comparison of morphologic and immunophenotypic features. Am J Surg Pathol. 1999;23(1):88–96.
40. Kuo, TT, Lo, SK, Chan, HL. Immunohistochemical analysis of dermal mononuclear cell infiltrates in cutaneous lupus erythematosus, polymorphous light eruption, lymphocytic infiltration of Jessner, and cutaneous lymphoid hyperplasia: a comparative differential study. J Cutan Pathol. 1994;21(5):430–436.
41. de la Fouchardiere, A, Balme, B, Chouvet, B, et al. Primary cutaneous marginal zone B-cell lymphoma: a report of 9 cases. J Am Acad Dermatol. 1999;41(2 Pt 1):181–188.
42. Cerroni, L, Zochling, N, Putz, B, Kerl, H. Infection by Borrelia burgdorferi and cutaneous B-cell lymphoma. J Cutan Pathol. 1997;24(8):457–461.
43. Goodlad, JR, Davidson, MM, Hollowood, K, et al. Borrelia burgdorferi-associated cutaneous marginal zone lymphoma: a clinicopathological study of two cases illustrating the temporal progression of B. burgdorferi-associated B-cell proliferation in the skin. Histopathology. 2000;37(6):501–508.
44. Roggero, E, Zucca, E, Mainetti, C, et al. Eradication of Borrelia burgdorferi infection in primary marginal zone B-cell lymphoma of the skin. Hum Pathol. 2000;31(2):263–268.
45. Bailey, EM, Ferry, JA, Harris, NL, et al. Marginal zone lymphoma (low-grade B-cell lymphoma of mucosa-associated lymphoid tissue type) of skin and subcutaneous tissue: a study of 15 patients. Am J Surg Pathol. 1996;20(8):1011–1023.
46. Cerroni, L, Signoretti, S, Hofler, G, et al. Primary cutaneous marginal zone B-cell lymphoma: a recently described entity of low-grade malignant cutaneous B-cell lymphoma. Am J Surg Pathol. 1997;21(11):1307–1315.
47. Tomaszewski, MM, Abbondanzo, SL, Lupton, GP. Extranodal marginal zone B-cell lymphoma of the skin: a morphologic and immunophenotypic study of 11 cases. Am J Dermatopathol. 2000;22(3):205–211.
48. Yildirim, FE, Karaduman, A, Hurmuz, P, Ozyar, E, et al. Symmetrical primary cutaneous marginal zone lymphoma associated with rheumatoid arthritis. J Cutan Pathol. 2010;37(5):600–604.
49. Gerami, P, Wickless, SC, Querfeld, C, et al. Cutaneous involvement with marginal zone lymphoma. J Am Acad Dermatol. 2010;63(1):142–145.
50. Valdez, R, Finn, WG, Ross, CW. Waldenstrom macroglobulinemia caused by extranodal marginal zone B-cell lymphoma: a report of six cases. Am J Clin Pathol. 2001;116(5):683–690.
51. Thieblemont, C, Berger, F, Dumontet, C, et al. Mucosa-associated lymphoid tissue lymphoma is a disseminated disease in one third of 158 patients analyzed. Blood. 2000;95(3):802–806.
52. Zhang, HY, Liu, AL, Zhou, LS, et al. Primary cutaneous marginal zone B-cell lymphoma with amyloid deposition: report of two cases with review of literature. Chin J Cancer. 2010;29(6):634–640.
53. Isaacson, PG, Muller-Hermelink, HK, Piris, MA, et al. Extranodal marginal zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT lymphoma). In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:157–160.
54. Kutzner, H, Kerl, H, Pfaltz, MC, et al. CD123-positive plasmacytoid dendritic cells in primary cutaneous marginal zone B-cell lymphoma: diagnostic and pathogenetic implications. Am J Surg Pathol. 2009;33(9):1307–1313.
55. Ott, G, Katzenberger, T, Greiner, A, et al. The t(11;18)(q21;q21) chromosome translocation is a frequent and specific aberration in low-grade but not high-grade malignant non-Hodgkin’s lymphomas of the mucosa-associated lymphoid tissue (MALT-) type. Cancer Res. 1997;57(18):3944–3948.
56. Wotherspoon, AC, Finn, TM, Isaacson, PG. Trisomy 3 in low-grade B-cell lymphomas of mucosa-associated lymphoid tissue. Blood. 1995;85(8):2000–2004.
57. Cho-Vega, JH, Vega, F, Rassidakis, G, et al. Primary cutaneous marginal zone B-cell lymphoma. Am J Clin Pathol. 2006;125(Suppl):S38–S49.
58. Takino, H, Li, C, Hu, S, et al. Primary cutaneous marginal zone B-cell lymphoma: a molecular and clinicopathological study of cases from Asia, Germany, and the United States. Mod Pathol. 2008;21(12):1517–1526.
59. Moody, BR, Bartlett, NL, George, DW, et al. Cyclin D1 as an aid in the diagnosis of mantle cell lymphoma in skin biopsies: a case report. Am J Dermatopathol. 2001;23(5):470–476.
60. Levin, C, Mirzamani, N, Zwerner, J, et al. A comparative analysis of cutaneous marginal zone lymphoma and cutaneous chronic lymphocytic leukemia. Am J Dermatopathol. 2012;34(1):18–23.
61. Aguilera, NS, Tomaszewski, MM, Moad, JC, et al. Cutaneous follicle center lymphoma: a clinicopathologic study of 19 cases. Mod Pathol. 2001;14(9):828–835.
62. Bergman, R, Kurtin, PJ, Gibson, LE, et al. Clinicopathologic, immunophenotypic, and molecular characterization of primary cutaneous follicular B-cell lymphoma. Arch Dermatol. 2001;137(4):432–439.
63. Cerroni, L, Kerl, H. Primary cutaneous follicle center cell lymphoma. Leuk Lymphoma. 2001;42(5):891–900.
64. Goodlad, JR, Krajewski, AS, Batstone, PJ, et al. Primary cutaneous follicular lymphoma: a clinicopathologic and molecular study of 16 cases in support of a distinct entity. Am J Surg Pathol. 2002;26(6):733–741.
65. Mann, RB, Berard, CW. Criteria for the cytologic subclassification of follicular lymphomas: a proposed alternative method. Hematol Oncol. 1983;1(2):187–192.
66. Barcus, ME, Karageorge, LS, Veloso, YL, et al. CD10 expression in follicular lymphoma versus reactive follicular hyperplasia: evaluation in paraffin-embedded tissue. Appl Immunohistochem Mol Morphol. 2000;8(4):263–266.
67. Chimenti, S, Cerroni, L, Zenahlik, P, et al. The role of MT2 and anti-bcl-2 protein antibodies in the differentiation of benign from malignant cutaneous infiltrates of B-lymphocytes with germinal center formation. J Cutan Pathol. 1996;23(4):319–322.
68. Child, FJ, Russell-Jones, R, Woolford, AJ, et al. Absence of the t(14;18) chromosomal translocation in primary cutaneous B-cell lymphoma. Br J Dermatol. 2001;144(4):735–744.
69. Kerl, H, Cerroni, L. Controversies in cutaneous lymphomas. Semin Cutan Med Surg. 2000;19(2):157–160.
70. Lawnicki, LC, Weisenburger, DD, Aoun, P, et al. The t(14;18) and bcl-2 expression are present in a subset of primary cutaneous follicular lymphoma: association with lower grade. Am J Clin Pathol. 2002;118(5):765–772.
71. Duray, PH, Steere, AC. Clinical pathologic correlations of Lyme disease by stage. Ann N Y Acad Sci. 1988;539:65–79.
72. Wick, MR, Ritter, JH, Humphrey, PA, et al. Immunopathology of nonneoplastic skin disease: a brief review. Am J Clin Pathol. 1996;105(4):417–429.
73. Viguier, M, Rivet, J, Agbalika, F, et al. B-cell lymphomas involving the skin associated with hepatitis C virus infection. Int J Dermatol. 2002;41(9):577–582.
74. Gatter, KC, Warnke, RA. Diffuse large B-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:171–174.
75. Armitage, JO, Weisenburger, DD. New approach to classifying non-Hodgkin’s lymphomas: clinical features of the major histologic subtypes. Non-Hodgkin’s Lymphoma Classification Project. J Clin Oncol. 1998;16(8):2780–2795.
76. Vermeer, MH, Geelen, FA, van Haselen, CW, et al. Primary cutaneous large B-cell lymphomas of the legs. A distinct type of cutaneous B-cell lymphoma with an intermediate prognosis. Dutch Cutaneous Lymphoma Working Group. Arch Dermatol. 1996;132(11):1304–1308.
77. Busschots, AM, Geerts, ML, Mecucci, C, et al. A translocation (8;14) in a cutaneous large B-cell lymphoma. Am J Clin Pathol. 1993;99(5):615–621.
78. Take, H, Kubota, K, Fukuda, T, et al. An indolent type of Epstein-Barr virus-associated T-cell-rich B-cell lymphoma of the skin: report of a case. Am J Hematol. 1996;52(3):221–223.
79. Hofbauer, GF, Kessler, B, Kempf, W, et al. Multilesional primary cutaneous diffuse large B-cell lymphoma responsive to antibiotic treatment. Dermatology. 2001;203(2):168–170.
80. Geelen, FA, Vermeer, MH, Meijer, CJ, et al. bcl-2 protein expression in primary cutaneous large B-cell lymphoma is site-related. J Clin Oncol. 1998;16(6):2080–2085.
81. Cerroni, L, El-Shabrawi-Caelen, L, Fink-Puches, R, et al. Cutaneous spindle-cell B-cell lymphoma: a morphologic variant of cutaneous large B-cell lymphoma. Am J Dermatopathol. 2000;22(4):299–304.
82. Garbea, A, Dippel, E, Hildenbrand, R, et al. Cutaneous large B-cell lymphoma of the leg masquerading as a chronic venous ulcer. Br J Dermatol. 2002;146(1):144–147.
83. Wollina, U, Mentzel, T, Graefe, T. Large B-cell lymphoma of the leg—complete remission with perilesional interferon alpha. Dermatology. 2001;203(2):165–167.
84. Aboulafia, DM. Primary cutaneous large B-cell lymphoma of the legs: a distinct clinical pathologic entity treated with CD20 monoclonal antibody (rituximab). Am J Clin Oncol. 2001;24(3):237–240.
85. Dommann, SN, Dommann-Scherrer, CC, Zimmerman, D, et al. Primary cutaneous T-cell-rich B-cell lymphoma. A case report with a 13-year follow-up. Am J Dermatopathol. 1995;17(6):618–624.
86. Li, S, Griffin, CA, Mann, RB, et al. Primary cutaneous T-cell-rich B-cell lymphoma: clinically distinct from its nodal counterpart? Mod Pathol. 2001;14(1):10–13.
87. Sander, CA, Kaudewitz, P, Kutzner, H, et al. T-cell-rich B-cell lymphoma presenting in skin. A clinicopathologic analysis of six cases. J Cutan Pathol. 1996;23(2):101–108.
88. Zinzani, PL, Quaglino, P, Pimpinelli, N, et al. Prognostic factors in primary cutaneous B-cell lymphoma: the Italian Study Group for Cutaneous Lymphomas. J Clin Oncol. 2006;24(9):1376–1382.
89. Grange, F, Bekkenk, MW, Wechsler, J, et al. Prognostic factors in primary cutaneous large B-cell lymphomas: a European multicenter study. J Clin Oncol. 2001;19(16):3602–3610.
90. Meijer, CJLM, Vergier, B, Duncan, LM, et al. Primary cutaneous diffuse large B-cell lymphoma, leg type. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008:242.
91. Falini, B, Fizzotti, M, Pucciarini, A, et al. A monoclonal antibody (MUM1p) detects expression of the MUM1/IRF4 protein in a subset of germinal center B cells, plasma cells, and activated T cells. Blood. 2000;95(6):2084–2092.
92. Miller, TP, Grogan, TM, Dahlberg, S, et al. Prognostic significance of the Ki-67-associated proliferative antigen in aggressive non-Hodgkin’s lymphomas: a prospective Southwest Oncology Group trial. Blood. 83(6), 1994. [1460-1406].
93. Piris, M, Brown, DC, Gatter, KC, et al. CD30 expression in non-Hodgkin’s lymphoma. Histopathology. 1990;17(3):211–218.
94. Wilson, WH, Teruya-Feldstein, J, Fest, T, et al. Relationship of p53, bcl-2, and tumor proliferation to clinical drug resistance in non-Hodgkin’s lymphomas. Blood. 1997;89(2):601–609.
95. Gogstetter, D, Brown, M, Seab, J, et al. Angiocentric primary cutaneous T-cell-rich B-cell lymphoma: a case report and review of the literature. J Cutan Pathol. 2000;27(10):516–525.
96. Grogan, TM, Van Camp, B, Kyle, RA, et al. Plasma Cell Neoplasms [myeloma]. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:142–156.
97. Lipford, EH, Jr., Margolick, JB, Longo, DL, et al. Angiocentric immunoproliferative lesions: a clinicopathologic spectrum of post-thymic T-cell proliferations. Blood. 1988;72(5):1674–1681.
98. Bhawan, J, Wolff, SM, Ucci, AA, et al. Malignant lymphoma and malignant angioendotheliomatosis: one disease. Cancer. 1985;55(3):570–576.
99. Dominguez, FE, Rosen, LB, Kramer, HC. Malignant angioendotheliomatosis proliferans. Report of an autopsied case studied with immunoperoxidase. Am J Dermatopathol. 1986;8(5):419–425.
100. Wick, MR, Rocamora, A. Reactive and malignant “angioendotheliomatosis”: a discriminant clinicopathological study. J Cutan Pathol. 1988;15(5):260–271.
101. Wrotnowski, U, Mills, SE, Cooper, PH. Malignant angioendotheliomatosis. An angiotropic lymphoma? Am J Clin Pathol. 1985;83(2):244–248.
102. Chang, A, Zic, JA, Boyd, AS. Intravascular large cell lymphoma: a patient with asymptomatic purpuric patches and a chronic clinical course. J Am Acad Dermatol. 1998;39(2 Pt 2):318–321.
103. Khalidi, HS, Brynes, RK, Browne, P, et al. Intravascular large B-cell lymphoma: the CD5 antigen is expressed by a subset of cases. Mod Pathol. 1998;11(10):983–988.
104. Ferreri, AJ, Dognini, GP, Campo, E, et al. Variations in clinical presentation, frequency of hemophagocytosis and clinical behavior of intravascular lymphoma diagnosed in different geographical regions. Haematologica. 2007;92(4):486–492.
105. Ponzoni, M, Ferreri, AJ. Intravascular lymphoma: a neoplasm of ‘homeless’ lymphocytes? Hematol Oncol. 2006;24(3):105–112.
106. Ferreri, AJ, Campo, E, Seymour, JF, et al. Intravascular lymphoma: clinical presentation, natural history, management and prognostic factors in a series of 38 cases, with special emphasis on the ‘cutaneous variant’. Br J Haematol. 2004;127(2):173–183.
107. Gatter, KC, Warnke, RA. Intravascular large B-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:177–178.
108. DiGiuseppe, JA, Nelson, WG, Seifter, EJ, et al. Intravascular lymphomatosis: a clinicopathologic study of 10 cases and assessment of response to chemotherapy. J Clin Oncol. 1994;12(12):2573–2579.
109. Kiyohara, T, Kumakiri, M, Kobayashi, H, et al. A case of intravascular large B-cell lymphoma mimicking erythema nodosum: the importance of multiple skin biopsies. J Cutan Pathol. 2000;27(8):413–418.
110. Au, WY, Shek, WH, Nicholls, J, et al. T-cell intravascular lymphomatosis (angiotropic large cell lymphoma): association with Epstein-Barr viral infection. Histopathology. 1997;31(6):563–567.
111. Lakhani, SR, Hulman, G, Hall, JM, et al. Intravascular malignant lymphomatosis (angiotropic large-cell lymphoma). A case report with evidence for T-cell lineage with polymerase chain reaction analysis. Histopathology. 1994;25(3):283–286.
112. Perniciaro, C, Winkelmann, RK, Daoud, MS, et al. Malignant angioendotheliomatosis is an angiotropic intravascular lymphoma. Immunohistochemical, ultrastructural, and molecular genetics studies. Am J Dermatopathol. 1995;17(3):242–248.
113. Kanda, M, Suzumiya, J, Ohshima, K, et al. Intravascular large cell lymphoma: clinicopathological, immuno-histochemical and molecular genetic studies. Leuk Lymphoma. 1999;34(5-6):569–580.
114. Setoyama, M, Mizoguchi, S, Orikawa, T, et al. A case of intravascular malignant lymphomatosis (angiotropic large-cell lymphoma) presenting memory T cell phenotype and its expression of adhesion molecules. J Dermatol. 1992;19(5):263–269.
115. Ponzoni, M, Arrigoni, G, Gould, VE, et al. Lack of CD 29 (beta1 integrin) and CD 54 (ICAM-1) adhesion molecules in intravascular lymphomatosis. Hum Pathol. 2000;31(2):220–226.
116. Beaty, MW, Toro, J, Sorbara, L, et al. Cutaneous lymphomatoid granulomatosis: correlation of clinical and biologic features. Am J Surg Pathol. 2001;25(9):1111–1120.
117. McNiff, JM, Cooper, D, Howe, G, et al. Lymphomatoid granulomatosis of the skin and lung. An angiocentric T-cell-rich B-cell lymphoproliferative disorder. Arch Dermatol. 1996;132(12):1464–1470.
118. Haque, AK, Myers, JL, Hudnall, SD, et al. Pulmonary lymphomatoid granulomatosis in acquired immunodeficiency syndrome: lesions with Epstein-Barr virus infection. Mod Pathol. 1998;11(4):347–356.
119. Sordillo, PP, Epremian, B, Koziner, B, et al. Lymphomatoid granulomatosis: an analysis of clinical and immunologic characteristics. Cancer. 1982;49(10):2070–2206.
120. Katzenstein, AL, Carrington, CB, Liebow, AA. Lymphomatoid granulomatosis: a clinicopathologic study of 152 cases. Cancer. 1979;43(1):360–373.
121. Jaffe, ES, Wilson, WH. Lymphomatoid granulomatosis. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:185–187.
122. Minars, N, Kay, S, Escobar, MR. Lymphomatoid granulomatosis of the skin. A new clinocopathologic entity. Arch Dermatol. 1975;111(4):493–496.
123. Wood, ML, Harrington, CI, Slater, DN, et al. Cutaneous lymphomatoid granulomatosis: a rare cause of recurrent skin ulceration. Br J Dermatol. 1984;110(5):619–625.
124. Tas, S, Simonart, T, Dargent, J, et al. [Primary and isolated cutaneous lymphomatoid granulomatosis following heart-lung transplantation]. Ann Dermatol Venereol. 2000;127(5):488–491.
125. James, WD, Odom, RB, Katzenstein, AL. Cutaneous manifestations of lymphomatoid granulomatosis. Report of 44 cases and a review of the literature. Arch Dermatol. 1981;117(4):196–202.
126. Brodell, RT, Miller, CW, Eisen, AZ. Cutaneous lesions of lymphomatoid granulomatosis. Arch Dermatol. 1986;122(3):303–306.
127. Akagi, M, Taniguchi, S, Ozaki, M, et al. Necrobiosis-lipoidica–like skin manifestation in lymphomatoid granulomatosis (Liebow). Dermatologica. 1987;174(2):84–92.
128. Carlson, KC, Gibson, LE. Cutaneous signs of lymphomatoid granulomatosis. Arch Dermatol. 1991;127(11):1693–1698.
129. Imaoka, K, Furumura, M, Maruyama, R, et al. Erythrodermic lymphomatoid granulomatosis: a case report. Case Rep Dermatol. 2011;3(3):244–250.
130. Kessler, S, Lund, HZ, Leonard, DD. Cutaneous lesions of lymphomatoid granulomatosis. Comparison with lymphomatoid papulosis. Am J Dermatopathol. 1981;3(2):115–127.
131. Murphy, GF, Harrist, TJ, Sato, S, et al. Microvascular injury in lymphomatoid granulomatosis involving the skin. An ultrastructural study. Arch Dermatol. 1981;117(12):804–808.
132. Magro, CM, Schaefer, JT, Crowson, AN, et al. Pigmented purpuric dermatosis: classification by phenotypic and molecular profiles. Am J Clin Pathol. 2007;128(2):218–229.
133. Kwon, EJ, Katz, KA, Draft, KS, et al. Posttransplantation lymphoproliferative disease with features of lymphomatoid granulomatosis in a lung transplant patient. J Am Acad Dermatol. 2006;54(4):657–663.
134. Brunning, RD, Borowitz, M, Matutes, E, et al. Precursor B lymphoblastic leukaemia/lymphoblastic lymphoma (precursor B-cell acute lymphoblastic leukaemia. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:111–114.
135. Kahwash, SB, Qualman, SJ. Cutaneous lymphoblastic lymphoma in children: report of six cases with precursor B-cell lineage. Pediatr Dev Pathol. 2002;5(1):45–53.
136. Lin, P, Jones, D, Dorfman, DM, et al. Precursor B-cell lymphoblastic lymphoma: a predominantly extranodal tumor with low propensity for leukemic involvement. Am J Surg Pathol. 2000;24(11):1480–1490.
137. Maitra, A, McKenna, RW, Weinberg, AG, et al. Precursor B-cell lymphoblastic lymphoma. A study of nine cases lacking blood and bone marrow involvement and review of the literature. Am J Clin Pathol. 2001;115(6):868–875.
138. Du, MQ, Diss, TC, Xu, CF, et al. Ongoing immunoglobulin gene mutations in mantle cell lymphomas. Br J Haematol. 1997;96(1):124–131.
139. Swerdlow, SH, Berger, F, Isaacson, I, et al. Mantle cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:168–170.
140. Marti, RM, Campo, E, Bosch, F, et al. Cutaneous lymphocyte-associated antigen (CLA) expression in a lymphoblastoid mantle cell lymphoma presenting with skin lesions. Comparison with other clinicopathologic presentations of mantle cell lymphoma. J Cutan Pathol. 2001;28(5):256–264.
141. Sarikaya, I, Patel, M, Holder, L. Cutaneous mantle cell lymphoma detected with Ga-67 Citrate. Clin Nucl Med. 2000;25(10):849–851.
142. Diebold, J, Jaffe, ES, Raphael, M, Warnke, R.A, et al. Burkitt lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:181–184.
143. Bachmeyer, C, Bazarbachi, A, Rio, B, et al. Specific cutaneous involvement indicating relapse of Burkitt’s lymphoma. Am J Hematol. 1997;54(2):176.
144. Rogge, T. [Burkitt’s lymphoma with skin infiltrates]. Hautarzt. 1975;26(7):379–382.
145. Pelicci, PG, Knowles, DM, II., Magrath, I, et al. Chromosomal breakpoints and structural alterations of the c-myc locus differ in endemic and sporadic forms of Burkitt lymphoma. Proc Natl Acad Sci U S A. 1986;83(9):2984–2988.
146. Guitart, J, Kennedy, J, Ronan, S, et al. Histologic criteria for the diagnosis of mycosis fungoides: proposal for a grading system to standardize pathology reporting. J Cutan Pathol. 2001;28(4):174–183.
147. Ralfkiaer, E, Jaffe, ES. Mycosis fungoides and Sezary syndrome. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:216–220.
148. Weinstock, MA, Horm, JW. Population-based estimate of survival and determinants of prognosis in patients with mycosis fungoides. Cancer. 1988;62(8):1658–1661.
149. Kim, YH, Hoppe, RT. Mycosis fungoides and the Sezary syndrome. Semin Oncol. 1999;26(3):276–289.
150. Lambert, WC, Everett, MA. The nosology of parapsoriasis. J Am Acad Dermatol. 1981;5(4):373–395.
151. Lazar, AP, Caro, WA, Roenigk, HH, Jr., et al. Parapsoriasis and mycosis fungoides: the Northwestern University experience, 1970 to 1985. J Am Acad Dermatol. 1989;21(5 Pt 1):919–923.
152. Simon, M, Flaig, MJ, Kind, P, et al. Large plaque parapsoriasis: clinical and genotypic correlations. J Cutan Pathol. 2000;27(2):57–60.
153. Jones, REJE. Questions to the Editorial Board and other authorities. Am J Dermatopathol. 1986;8:534–545.
154. Haeffner, AC, Smoller, BR, Zepter, K, et al. Differentiation and clonality of lesional lymphocytes in small plaque parapsoriasis. Arch Dermatol. 1995;131(3):321–324.
155. Burg, G, Dummer, R. Small plaque (digitate) parapsoriasis is an ‘abortive cutaneous T-cell lymphoma’ and is not mycosis fungoides. Arch Dermatol. 1995;131(3):336–338.
156. King-Ismael, D, Ackerman, AB. Guttate parapsoriasis/digitate dermatosis (small plaque parapsoriasis) is mycosis fungoides. Am J Dermatopathol. 1992;14(6):518–530. [discussion 531-535].
157. Hodak, E, Phenig, E, Amichai, B, et al. Unilesional mycosis fungoides: a study of seven cases. Dermatology. 2000;201(4):300–306.
158. Oliver, GF, Winkelmann, RK. Unilesional mycosis fungoides: a distinct entity. J Am Acad Dermatol. 1989;20(1):63–70.
159. El-Shabrawi-Caelen, L, Cerroni, L, Medeiros, LJ, et al. Hypopigmented mycosis fungoides: frequent expression of a CD8+ T-cell phenotype. Am J Surg Pathol. 2002;26(4):450–457.
160. Whitmore, SE, Simmons-O’Brien, E, Rotter, FS. Hypopigmented mycosis fungoides. Arch Dermatol. 1994;130(4):476–480.
161. Kartsonis, J, Brettschneider, F, Weissmann, A, et al. Mycosis fungoides bullosa. Am J Dermatopathol. 1990;12(1):76–80.
162. Wolf, P, Cerroni, L, Kerl, H. Mycosis fungoides mimicking perioral dermatitis. Clin Exp Dermatol. 1992;17(2):132–134.
163. Patterson, JW, Ali, M, Murray, JC, et al. Bullous pemphigoid. Occurrence in a patient with mycosis fungoides receiving PUVA and topical nitrogen mustard therapy. Int J Dermatol. 1985;24(3):173–176.
164. Bonta, MD, Tannous, ZS, Demierre, MF, et al. Rapidly progressing mycosis fungoides presenting as follicular mucinosis. J Am Acad Dermatol. 2000;43(4):635–640.
165. Gibson, LE, Muller, SA, Leiferman, KM, et al. Follicular mucinosis: clinical and histopathologic study. J Am Acad Dermatol. 1989;20(3):441–446.
166. Cerroni, L, Fink-Puches, R, Back, B, et al. Follicular mucinosis: a critical reappraisal of clinicopathologic features and association with mycosis fungoides and Sezary syndrome. Arch Dermatol. 2002;138(2):182–189.
167. Rongioletti, F, De Lucchi, S, Meyes, D, et al. Follicular mucinosis: a clinicopathologic, histochemical, immunohistochemical and molecular study comparing the primary benign form and the mycosis fungoides-associated follicular mucinosis. J Cutan Pathol. 2010;37(1):15–19.
168. Klemke, CD, Dippel, E, Assaf, C, et al. Follicular mycosis fungoides. Br J Dermatol. 1999;141(1):137–140.
169. Gilliam, AC, Lessin, SR, Wilson, DM, et al. Folliculotropic mycosis fungoides with large-cell transformation presenting as dissecting cellulitis of the scalp. J Cutan Pathol. 1997;24(3):169–175.
170. Lehman, JS, Cook-Norris, RH, Weed, BR, et al. Folliculotropic mycosis fungoides: single-center study and systematic review. Arch Dermatol. 2010;146(6):607–613.
171. Zelger, B, Sepp, N, Weyrer, K, et al. Syringotropic cutaneous T-cell lymphoma: a variant of mycosis fungoides? Br J Dermatol. 1994;130(6):765–769.
172. Tannous, Z, Baldassano, MF, Li, VW, et al. Syringolymphoid hyperplasia and follicular mucinosis in a patient with cutaneous T-cell lymphoma. J Am Acad Dermatol. 1999;41(2 Pt 2):303–308.
173. Tomaszewski, MM, Lupton, GP, Krishnan, J, et al. Syringolymphoid hyperplasia with alopecia. A case report. J Cutan Pathol. 1994;21(6):520–526.
174. Haller, A, Elzubi, E, Petzelbauer, P. Localized syringolymphoid hyperplasia with alopecia and anhidrosis. J Am Acad Dermatol. 2001;45(1):127–130.
175. Veelken, H, Wood, GS, Sklar, J. Molecular staging of cutaneous T-cell lymphoma: evidence for systemic involvement in early disease. J Invest Dermatol. 1995;104(6):889–894.
176. Sausville, EA, Eddy, JL, Makuch, RW, et al. Histopathologic staging at initial diagnosis of mycosis fungoides and the Sezary syndrome. Definition of three distinctive prognostic groups. Ann Intern Med. 1988;109(5):372–382.
177. Cerroni, L, Rieger, E, Hodl, S, et al. Clinicopathologic and immunologic features associated with transformation of mycosis fungoides to large-cell lymphoma. Am J Surg Pathol. 1992;16(6):543–552.
178. Tan, RS, Butterworth, CM, McLaughlin, H, et al. Mycosis fungoides—a disease of antigen persistence. Br J Dermatol. 1974;91(6):607–616.
179. Thiers, BH. Controversies in mycosis fungoides. J Am Acad Dermatol. 1982;7(1):1–16.
180. Shapiro, PE, Pinto, FJ. The histologic spectrum of mycosis fungoides/Sezary syndrome (cutaneous T-cell lymphoma). A review of 222 biopsies, including newly described patterns and the earliest pathologic changes. Am J Surg Pathol. 1994;18(7):645–667.
181. Pancake, BA, Zucker-Franklin, D, Coutavas, EE. The cutaneous T cell lymphoma, mycosis fungoides, is a human T cell lymphotropic virus-associated disease. A study of 50 patients. J Clin Invest. 1995;95(2):547–554.
182. Boni, R, Davis-Daneshfar, A, Burg, G, et al. No detection of HTLV-I proviral DNA in lesional skin biopsies from Swiss and German patients with cutaneous T-cell lymphoma. Br J Dermatol. 1996;134(2):282–284.
183. Altman, J. Parapsoriasis: a histopathologic review and classification. Semin Dermatol. 1984;3:14–21.
184. McMillan, EM, Wasik, R, Martin, D, et al. Immuno-electron microscopy of “T” cells in large plaque parapsoriasis. J Cutan Pathol. 1981;8(5):385–392.
185. Sanchez, JL, Ackerman, AB. The patch stage of mycosis fungoides. Criteria for histologic diagnosis. Am J Dermatopathol. 1979;1(1):5–26.
186. Nickoloff, BJ. Epidermal mucinosis in mycosis fungoides. J Am Acad Dermatol. 1986;15(1):83–86.
187. Nickoloff, BJ. Light-microscopic assessment of 100 patients with patch/plaque-stage mycosis fungoides. Am J Dermatopathol. 1988;10(6):469–477.
188. Smoller, BR, Bishop, K, Glusac, E, et al. Reassessment of histologic parameters in the diagnosis of mycosis fungoides. Am J Surg Pathol. 1995;19(12):1423–1430.
189. Argenyi, ZB, Goeken, JA, Piette, WW, et al. Granulomatous mycosis fungoides. Clinicopathologic study of two cases. Am J Dermatopathol. 1992;14(3):200–210.
190. Su, LD, Kim, YH, LeBoit, PE, et al. Interstitial mycosis fungoides, a variant of mycosis fungoides resembling granuloma annulare and inflammatory morphea. J Cutan Pathol. 2002;29(3):135–141.
191. Horiuchi, Y, Tone, T, Umezawa, A, et al. Large cell mycosis fungoides at the tumor stage. Unusual T8, T4, T6 phenotypic expression. Am J Dermatopathol. 1988;10(1):54–58.
192. Scheffer, E, Meijer, CJ, Van Vloten, WA. Dermatopathic lymphadenopathy and lymph node involvement in mycosis fungoides. Cancer. 1980;45(1):137–148.
193. Colby, TV, Burke, JS, Hoppe, RT. Lymph node biopsy in mycosis fungoides. Cancer. 1981;47(2):351–359.
194. Pileri, A, Facchetti, F, Rutten, A, et al. Syringotropic mycosis fungoides: a rare variant of the disease with peculiar clinicopathologic features. Am J Surg Pathol. 2011;35(1):100–109.
195. Vonderheid, EC, Tan, E, Sobel, EL, Schwab, E, et al. Clinical implications of immunologic phenotyping in cutaneous T cell lymphoma. J Am Acad Dermatol. 1987;17(1):40–52.
196. Knapp, CF, Mathew, R, Messina, JL, et al. CD4/CD8 dual-positive mycosis fungoides: a previously unrecognized variant. Am J Dermatopathol. 2012;34(3):e37–39.
197. Sen, F, Kang, S, Cangiarella, J, Kamino, H, et al. CD20 positive mycosis fungoides: a case report. J Cutan Pathol. 2008;35(4):398–403.
198. Wood, GS, Abel, EA, Hoppe, RT, et al. Leu-8 and Leu-9 antigen phenotypes: immunologic criteria for the distinction of mycosis fungoides from cutaneous inflammation. J Am Acad Dermatol. 1986;14(6):1006–1013.
199. Lindae, ML, Abel, EA, Hoppe, RT, et al. Poikilodermatous mycosis fungoides and atrophic large-plaque parapsoriasis exhibit similar abnormalities of T-cell antigen expression. Arch Dermatol. 1988;124(3):366–372.
200. Ralfkiaer, E. Immunohistological markers for the diagnosis of cutaneous lymphomas. Semin Diagn Pathol. 1991;8(2):62–72.
201. Murphy, M, Fullen, D, Carlson, JA. Low CD7 expression in benign and malignant cutaneous lymphocytic infiltrates: experience with an antibody reactive with paraffin-embedded tissue. Am J Dermatopathol. 2002;24(1):6–16.
202. Ormsby, A, Bergfeld, WF, Tubbs, RR, et al. Evaluation of a new paraffin-reactive CD7 T-cell deletion marker and a polymerase chain reaction-based T-cell receptor gene rearrangement assay: implications for diagnosis of mycosis fungoides in community clinical practice. J Am Acad Dermatol. 2001;45(3):405–413.
203. Michie, SA, Abel, EA, Hoppe, RT, et al. Discordant expression of antigens between intraepidermal and intradermal T cells in mycosis fungoides. Am J Pathol. 1990;137(6):1447–1451.
204. Agnarsson, BA, Vonderheid, EC, Kadin, ME. Cutaneous T cell lymphoma with suppressor/cytotoxic (CD8) phenotype: identification of rapidly progressive and chronic subtypes. J Am Acad Dermatol. 1990;22(4):569–577.
205. Quarterman, MJ, Lesher, JL, Jr., Davis, LS, et al. Rapidly progressive CD8-positive cutaneous T-cell lymphoma with tongue involvement. Am J Dermatopathol. 1995;17(3):287–291.
206. Dummer, R, Kamarashev, J, Kempf, W, et al. Junctional CD8+ cutaneous lymphomas with nonaggressive clinical behavior: a CD8+ variant of mycosis fungoides? Arch Dermatol. 2002;138(2):199–203.
207. Whittam, LR, Calonje, E, Orchard, G, et al. CD8-positive juvenile onset mycosis fungoides: an immunohistochemical and genotypic analysis of six cases. Br J Dermatol. 2000;143(6):1199–1204.
208. Hoppe, RT, Medeiros, LJ, Warnke, RA, et al. CD8-positive tumor-infiltrating lymphocytes influence the long-term survival of patients with mycosis fungoides. J Am Acad Dermatol. 1995;32(3):448–453.
209. Willemze, R, Kerl, H, Sterry, W, et al. EORTC classification for primary cutaneous lymphomas: a proposal from the Cutaneous Lymphoma Study Group of the European Organization for Research and Treatment of Cancer. Blood. 1997;90(1):354–371.
210. Dommann, SN, Ziegler, T, Dommann-Schener, CC, et al. CD44v6 is a marker for systemic spread in cutaneous T-cell lymphomas. A comparative study between nodal and cutaneous lymphomas. J Cutan Pathol. 1995;22(5):407–412.
211. Vermeer, MH, Geelen, FA, Kummer, JA, et al. Expression of cytotoxic proteins by neoplastic T cells in mycosis fungoides increases with progression from plaque stage to tumor stage disease. Am J Pathol. 1999;154(4):1203–1210.
212. Barzilai, A, Goldberg, I, Shibi, R, et al. Mycosis fungoides expressing gamma/delta T-cell receptors. J Am Acad Dermatol. 1996;34(2 Pt 1):301–302.
213. McNutt, NS, Crain, WR. Quantitative electron microscopic comparison of lymphocyte nuclear contours in mycosis fungoides and in benign infiltrates in skin. Cancer. 1981;47(4):698–709.
214. Navas, IC, Ortiz-Romero, PL, Villuendas, R, et al. p16(INK4a) gene alterations are frequent in lesions of mycosis fungoides. Am J Pathol. 2000;156(5):1565–1572.
215. Scarisbrick, JJ, Woolford, AJ, Russell-Jones, R, et al. Loss of heterozygosity on 10q and microsatellite instability in advanced stages of primary cutaneous T-cell lymphoma and possible association with homozygous deletion of PTEN. Blood. 2000;95(9):2937–2942.
216. LeBoit, PE, Epstein, BA. A vase-like shape characterizes the epidermal-mononuclear cell collections seen in spongiotic dermatitis. Am J Dermatopathol. 1990;12(6):612–616.
217. Olerud, JE, Kulin, PA, Chew, DE, et al. Cutaneous T-cell lymphoma. Evaluation of pretreatment skin biopsy specimens by a panel of pathologists. Arch Dermatol. 1992;128(4):501–507.
218. Rieger, E, Smolle, J, Hoedl, S, et al. Morphometrical analysis of mycosis fungoides on paraffin-embedded sections. J Cutan Pathol. 1989;16(1):7–13.
219. De Silva, BD, McLaren, K, Kavanagh, GM. Photosensitive mycosis fungoides or actinic reticuloid? Br J Dermatol. 2000;142(6):1221–1227.
220. Rijlaarsdam, U, Scheffer, E, Meijer, CJ, et al. Mycosis fungoides-like lesions associated with phenytoin and carbamazepine therapy. J Am Acad Dermatol. 1991;24(2 Pt 1):216–220.
221. Wolf, R, Kahane, E, Sandbank, M. Mycosis fungoides-like lesions associated with phenytoin therapy. Arch Dermatol. 1985;121(9):1181–1182.
222. Magro, CM, Crowson, AN, Kovatich, AJ, et al. Drug-induced reversible lymphoid dyscrasia: a clonal lymphomatoid dermatitis of memory and activated T cells. Hum Pathol. 2003;34(2):119–129.
223. Plaza, JA, Morrison, C, Magro, CM. Assessment of TCR-beta clonality in a diverse group of cutaneous T-cell infiltrates. J Cutan Pathol. 2008;35(4):358–365.
224. Mandojana, RM, Helwig, EB. Localized epidermotropic reticulosis (Woringer-Kolopp disease). J Am Acad Dermatol. 1983;8(6):813–829.
225. Haghighi, B, Smoller, BR, LeBoit, PE, et al. Pagetoid reticulosis (Woringer-Kolopp disease): an immunophenotypic, molecular, and clinicopathologic study. Mod Pathol. 2000;13(5):502–510.
226. Yagi, H, Hagiwara, T, Shirahama, S, et al. Disseminated pagetoid reticulosis: need for long-term follow-up. J Am Acad Dermatol. 1994;30(2 Pt 2):345–349.
227. McNiff, JM, Schechner, JS, Crotty, PL, et al. Mycosis fungoides palmaris et plantaris or acral pagetoid reticulosis? Am J Dermatopathol. 1998;20(3):271–275.
228. Berti, E, Cerri, A, Cavicchini, S, et al. Primary cutaneous gamma/delta T-cell lymphoma presenting as disseminated pagetoid reticulosis. J Invest Dermatol. 1991;96(5):718–723.
229. Cotten, H, Janin, A, Gross, S, et al. [Strictly epidermotropic “Ketron Goodman”-type lymphoma. Immunohistochemical and ultrastructural analysis of a case]. Ann Pathol. 1991;11(2):117–121.
230. Luther, H, Bacharach-Buhles, M, Schultz-Ehrenburg, U, et al. [Pagetoid reticulosis of the Ketron-Goodman type]. Hautarzt. 1989;40(8):530–535.
231. Ringel, E, Medenica, M, Lorincz, A. Localized mycosis fungoides not manifesting as Woringer-Kolopp disease. Arch Dermatol. 1983;119(9):756–760.
232. Wood, WS, Killby, VA, Stewart, WD. Pagetoid reticulosis (Woringer-Kolopp disease). J Cutan Pathol. 1979;6(2):113–123.
233. Burns, MK, Chan, LS, Cooper, KD. Woringer-Kolopp disease (localized pagetoid reticulosis) or unilesional mycosis fungoides? An analysis of eight cases with benign disease. Arch Dermatol. 1995;131(3):325–329.
234. Crowson, AN, Magro, CM. Woringer-Kolopp disease. A lymphomatoid hypersensitivity reaction. Am J Dermatopathol. 1994;16(5):542–548.
235. Deneau, DG, Wood, GS, Beckstead, J, et al. Woringer-Kolopp disease (pagetoid reticulosis). Four cases with histopathologic, ultrastructural, and immunohistologic observations. Arch Dermatol. 1984;120(8):1045–1051.
236. Smoller, BR, Stewart, M, Warnke, R. A case of Woringer-Kolopp disease with Ki-1 (CD30) + cytotoxic/suppressor cells. Arch Dermatol. 1992;128(4):526–529.
237. Drillenburg, P, Bronkhorst, CM, van der Wal, AC, et al. Expression of adhesion molecules in pagetoid reticulosis (Woringer-Kolopp disease). Br J Dermatol. 1997;136(4):613–636.
238. Benton, EC, Morris, SL, Robson, A, et al. An unusual case of granulomatous slack skin disease with necrobiosis. Am J Dermatopathol. 2008;30(5):462–465.
239. Camacho, FM, Burg, G, Moreno, JC, et al. Granulomatous slack skin in childhood. Pediatr Dermatol. 1997;14(3):204–208.
240. Noto, G, Pravata, G, Miceli, S, et al. Granulomatous slack skin: report of a case associated with Hodgkin’s disease and a review of the literature. Br J Dermatol. 1994;131(2):275–279.
241. Balus, L, Bassetti, F, Gentili, G. Granulomatous slack skin. Arch Dermatol. 1985;121(2):250–252.
242. LeBoit, PE. Granulomatous slack skin. Dermatol Clin. 1994;12(2):375–389.
243. LeBoit, PE, Zackheim, HS, White, CR, Jr. Granulomatous variants of cutaneous T-cell lymphoma. The histopathology of granulomatous mycosis fungoides and granulomatous slack skin. Am J Surg Pathol. 1988;12(2):83–95.
244. van Haselen, CW, Toonstra, J, van der Putte, SJ, et al. Granulomatous slack skin. Report of three patients with an updated review of the literature. Dermatology. 1998;196(4):382–391.
245. DeGregorio, R, Fenske, NA, Glass, LF. Granulomatous slack skin: a possible precursor of Hodgkin’s disease. J Am Acad Dermatol. 1995;33(6):1044–1047.
246. Tsang, WY, Chan, JK, Loo, KT, et al. Granulomatous slack skin. Histopathology. 1994;25(1):49–55.
247. Kempf, W, Ostheeren-Michaelis, S, Paulli, M, et al. Granulomatous mycosis fungoides and granulomatous slack skin: a multicenter study of the Cutaneous Lymphoma Histopathology Task Force Group of the European Organization For Research and Treatment of Cancer (EORTC). Arch Dermatol. 2008;144(12):1609–1617.
248. Tsuruta, D, Kono, T, Kutsuna, H, et al. Granulomatous slack skin: an ultrastructural study. J Cutan Pathol. 2001;28(1):44–48.
249. Helm, KF, Cerio, R, Winkelmann, RK. Granulomatous slack skin: a clinicopathological and immunohistochemical study of three cases. Br J Dermatol. 1992;126(2):142–147.
250. LeBoit, PE, Beckstead, JH, Bond, B, et al. Granulomatous slack skin: clonal rearrangement of the T-cell receptor beta gene is evidence for the lymphoproliferative nature of a cutaneous elastolytic disorder. J Invest Dermatol. 1987;89(2):183–186.
251. Grammatico, P, Balus, L, Scarpa, S, et al. Granulomatous slack skin: cytogenetic and molecular analyses. Cancer Genet Cytogenet. 1994;72(2):96–100.
252. Payne, CM, Glasser, L. Ultrastructural morphometry in the diagnosis of Sezary syndrome. Arch Pathol Lab Med. 1990;114(7):661–671.
253. Vonderheid, EC, Bigler, RD, Kotecha, A, et al. Variable CD7 expression on T cells in the leukemic phase of cutaneous T cell lymphoma (Sezary syndrome). J Invest Dermatol. 2001;117(3):654–662.
254. Ikai, K, Uchiyama, T, Maeda, M, et al. Sezary-like syndrome in a 10-year-old girl with serologic evidence of human T-cell lymphotropic virus type I infection. Arch Dermatol. 1987;123(10):1351–1355.
255. Meister, L, Duarte, AM, Davis, J, et al. Sezary syndrome in an 11-year-old girl. J Am Acad Dermatol. 1993;28(1):93–95.
256. Buzzanga, J, Banks, PM, Winkelmann, RK. Lymph node histopathology in Sezary syndrome. J Am Acad Dermatol. 1984;11(5 Pt 1):880–888.
257. Ikari, Y, Ohkura, M, Morita, M, Seki, K, et al. Leser-Trelat sign associated with Sezary syndrome. J Dermatol. 1995;22(1):62–67.
258. Barth, G, Basten, O, Ruschoff, J, et al. [Clinical and histopathological characteristics of early Leser-Trelat syndrome]. Hautarzt. 2001;52(7):649–652.
259. Agar, NS, Wedgeworth, E, Crichton, S, et al. Survival outcomes and prognostic factors in mycosis fungoides/Sezary syndrome: validation of the revised International Society for Cutaneous Lymphomas/European Organisation for Research and Treatment of Cancer staging proposal. J Clin Oncol. 2010;28(31):4730–4739.
260. Russell-Jones, R, Whittaker, S. T-cell receptor gene analysis in the diagnosis of Sezary syndrome. J Am Acad Dermatol. 1999;41(2 Pt 1):254–259.
261. Kim, YH, Bishop, K, Varghese, A, et al. Prognostic factors in erythrodermic mycosis fungoides and the Sezary syndrome. Arch Dermatol. 1995;131(9):1003–1008.
262. Campbell, JJ, Clark, RA, Watanabe, R, et al. Sezary syndrome and mycosis fungoides arise from distinct T-cell subsets: a biologic rationale for their distinct clinical behaviors. Blood. 2010;116(5):767–771.
263. Heid, JB, Schmidt, A, Oberle, N, et al. FOXP3+CD25– tumor cells with regulatory function in Sezary syndrome. J Invest Dermatol. 2009;129(12):2875–2885.
264. Clark, RA. Regulation gone wrong: a subset of Sezary patients have malignant regulatory T cells. J Invest Dermatol. 2009;129(12):2747–2750.
265. Bernengo, MG, Quaglino, P, Novelli, M, et al. Prognostic factors in Sezary syndrome: a multivariate analysis of clinical, haematological and immunological features. Ann Oncol. 1998;9(8):857–863.
266. Caudron, A, Marie-Cardine, A, Bensussan, A, et al. [New developments in Sezary syndrome]. Ann Dermatol Venereol. 2012;139(1):31–40.
267. Wieselthier, JS, Koh, HK. Sezary syndrome: diagnosis, prognosis, and critical review of treatment options. J Am Acad Dermatol. 1990;22(3):381–401.
268. Buechner, SA, Winkelmann, RK. Sezary syndrome. A clinicopathologic study of 39 cases. Arch Dermatol. 1983;119(12):979–986.
269. Sentis, HJ, Willemze, R, Scheffer, E. Histopathologic studies in Sezary syndrome and erythrodermic mycosis fungoides: a comparison with benign forms of erythroderma. J Am Acad Dermatol. 1986;15(6):1217–1226.
270. Kohler, S, Kim, YH, Smoller, BR. Histologic criteria for the diagnosis of erythrodermic mycosis fungoides and Sezary syndrome: a critical reappraisal. J Cutan Pathol. 1997;24(5):292–297.
271. Trotter, MJ, Whittaker, SJ, Orchard, GE, et al. Cutaneous histopathology of Sezary syndrome: a study of 41 cases with a proven circulating T-cell clone. J Cutan Pathol. 1997;24(5):286–291.
272. Hofman, FM, Meyer, PR, Yanagihara, E, et al. Demonstration of a subpopulation of Ia+ T-helper cells in mycosis fungoides and the Sezary syndrome. Am J Dermatopathol. 1983;5(2):135–143.
273. Piepkorn, M, Marty, J, Kjeldsberg, CR. T cell subset heterogeneity in a series of patients with mycosis fungoides and Sezary syndrome. J Am Acad Dermatol. 1984;11(3):427–432.
274. Dummer, R, Nestle, FO, Niederer, E, et al. Genotypic, phenotypic and functional analysis of CD4+CD7+ and CD4+CD7– T lymphocyte subsets in Sezary syndrome. Arch Dermatol Res. 1999;291(6):307–311.
275. Heald, P, Yan, SL, Edelson, R. Profound deficiency in normal circulating T cells in erythrodermic cutaneous T-cell lymphoma. Arch Dermatol. 1994;130(2):198–203.
276. Diamandidou, E, Colome-Grimmer, M, Fayad, L, et al. Transformation of mycosis fungoides/Sezary syndrome: clinical characteristics and prognosis. Blood. 1998;92(4):1150–1159.
277. Duncan, SC, Winkelmann, RK. Circulating Sezary cells in hospitalized dermatology patients. Br J Dermatol. 1978;99(2):171–178.
278. Macaulay, WL. Lymphomatoid papulosis. A continuing self-healing eruption, clinically benign—histologically malignant. Arch Dermatol. 1968;97(1):23–30.
279. Macaulay, WL. Lymphomatoid papulosis update. A historical perspective. Arch Dermatol. 1989;125(10):1387–1389.
280. Jaffe, ES, Harris, NL, Stein, H, et al. Summary of the WHO classification of tumours of haematopooietic and lymphoid tissues. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:10–13.
281. Rogers, M. Pityriasis lichenoides and lymphomatoid papulosis. Semin Dermatol. 1992;11(1):73–79.
282. Thomsen, K, Wantzin, GL. Lymphomatoid papulosis. A follow-up study of 30 patients. J Am Acad Dermatol. 1987;17(4):632–636.
283. Rifkin, S, Valderrama, E, Lipton, JM, et al. Lymphomatoid papulosis and Ki-1+ anaplastic large cell lymphoma occurring concurrently in a pediatric patient. J Pediatr Hematol Oncol. 2001;23(5):321–333.
284. Van Neer, FJ, Toonstra, J, Van Voorst Vader, PC, et al. Lymphomatoid papulosis in children: a study of 10 children registered by the Dutch Cutaneous Lymphoma Working Group. Br J Dermatol. 2001;144(2):351–354.
285. Sanchez, NP, Pittelkow, MR, Muller, SA, et al. The clinicopathologic spectrum of lymphomatoid papulosis: study of 31 cases. J Am Acad Dermatol. 1983;8(1):81–94.
286. Allabert, C, Esteve, E, Joly, P, et al. [Mucosal involvement in lymphomatoid papulosis: four cases]. Ann Dermatol Venereol. 2008;135(4):273–278.
287. Pileri, A, Bacci, F, Neri, I, et al. Persistent agmination of lymphomatoid papulosis: an ongoing debate. Dermatology. 2012. [[Epub ahead of print] Sep 14].
288. Torrelo, A, Colmenero, I, Hernandez, A, et al. Persistent agmination of lymphomatoid papulosis. Pediatr Dermatol. 2009;26(6):762–764.
289. Kadin, ME. Lymphomatoid papulosis, Ki-1+ lymphoma, and primary cutaneous Hodgkin’s disease. Semin Dermatol. 1991;10(3):164–171.
290. Chott, A, Vonderheid, EC, Olbricht, S, et al. The dominant T cell clone is present in multiple regressing skin lesions and associated T cell lymphomas of patients with lymphomatoid papulosis. J Invest Dermatol. 1996;106(4):696–700.
291. Lish, KM, Ramsay, DL, Raphael, BG, et al. Lymphomatoid papulosis followed by acute myeloblastic leukemia. J Am Acad Dermatol. 1993;29(1):112–115.
292. Aoki, M, Niimi, Y, Takezaki, S, et al. CD30+ lymphoproliferative disorder: primary cutaneous anaplastic large cell lymphoma followed by lymphomatoid papulosis. Br J Dermatol. 2001;145(1):123–126.
293. Kardashian, JL, Zackheim, HS, Egbert, BM. Lymphomatoid papulosis associated with plaque-stage and granulomatous mycosis fungoides. Arch Dermatol. 1985;121(9):1175–1180.
294. Ralfkiaer, E, Delsol, G, Willemze, R, et al. Primary cutaneous CD30-positive T-cell lymphoproliferative disorders. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lympholid Tissues. Lyon: IARC Press; 2001:222–224.
295. Cockerell, CJ, Stetler, LD. Accuracy in diagnosis of lymphomatoid papulosis. Am J Dermatopathol. 1991;13(1):20–25.
296. Tomaszewski, MM, Lupton, GP, Krishnan, J, et al. A comparison of clinical, morphological and immunohistochemical features of lymphomatoid papulosis and primary cutaneous CD30(Ki-1)-positive anaplastic large cell lymphoma. J Cutan Pathol. 1995;22(4):310–318.
297. Pierard, GE, Ackerman, AB, Lapiere, CM. Follicular lymphomatoid papulosis. Am J Dermatopathol. 1980;2(2):173–180.
298. Requena, L, Sanchez, M, Coca, S, et al. Follicular lymphomatoid papulosis. Am J Dermatopathol. 1990;12(1):67–75.
299. Willemze, R, Meyer, CJ, Van Vloten, WA, et al. The clinical and histological spectrum of lymphomatoid papulosis. Br J Dermatol. 1982;107(2):131–144.
300. Willemze, R, Scheffer, E. Clinical and histologic differentiation between lymphomatoid papulosis and pityriasis lichenoides. J Am Acad Dermatol. 1985;13(3):418–428.
301. Willemze, R, Scheffer, E, Ruiter, DJ, et al. Immunological, cytochemical and ultrastructural studies in lymphomatoid papulosis. Br J Dermatol. 1983;108(4):381–394.
302. Vonderheid, EC, Sajjadian, A, Kadin, ME. Methotrexate is effective therapy for lymphomatoid papulosis and other primary cutaneous CD30-positive lymphoproliferative disorders. J Am Acad Dermatol. 1996;34(3):470–481.
303. Saggini, A, Gulia, A, Argenyi, Z, et al. A variant of lymphomatoid papulosis simulating primary cutaneous aggressive epidermotropic CD8+ cytotoxic T-cell lymphoma. Description of 9 cases. Am J Surg Pathol. 2010;34(8):1168–1175.
304. Cardoso, J, Duhra, P, Thway, Y, et al. Lymphomatoid papulosis type D: a newly described variant easily confused with cutaneous aggressive CD8-positive cytotoxic T-cell lymphoma. Am J Dermatopathol. 2012;34(7):762–765.
305. Agnarsson, BA, Kadin, ME. Host response in lymphomatoid papulosis. Hum Pathol. 1989;20(8):747–752.
306. Davis, TH, Morton, CC, Miller-Cassman, R, et al. Hodgkin’s disease, lymphomatoid papulosis, and cutaneous T-cell lymphoma derived from a common T-cell clone. N Engl J Med. 1992;326(17):1115–1122.
307. Ralfkiaer, E, Stein, H, Wantzin, GL, et al. Lymphomatoid papulosis. Characterization of skin infiltrates by monoclonal antibodies. Am J Clin Pathol. 1985;84(5):587–593.
308. Bekkenk, MW, Kluin, PM, Jansen, PM, et al. Lymphomatoid papulosis with a natural killer-cell phenotype. Br J Dermatol. 2001;145(2):318–322.
309. Jang, KA, Choi, JC, Choi, JH. Expression of cutaneous lymphocyte-associated antigen and TIA-1 by lymphocytes in pityriasis lichenoides et varioliformis acuta and lymphomatoid papulosis: immunohistochemical study. J Cutan Pathol. 2001;28(9):453–459.
310. Whittaker, S, Smith, N, Jones, RR, et al. Analysis of beta, gamma, and delta T-cell receptor genes in lymphomatoid papulosis: cellular basis of two distinct histologic subsets. J Invest Dermatol. 1991;96(5):786–791.
311. Boulland, ML, Wechsler, J, Bagot, M, et al. Primary CD30-positive cutaneous T-cell lymphomas and lymphomatoid papulosis frequently express cytotoxic proteins. Histopathology. 2000;36(2):136–144.
312. Horiguchi, Y, Horiguchi, M, Toda, K, et al. The ultrastructural observation of a case of lymphomatoid papulosis. Acta Derm Venereol. 1984;64(4):308–315.
313. Weiss, LM, Wood, GS, Trela, M, et al. Clonal T-cell populations in lymphomatoid papulosis. Evidence of a lymphoproliferative origin for a clinically benign disease. N Engl J Med. 1986;315(8):475–479.
314. Wood, GS, Hardman, DL, Boni, R, et al. Lack of the t(2;5) or other mutations resulting in expression of anaplastic lymphoma kinase catalytic domain in CD30+ primary cutaneous lymphoproliferative disorders and Hodgkin’s disease [lymphomatoid papulosis]. Blood. 1996;88(5):1765–1770.
315. Kempf, W, Kadin, ME, Kutzner, H, et al. Lymphomatoid papulosis and human herpesviruses—a PCR-based evaluation for the presence of human herpesvirus 6, 7 and 8 related herpesviruses. J Cutan Pathol. 2001;28(1):29–33.
316. Ortiz Romero, PL, Vallejo, A, Lopez Estebaranz, JL, et al. Absence of HTLV-1 proviral sequences in patients with lymphomatoid papulosis. J Invest Dermatol. 1997;109(6):817–818.
317. Bekkenk, MW, Geelen, FA, van Voorst Vader, PC, et al. Primary and secondary cutaneous CD30(+) lymphoproliferative disorders: a report from the Dutch Cutaneous Lymphoma Group on the long-term follow-up data of 219 patients and guidelines for diagnosis and treatment [lymphomatoid papulosis]. Blood. 2000;95(12):3653–3661.
318. Sioutos, N, Kerl, H, Murphy, SB, et al. Primary cutaneous Hodgkin’s disease. Unique clinical, morphologic, and immunophenotypic findings. Am J Dermatopathol. 1994;16(1):2–8.
319. Kempf, W, Kutzner, H, Cozzio, A, et al. MUM1 expression in cutaneous CD30+ lymphoproliferative disorders: a valuable tool for the distinction between lymphomatoid papulosis and primary cutaneous anaplastic large-cell lymphoma. Br J Dermatol. 2008;158(6):1280–1287.
320. Agnarsson, BA, Kadin, ME. Ki-1 positive large cell lymphoma. A morphologic and immunologic study of 19 cases. Am J Surg Pathol. 1988;12(4):264–274.
321. Kadin, ME. Ki-1-positive anaplastic large-cell lymphoma: a clinicopathologic entity? J Clin Oncol. 1991;9(4):533–536.
322. Tashiro, K, Kikuchi, M, Takeshita, M, et al. Clinicopathological study of Ki-1-positive lymphomas. Pathol Res Pract. 1989;185(4):461–467.
323. Anagnostopoulos, I, Herbst, H, Niedobitek, G, et al. Demonstration of monoclonal EBV genomes in Hodgkin’s disease and Ki-1-positive anaplastic large cell lymphoma by combined Southern blot and in situ hybridization. Blood. 1989;74(2):810–816.
324. Frizzera, G. The distinction of Hodgkin’s disease from anaplastic large cell lymphoma. Semin Diagn Pathol. 1992;9(4):291–296.
325. Rosso, R, Paulli, M, Magrini, U, et al. Anaplastic large cell lymphoma, CD30/Ki-1 positive, expressing the CD15/Leu-M1 antigen. Immunohistochemical and morphological relationships to Hodgkin’s disease. Virchows Arch A Pathol Anat Histopathol. 1990;416(3):229–235.
326. Penny, RJ, Blaustein, JC, Longtine, JA, et al. Ki-1-positive large cell lymphomas, a heterogenous group of neoplasms. Morphologic, immunophenotypic, genotypic, and clinical features of 24 cases. Cancer. 1991;68(2):362–373.
327. Kadin, M, Nasu, K, Sako, D, et al. Lymphomatoid papulosis. A cutaneous proliferation of activated helper T cells expressing Hodgkin’s disease-associated antigens. Am J Pathol. 1985;119(2):315–325.
328. Miyake, K, Yoshino, T, Sarker, AB, et al. CD30 antigen in non-Hodgkin’s lymphoma. Pathol Int. 1994;44(6):428–434.
329. Falini, B. Anaplastic large cell lymphoma: pathological, molecular and clinical features. Br J Haematol. 2001;114(4):741–760.
330. Cataldo, KA, Jalal, SM, Law, ME, et al. Detection of t(2;5) in anaplastic large cell lymphoma: comparison of immunohistochemical studies, FISH, and RT-PCR in paraffin-embedded tissue. Am J Surg Pathol. 1999;23(11):1386–1392.
331. Kinney, MC, Kadin, ME. The pathologic and clinical spectrum of anaplastic large cell lymphoma and correlation with ALK gene dysregulation. Am J Clin Pathol. 1999;111(1 Suppl 1):S56–S67.
332. Mason, DY, Bastard, C, Rimokh, R, et al. CD30-positive large cell lymphomas (‘Ki-1 lymphoma’) are associated with a chromosomal translocation involving 5q35. Br J Haematol. 1990;74(2):161–168.
333. Bittencourt, AL, Rothers, S, Boente, P, et al. Primary cutaneous eosinophil-rich anaplastic large cell lymphoma: report of an unusual case and literature review. J Cutan Med Surg. 2008;12(2):88–92.
334. Kong, YY, Dai, B, Kong, JC, et al. Neutrophil/eosinophil-rich type of primary cutaneous anaplastic large cell lymphoma: a clinicopathological, immunophenotypic and molecular study of nine cases. Histopathology. 2009;55(2):189–196.
335. Chan, JK, Buchanan, R, Fletcher, CD. Sarcomatoid variant of anaplastic large-cell Ki-1 lymphoma. Am J Surg Pathol. 14(10), 1990. [983-938].
336. Iacobelli, J, Spagnolo, DV, Tesfai, Y, et al. Cutaneous intravascular anaplastic large T-cell lymphoma: a case report and review of the literature. Am J Dermatopathol. 2012. [[Epub ahead of print] Sep 27].
337. Wang, L, Li, C, Gao, T. Cutaneous intravascular anaplastic large cell lymphoma. J Cutan Pathol. 2011;38(2):221–226.
338. Kinney, MC, Glick, AD, Stein, H, et al. Comparison of anaplastic large cell Ki-1 lymphomas and microvillous lymphomas in their immunologic and ultrastructural features. Am J Surg Pathol. 1990;14(11):1047–1060.
339. Delsol, G, Al Saati, T, Gatter, KC, et al. Coexpression of epithelial membrane antigen (EMA), Ki-1, and interleukin-2 receptor by anaplastic large cell lymphomas. Diagnostic value in so-called malignant histiocytosis. Am J Pathol. 1988;130(1):59–70.
340. Falini, B, Pileri, S, Stein, H, et al. Variable expression of leucocyte-common (CD45) antigen in CD30 (Ki1)-positive anaplastic large-cell lymphoma: implications for the differential diagnosis between lymphoid and nonlymphoid malignancies. Hum Pathol. 1990;21(6):624–629.
341. Frierson, HF, Jr., Bellafiore, FJ, Gaffey, MJ, et al. Cytokeratin in anaplastic large cell lymphoma. Mod Pathol. 1994;7(3):317–321.
342. Gustmann, C, Altmannsberger, M, Osborn, M, et al. Cytokeratin expression and vimentin content in large cell anaplastic lymphomas and other non-Hodgkin’s lymphomas. Am J Pathol. 1991;138(6):1413–1422.
343. Stein, H, Mason, DY, Gerdes, J, et al. The expression of the Hodgkin’s disease associated antigen Ki-1 in reactive and neoplastic lymphoid tissue: evidence that Reed-Sternberg cells and histiocytic malignancies are derived from activated lymphoid cells. Blood. 1985;66(4):848–858.
344. Carbone, A, Gloghini, A, Volpe, R, et al. High frequency of Epstein-Barr virus latent membrane protein-1 expression in acquired immunodeficiency syndrome-related Ki-1 (CD30)-positive anaplastic large-cell lymphomas. Italian Cooperative Group on AIDS and Tumors. Am J Clin Pathol. 1994;101(6):768–772.
345. Fan, G, Kotylo, P, Neiman, RS, et al. Comparison of fascin expression in anaplastic large cell lymphoma and Hodgkin disease. Am J Clin Pathol. 2003;119(2):199–204.
346. Flynn, KJ, Dehner, LP, Gajl-Peczalska, KJ, et al. Regressing atypical histiocytosis: a cutaneous proliferation of atypical neoplastic histiocytes with unexpectedly indolent biologic behavior. Cancer. 1982;49(5):959–970.
347. Wick, MR, Sanchez, NP, Crotty, CP, et al. Cutaneous malignant histiocytosis: a clinical and histopathologic study of eight cases, with immunohistochemical analysis. J Am Acad Dermatol. 1983;8(1):50–62.
348. Jaffe, ES, Ralfkiaer, E. Subcutaneous panniculitis-like T-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:212–213.
349. Ali, SK, Othman, NM, Tagoe, AB, et al. Subcutaneous panniculitic T cell lymphoma mimicking histiocytic cytophagic panniculitis in a child. Saudi Med J. 2000;21(11):1074–1077.
350. Chan, YF, Lee, KC, Llewellyn, H. Subcutaneous T-cell lymphoma presenting as panniculitis in children: report of two cases. Pediatr Pathol. 1994;14(4):595–608.
351. Au, WY, Ng, WM, Choy, C, et al. Aggressive subcutaneous panniculitis-like T-cell lymphoma: complete remission with fludarabine, mitoxantrone and dexamethasone. Br J Dermatol. 2000;143(2):408–410.
352. Ikeda, E, Endo, M, Uchigasaki, S, et al. Phagocytized apoptotic cells in subcutaneous panniculitis-like T-cell lymphoma. J Eur Acad Dermatol Venereol. 2000;15(2):159–162.
353. Ohtake, N, Shimada, S, Mizoguchi, S, et al. Membranocystic lesions in a patient with cytophagic histiocytic panniculitis associated with subcutaneous T-cell lymphoma. Am J Dermatopathol. 1998;20(3):276–280.
354. Wang, L, Yang, Y, Liu, W, et al. [Subcutaneous panniculitis-like T-cell lymphoma: expression of cytotoxic-granule-associated protein TIA-1 and its relation with Epstein-Barr virus infection]. Zhonghua Bing Li Xue Za Zhi. 2000;29(2):103–106.
355. Marzano, AV, Berti, E, Paulli, M, et al. Cytophagic histiocytic panniculitis and subcutaneous panniculitis-like T-cell lymphoma: report of 7 cases. Arch Dermatol. 2000;136(7):889–896.
356. Yung, A, Snow, J, Jarrett, P. Subcutaneous panniculitic T-cell lymphoma and cytophagic histiocytic panniculitis. Australas J Dermatol. 2001;42(3):183–187.
357. Salhany, KE, Macon, WR, Choi, JK, et al. Subcutaneous panniculitis-like T-cell lymphoma: clinicopathologic, immunophenotypic, and genotypic analysis of alpha/beta and gamma/delta subtypes. Am J Surg Pathol. 1998;22(7):881–893.
358. Winkelmann, RK, Bowie, EJ. Hemorrhagic diathesis associated with benign histiocytic, cytophagic panniculitis and systemic histiocytosis. Arch Intern Med. 1980;140(11):1460–1463.
359. Wang, CY, Su, WP, Kurtin, PJ. Subcutaneous panniculitic T-cell lymphoma. Int J Dermatol. 1996;35(1):1–8.
360. Harada, H, Iwatsuki, K, Kaneko, F. Detection of Epstein-Barr virus genes in malignant lymphoma with clinical and histologic features of cytophagic histiocytic panniculitis. J Am Acad Dermatol. 1994;31(2 Pt 2):379–383.
361. Wick, MR, Patterson, JW. Cytophagic histiocytic panniculitis—a critical reappraisal. Arch Dermatol. 2000;136(7):922–924.
362. Kumar, S, Krenacs, L, Medeiros, J, et al. Subcutaneous panniculitic T-cell lymphoma is a tumor of cytotoxic T lymphocytes. Hum Pathol. 1998;29(4):397–403.
363. Weenig, RH, Ng, CS, Perniciaro, C. Subcutaneous panniculitis-like T-cell lymphoma: an elusive case presenting as lipomembranous panniculitis and a review of 72 cases in the literature. Am J Dermatopathol. 2001;23(3):206–215.
364. Mizutani, Y, Iwamasa, K, Arai, J, et al. [Subcutaneous panniculitic T-cell lymphoma with chromosomal abnormalities and large granular lymphocytes morphology]. Rinsho Ketsueki. 2000;41(6):519–523.
365. Iwatsuki, K, Harada, H, Ohtsuka, M, et al. Latent Epstein-Barr virus infection is frequently detected in subcutaneous lymphoma associated with hemophagocytosis but not in nonfatal cytophagic histiocytic panniculitis. Arch Dermatol. 1997;133(6):787–788.
366. Pincus, LB, LeBoit, PE, McCalmont, TH, et al. Subcutaneous panniculitis-like T-cell lymphoma with overlapping clinicopathologic features of lupus erythematosus: coexistence of 2 entities? Am J Dermatopathol. 2009;31(6):520–526.
367. Aguilera, P, Mascaro, JM, Jr., Martinez, A, et al. Cutaneous gamma/delta T-cell lymphoma: a histopathologic mimicker of lupus erythematosus profundus (lupus panniculitis). J Am Acad Dermatol. 2007;56(4):643–647.
368. Wang, X, Magro, CM. Human myxovirus resistance protein 1 (MxA) as a useful marker in the differential diagnosis of subcutaneous lymphoma vs. lupus erythematosus profundus. Eur J Dermatol. 2012;22(5):629–633.
369. Chang, SE, Huh, J, Choi, JH, Sung, KJ, et al. Clinicopathological features of CD56+ nasal-type T/natural killer cell lymphomas with lobular panniculitis. Br J Dermatol. 2000;142(5):924–930.
370. Pileri, SA, Weisenburger, DD, Sn, I, et al. Peripheral T-cell lymphoma, not otherwise specified. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008:306–308.
371. Giam, YC, Ong, BH. Clinicopathological and immunohistological correlation of malignant lymphomas of the skin. Ann Acad Med Singapore. 1994;23(3):412–417.
372. Gilliam, AC, Wood, GS. Primary cutaneous lymphomas other than mycosis fungoides. Semin Oncol. 1999;26(3):290–306.
373. Gordon, BG, Weisenburger, DD, Warkentin, PI, et al. Peripheral T-cell lymphoma in childhood and adolescence. A clinicopathologic study of 22 patients. Cancer. 1993;71(1):257–263.
374. Nakamura, S, Suchi, T, Koshikawa, T, et al. Clinicopathologic study of 212 cases of peripheral T-cell lymphoma among the Japanese. Cancer. 1993;72(5):1762–1772.
375. Rencic, A, Laman, S, Nousari, HC. Peripheral T cell lymphoma presenting as dermatomyositis-like eruption. J Cutan Med Surg. 2002;6(3):218–220.
376. Bhushan, M, Craven, NM, Armstrong, GR, et al. Lymphoepithelioid cell lymphoma (Lennert’s lymphoma) presenting as atypical granuloma annulare. Br J Dermatol. 2000;142(4):776–780.
377. Seeburger, J, Anderson-Wilms, N, Jacobs, R. Lennert’s lymphoma presenting as prurigo nodularis. Cutis. 1993;51(5):355–358.
378. Arnulf, B, Copie-Bergman, C, Delfau-Larue, MH, et al. Nonhepatosplenic gammadelta T-cell lymphoma: a subset of cytotoxic lymphomas with mucosal or skin localization. Blood. 1998;91(5):1723–1731.
379. de Wolf-Peeters, C, Achten, R. Gammadelta T-cell lymphomas: a homogeneous entity? Histopathology. 2000;36(4):294–305.
380. Berti, E, Tomasini, D, Vermeer, MH, et al. Primary cutaneous CD8-positive epidermotropic cytotoxic T cell lymphomas. A distinct clinicopathological entity with an aggressive clinical behavior. Am J Pathol. 1999;155(2):483–492.
381. Massone, C, Chott, A, Metze, D, et al. Subcutaneous, blastic natural killer (NK), NK/T-cell, and other cytotoxic lymphomas of the skin: a morphologic, immunophenotypic, and molecular study of 50 patients. Am J Surg Pathol. 2004;28(6):719–735.
382. Bekkenk, MW, Vermeer, MH, Jansen, PM, et al. Peripheral T-cell lymphomas unspecified presenting in the skin: analysis of prognostic factors in a group of 82 patients. Blood. 2003;102(6):2213–2219.
383. von den Driesch, P, Coors, EA. Localized cutaneous small to medium-sized pleomorphic T-cell lymphoma: a report of 3 cases stable for years. J Am Acad Dermatol. 2002;46(4):531–535.
384. Vaillant, L, Monegier du Sorbier, C, et al. Cutaneous T cell lymphoma of signet ring cell type: a specific clinico-pathologic entity. Acta Derm Venereol. 1993;73(4):255–258.
385. Zelickson, BD, Peters, MS, Muller, SA, et al. T-cell receptor gene rearrangement analysis: cutaneous T cell lymphoma, peripheral T cell lymphoma, and premalignant and benign cutaneous lymphoproliferative disorders. J Am Acad Dermatol. 1991;25(5 Pt 1):787–796.
386. Schlegelberger, B, Himmler, A, Godde, E, et al. Cytogenetic findings in peripheral T-cell lymphomas as a basis for distinguishing low-grade and high-grade lymphomas. Blood. 1994;83(2):505–511.
387. Willemze, R, Jaffe, ES, Burg, G, et al. WHO-EORTC classification for cutaneous lymphomas. Blood. 2005;105(10):3768–3785.
388. Garcia-Herrera, A, Colomo, L, Camos, M, et al. Primary cutaneous small/medium CD4+ T-cell lymphomas: a heterogeneous group of tumors with different clinicopathologic features and outcome. J Clin Oncol. 2008;26(20):3364–3371.
389. Jaffe, ES. Post-thymic T-cell lymphomas. Surgical Pathology of the Lymph Nodes and Related Organs. Philadelphia: W.B. Saunders; 1995. [344-389].
390. Ritter, JH, Goldstein, NS, Argenyi, Z, et al. Granulocytic sarcoma: an immunohistologic comparison with peripheral T-cell lymphoma in paraffin sections. J Cutan Pathol. 1994;21(3):207–216.
391. Goldstein, NS, Ritter, JH, Argenyi, ZB, et al. Granulocytic sarcoma: potential diagnostic clues from immunostaining patterns seen with “anti-lymphoid” antibodies. Int J Surg Pathol. 1995;2:199–206.
392. Scarabello, A, Leinweber, B, Ardigo, M, et al. Cutaneous lymphomas with prominent granulomatous reaction: a potential pitfall in the histopathologic diagnosis of cutaneous T- and B-cell lymphomas. Am J Surg Pathol. 2002;26(10):1259–1268.
393. Jaffe, ES. Lymphoid lesions of the head and neck: a model of lymphocyte homing and lymphomagenesis. Mod Pathol. 2002;15(3):255–263.
394. Ansai, S, Maeda, K, Yamakawa, M, et al. CD56-positive (nasal-type T/NK cell) lymphoma arising on the skin. Report of two cases and review of the literature. J Cutan Pathol. 1997;24(8):468–476.
395. Chang, SE, Yoon, GS, Huh, J, et al. Comparison of primary and secondary cutaneous CD56+ NK/T cell lymphomas. Appl Immunohistochem Mol Morphol. 2002;10(2):163–170.
396. Jaffe, ES, Chan, JK, Su, IJ, et al. Report of the Workshop on Nasal and Related Extranodal Angiocentric T/Natural Killer Cell Lymphomas. Definitions, differential diagnosis, and epidemiology. Am J Surg Pathol. 1996;20(1):103–111.
397. Jaffe, ES, Krenacs, L, Kumar, S, et al. Extranodal peripheral T-cell and NK-cell neoplasms. Am J Clin Pathol. 1999;111(1 Suppl 1):S46–S55.
398. Kato, N, Yasukawa, K, Onozuka, T, et al. Nasal and nasal-type T/NK-cell lymphoma with cutaneous involvement. J Am Acad Dermatol. 1999;40(5 Pt 2):850–856.
399. Rodriguez, J, Romaguera, JE, Manning, J, et al. Nasal-type T/NK lymphomas: a clinicopathologic study of 13 cases. Leuk Lymphoma. 2000;39(1-2):139–144.
400. Hwang, DK, Kwon, HM, Park, JW, et al. TCR gene-rearranged, extranodal NK/T-cell lymphoma, nasal type, presenting as gyrate patches. J Dermatol. 2002;29(10):648–652.
401. Au, WY, Liang, R. Peripheral T-cell lymphoma. Curr Oncol Rep. 2002;4(5):434–442.
402. de Campos-Lima, PO, Levitsky, V, Brooks, J, et al. T cell responses and virus evolution: loss of HLA A11-restricted CTL epitopes in Epstein-Barr virus isolates from highly A11-positive populations by selective mutation of anchor residues. J Exp Med. 1994;179(4):1297–1305.
403. Gaal, K, Weiss, LM, Chen, WG, et al. Epstein-Barr virus nuclear antigen (EBNA)-1 carboxy-terminal and EBNA-4 sequence polymorphisms in nasal natural killer/T-cell lymphoma in the United States. Lab Invest. 2002;82(7):957–962.
404. Harabuchi, Y, Imai, S, Wakashima, J, et al. Nasal T-cell lymphoma causally associated with Epstein-Barr virus: clinicopathologic, phenotypic, and genotypic studies. Cancer. 1996;77(10):2137–2149.
405. Canioni, D, Arnulf, B, Asso-Bonnet, M, et al. Nasal natural killer lymphoma associated with Epstein-Barr virus in a patient infected with human immunodeficiency virus. Arch Pathol Lab Med. 2001;125(5):660–662.
406. Shen, L, Liang, AC, Lu, L, et al. Frequent deletion of Fas gene sequences encoding death and transmembrane domains in nasal natural killer/T-cell lymphoma. Am J Pathol. 2002;161(6):2123–2131.
407. Siu, LL, Chan, JK, Kwong, YL. Natural killer cell malignancies: clinicopathologic and molecular features. Histol Histopathol. 2002;17(2):539–554.
408. Santucci, M, Pimpinelli, N, Massi, D, et al. Cytotoxic/natural killer cell cutaneous lymphomas. Report of EORTC Cutaneous Lymphoma Task Force Workshop. Cancer. 2003;97(3):610–627.
409. Vidal, RW, Devaney, K, Ferlito, A, et al. Sinonasal malignant lymphomas: a distinct clinicopathological category. Ann Otol Rhinol Laryngol. 1999;108(4):411–419.
410. Blakolmer, K, Vesely, M, Kummer, JA, et al. Immunoreactivity of B-cell markers (CD79a, L26) in rare cases of extranodal cytotoxic peripheral T- (NK/T-) cell lymphomas. Mod Pathol. 2000;13(7):766–772.
411. Facchetti, F, Jones, DM, Petrella, T. Blastic plasmacytoid dendritic cell neoplasm. In: Swerdlow SH, Campo E, Harris NL, et al, eds. WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2008:145–147.
412. Ginarte, M, Abalde, MT, Peteiro, C, et al. Blastoid NK cell leukemia/lymphoma with cutaneous involvement. Dermatology. 2000;201(3):268–271.
413. Taddesse-Heath, L, Feldman, JI, Fahle, GA, et al. Florid CD4+, CD56+ T-cell infiltrate associated with herpes simplex infection simulating nasal NK-/T-cell lymphoma. Mod Pathol. 2003;16(2):166–172.
414. Jaffe, ES, Blattner, WA, Blayney, DW, et al. The pathologic spectrum of adult T-cell leukemia/lymphoma in the United States. Human T-cell leukemia/lymphoma virus-associated lymphoid malignancies. Am J Surg Pathol. 1984;8(4):263–275.
415. Kikuchi, M, Jaffe, ES, Ralfkiaer, E. Adult T-cell leukaemia/lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:200–203.
416. Shimoyama, M. Diagnostic criteria and classification of clinical subtypes of adult T-cell leukaemia-lymphoma. A report from the Lymphoma Study Group (1984- 87). Br J Haematol. 1991;79(3):428–437.
417. Chan, HL, Su, IJ, Kuo, TT, et al. Cutaneous manifestations of adult T cell leukemia/lymphoma. Report of three different forms. J Am Acad Dermatol. 1985;13(2 Pt 1):213–219.
418. Kikuchi, M, Mitsui, T, Kozuru, M, et al. Case report of adult T-cell leukemia with preceding long-standing cutaneous involvement. Jpn J Clin Oncol. 1983;13(Suppl 2):201–207.
419. Takahashi, K, Tanaka, T, Fujita, M, et al. Cutaneous-type adult T-cell leukemia/lymphoma. A unique clinical feature with monoclonal T-cell proliferation detected by Southern blot analysis. Arch Dermatol. 1988;124(3):399–404.
420. Setoyama, M, Katahira, Y, Kanzaki, T. Clinicopathologic analysis of 124 cases of adult T-cell leukemia/lymphoma with cutaneous manifestations: the smouldering type with skin manifestations has a poorer prognosis than previously thought. J Dermatol. 1999;26(12):785–790.
421. Maeda, K, Takahashi, M. Characterization of skin infiltrating cells in adult T-cell leukaemia/lymphoma (ATLL): clinical, histological and immunohistochemical studies on eight cases. Br J Dermatol. 1989;121(5):603–612.
422. Johno, M, Ono, T. [Clinicopathological differential diagnosis of mycosis fungoides/Sezary syndrome from the cutaneous type of adult T-cell leukemia/lymphoma]. Nippon Rinsho. 2000;58(3):660–664.
423. Dosaka, N, Tanaka, T, Miyachi, Y, et al. Examination of HTLV-I integration in the skin lesions of various types of adult T-cell leukemia (ATL): independence of cutaneous-type ATL confirmed by Southern blot analysis. J Invest Dermatol. 1991;96(2):196–200.
424. Gessain, A, Moulonguet, I, Flageul, B, et al. Cutaneous type of adult T cell leukemia/lymphoma in a French West Indian woman. Clonal rearrangement of T-cell receptor beta and gamma genes and monoclonal integration of HTLV-I proviral DNA in the skin infiltrate. J Am Acad Dermatol. 1990;23(5 Pt 2):994–1000.
425. Maeda, K, Yamana, K, Takahashi, H, et al. Dual surface makers and HTLV-I proviral DNA in a cutaneous tumour nodule in a case of adult T cell leukaemia/lymphoma. Br J Dermatol. 1987;117(5):561–568.
426. Haynes, BF, Miller, SE, Palker, TJ, et al. Identification of human T cell leukemia virus in a Japanese patient with adult T cell leukemia and cutaneous lymphomatous vasculitis. Proc Natl Acad Sci U S A. 1983;80(7):2054–2058.
427. Manabe, T, Hirokawa, M, Sugihara, K, et al. Angiocentric and angiodestructive infiltration of adult T-cell leukemia/lymphoma (ATLL) in the skin. Report of two cases. Am J Dermatopathol. 1988;10(6):487–496.
428. Setoyama, M, Katahira, Y, Kanzaki, T, et al. Adult T-cell leukemia/lymphoma associated with noninfectious epithelioid granuloma in the skin: a clinicopathologic study. Am J Dermatopathol. 1997;19(6):591–595.
429. Nagatani, T, Miyazawa, M, Matsuzaki, T, et al. Comparative study of cutaneous T-cell lymphoma and adult T-cell leukemia/lymphoma. Semin Dermatol. 1994;13(3):216–222.
430. Arai, E, Chow, KC, Li, CY, et al. Differentiation between cutaneous form of adult T cell leukemia/lymphoma and cutaneous T cell lymphoma by in situ hybridization using a human T cell leukemia virus-1 DNA probe. Am J Pathol. 1994;144(1):15–20.
431. Yoshinaga, H. [A case of adult T-cell leukemia (ATL)–various skin eruptions and virus-like particles in the fresh tumor cells]. Nippon Hifuka Gakkai Zasshi. 1989;99(1):25–35.
432. Hasui, K, Sato, E, Tokudome, T, et al. A comparative microphotometric analysis of adult T-cell leukemia/lymphoma (ATLL) in the skin and mycosis fungoides (MF). Acta Pathol Jpn. 1987;37(9):1405–1414.
433. Oishi, M, Johno, M, Ono, T, et al. Differences in IL-2 receptor levels between mycosis fungoides and cutaneous type adult T-cell leukemia/lymphoma in the early stages of the disease. J Invest Dermatol. 1994;102(5):710–715.
434. Fouchard, N, Mahe, A, Huerre, M, et al. Cutaneous T cell lymphomas: mycosis fungoides, Sezary syndrome and HTLV- I-associated adult T cell leukemia (ATL) in Mali, West Africa: a clinical, pathological and immunovirological study of 14 cases and a review of the African ATL cases. Leukemia. 1998;12(4):578–585.
435. Bernstein, JE, Soltani, K, Lorincz, AL. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1979;1(3):227–232.
436. Schmuth, M, Ramaker, J, Trautmann, C, et al. Cutaneous involvement in prelymphomatous angioimmunoblastic lymphadenopathy. J Am Acad Dermatol. 1997;36(2 Pt 2):290–295.
437. Jaffe, ES, Ralfkiaer, E. Angioimmunoblastic T-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:225–226.
438. Siegert, W, Nerl, C, Agthe, A, et al. Angioimmunoblastic lymphadenopathy (AILD)-type T-cell lymphoma: prognostic impact of clinical observations and laboratory findings at presentation. The Kiel Lymphoma Study Group. Ann Oncol. 1995;6(7):659–664.
439. Schotte, U, Megahed, M, Jansen, T, et al. [Angioimmunoblastic lymphadenopathy with cutaneous manifestations in a 13-year-old girl]. Hautarzt. 1992;43(11):728–734.
440. Ree, HJ, Kadin, ME, Kikuchi, M, et al. Angioimmunoblastic lymphoma (AILD-type T-cell lymphoma) with hyperplastic germinal centers. Am J Surg Pathol. 1998;22(6):643–655.
441. Matloff, RB, Neiman, RS. Angioimmunoblastic lymphadenopathy. A generalized lymphoproliferative disorder with cutaneous manifestations. Arch Dermatol. 1978;114(1):92–94.
442. Seehafer, JR, Goldberg, NC, Dicken, CH, et al. Cutaneous manifestations of angioimmunoblastic lymphadenopathy. Arch Dermatol. 1980;116(1):41–45.
443. Martel, P, Laroche, L, Courville, P, et al. Cutaneous involvement in patients with angioimmunoblastic lymphadenopathy with dysproteinemia: a clinical, immunohistological, and molecular analysis. Arch Dermatol. 2000;136(7):881–886.
444. Bernengo, MG, Levi, L, Zina, G. Skin lesions in angioimmunoblastic lymphadenopathy: histological and immunological studies. Br J Dermatol. 1981;104(2):131–139.
445. Quintanilla-Martinez, L, Fend, F, Moguel, LR, et al. Peripheral T-cell lymphoma with Reed-Sternberg-like cells of B-cell phenotype and genotype associated with Epstein-Barr virus infection. Am J Surg Pathol. 1999;23(10):1233–1240.
446. Murakami, T, Ohtsuki, M, Nakagawa, H. Angioimmunoblastic lymphadenopathy-type peripheral T-cell lymphoma with cutaneous infiltration: report of a case and its gene expression profile. Br J Dermatol. 2001;144(4):878–884.
447. Brown, HA, Macon, WR, Kurtin, PJ, et al. Cutaneous involvement by angioimmunoblastic T-cell lymphoma with remarkable heterogeneous Epstein-Barr virus expression. J Cutan Pathol. 2001;28(8):432–438.
448. Dorfman, DM, Shahsafaei, A. CD200 (OX-2 membrane glycoprotein) is expressed by follicular T helper cells and in angioimmunoblastic T-cell lymphoma. Am J Surg Pathol. 2011;35(1):76–83.
449. Ponciano, A, de Muret, A, Machet, L, et al. Epidermotropic secondary cutaneous involvement by relapsed angioimmunoblastic T-cell lymphoma mimicking mycosis fungoides: a case report. J Cutan Pathol. 2012. [[Epub ahead of print] Oct 10].
450. Ren, YL, Hong, L, Nong, L, Zhang, S, et al. [Clinicopathologic, immunohistochemical and molecular analysis in 15 cases of angioimmunoblastic T-cell lymphomas]. Beijing Da Xue Xue Bao. 2008;40(4):352–357.
451. Mourad, N, Mounier, N, Briere, J, et al. Clinical, biologic, and pathologic features in 157 patients with angioimmunoblastic T-cell lymphoma treated within the Groupe d’Etude des Lymphomes de l’Adulte (GELA) trials. Blood. 2008;111(9):4463–4470.
452. Iannitto, E, Ferreri, AJ, Minardi, V, et al. Angioimmunoblastic T-cell lymphoma. Crit Rev Oncol Hematol. 2008;68(3):264–271.
453. Liao, YL, Chang, ST, Kuo, SY, et al. Angioimmunoblastic T-cell lymphoma of cytotoxic T-cell phenotype containing a large B-cell proliferation with an undersized B-cell clonal product. Appl Immunohistochem Mol Morphol. 2010;18(2):185–189.
454. Jones, D, Jorgensen, JL, Shahsafaei, A, et al. Characteristic proliferations of reticular and dendritic cells in angioimmunoblastic lymphoma. Am J Surg Pathol. 1998;22(8):956–964.
455. Anagnostopoulos, I, Hummel, M, Finn, T, et al. Heterogeneous Epstein-Barr virus infection patterns in peripheral T-cell lymphoma of angioimmunoblastic lymphadenopathy type. Blood. 1992;80(7):1804–1812.
456. Weiss, LM, Jaffe, ES, Liu, XF, et al. Detection and localization of Epstein-Barr viral genomes in angioimmunoblastic lymphadenopathy and angioimmunoblastic lymphadenopathy-like lymphoma. Blood. 1992;79(7):1789–1795.
457. Lee, Y, Lee, KW, Kim, JH, et al. Epstein-Barr virus-positivity in tumor has no correlation with the clinical outcomes of patients with angioimmunoblastic T-cell lymphoma. Korean J Intern Med. 2008;23(1):30–36.
458. Lipford, EH, Smith, HR, Pittaluga, S, et al. Clonality of angioimmunoblastic lymphadenopathy and implications for its evolution to malignant lymphoma. J Clin Invest. 1987;79(2):637–642.
459. O’Connor, NT, Crick, JA, Wainscoat, JS, et al. Evidence for monoclonal T lymphocyte proliferation in angioimmunoblastic lymphadenopathy. J Clin Pathol. 1986;39(11):1229–1232.
460. Abruzzo, LV, Schmidt, K, Weiss, LM, et al. B-cell lymphoma after angioimmunoblastic lymphadenopathy: a case with oligoclonal gene rearrangements associated with Epstein-Barr virus. Blood. 1993;82(1):241–246.
461. Huang, J, Zhang, PH, Gao, YH, et al. Sequential development of diffuse large B-cell lymphoma in a patient with angioimmunoblastic T-cell lymphoma. Diagn Cytopathol. 2012;40(4):346–351.
462. Bayerl, MG, Hennessy, J, Ehmann, WC, et al. Multiple cutaneous monoclonal B-cell proliferations as harbingers of systemic angioimmunoblastic T-cell lymphoma. J Cutan Pathol. 2010;37(7):777–786.
463. Cella, M, Jarrossay, D, Facchetti, F, et al. Plasmacytoid monocytes migrate to inflamed lymph nodes and produce large amounts of type I interferon. Nat Med. 1999;5(8):919–923.
464. Chan, JKC, Jaffe, ES, Ralfkiaer, E. Blastic NK-cell lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Hematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:214–215.
465. Uchiyama, N, Ito, K, Kawai, K, et al. CD2-, CD4+, CD56+ agranular natural killer cell lymphoma of the skin. Am J Dermatopathol. 1998;20(5):513–517.
466. Aoyama, Y, Yamane, T, Hino, M, et al. Blastic NK-cell lymphoma/leukemia with T-cell receptor gamma rearrangement. Ann Hematol. 2001;80(12):752–754.
467. Mukai, HY, Kojima, H, Suzukawa, K, et al. High-dose chemotherapy with peripheral blood stem cell rescue in blastoid natural killer cell lymphoma. Leuk Lymphoma. 1999;32(5-6):583–588.
468. Bower, CP, Standen, GR, Pawade, J, et al. Cutaneous presentation of steroid responsive blastoid natural killer cell lymphoma. Br J Dermatol. 2000;142(5):1017–1020.
469. Matano, S, Nakamura, S, Annen, Y, et al. Monomorphic agranular natural killer cell lymphoma/leukemia with no Epstein-Barr virus association. Acta Haematol. 1999;101(4):206–208.
470. Herling, M, Jones, D. CD4+/CD56+ hematodermic tumor: the features of an evolving entity and its relationship to dendritic cells. Am J Clin Pathol. 2007;127(5):687–700.
471. Petrella, T, Dalac, S, Maynadie, M, et al. CD4+ CD56+ cutaneous neoplasms: a distinct hematological entity? Groupe Francais d’Etude des Lymphomes Cutanes (GFELC). Am J Surg Pathol. 1999;23(2):137–146.
472. Cota, C, Vale, E, Viana, I, et al. Cutaneous manifestations of blastic plasmacytoid dendritic cell neoplasm-morphologic and phenotypic variability in a series of 33 patients. Am J Surg Pathol. 2010;34(1):75–87.
473. Piccin, A, Morello, E, Svaldi, M, et al. Post-transplant lymphoproliferative disease of donor origin, following haematopoietic stem cell transplantation in a patient with blastic plasmacytoid dendritic cell neoplasm. Hematol Oncol. 2012. [[Epub ahead of print] Aug 23].
474. Campbell, SM, Moad, JC, Sammons, DL, et al. CD4+ CD56+ hematodermic neoplasm and plasmacytoid dendritic cell tumor: case report and review of the literature. Cutis. 2012;89(6):278–283.
475. Pilichowska, ME, Fleming, MD, Pinkus, JL, et al. CD4+/CD56+ hematodermic neoplasm (“blastic natural killer cell lymphoma”): neoplastic cells express the immature dendritic cell marker BDCA-2 and produce interferon. Am J Clin Pathol. 2007;128(3):445–453.
476. Patel, JL, Shetty, S, Salama, ME. An unusual case of cutaneous blastic plasmacytoid dendritic cell neoplasm with concomitant B-cell lymphoproliferative disorder. Am J Dermatopathol. 2011;33(3):e31–36.
477. Chang, SE, Choi, HJ, Huh, J, et al. A case of primary cutaneous CD56+, TdT+, CD4+, blastic NK-cell lymphoma in a 19-year-old woman. Am J Dermatopathol. 2002;24(1):72–75.
478. Dargent, JL, Delannoy, A, Pieron, P, et al. Cutaneous accumulation of plasmacytoid dendritic cells associated with acute myeloid leukemia: a rare condition distinct from blastic plasmacytoid dendritic cell neoplasm. J Cutan Pathol. 2011;38(11):893–898.
479. Brunning, RD, Borowitz, M, Matutes, E, et al. Precursor T lymphoblastic leukaemia/lymphoblastic lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumoursof Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:115–117.
480. Chimenti, S, Fink-Puches, R, Peris, K, et al. Cutaneous involvement in lymphoblastic lymphoma. J Cutan Pathol. 1999;26(8):379–385.
481. Sander, CA, Medeiros, LJ, Abruzzo, LV, et al. Lymphoblastic lymphoma presenting in cutaneous sites. A clinicopathologic analysis of six cases. J Am Acad Dermatol. 1991;25(6 Pt 1):1023–1031.
482. Millot, F, Robert, A, Bertrand, Y, et al. Cutaneous involvement in children with acute lymphoblastic leukemia or lymphoblastic lymphoma. The Children’s Leukemia Cooperative Group of the European Organization of Research and Treatment of Cancer (EORTC). Pediatrics. 1997;100(1):60–64.
483. Okuda, T, Fisher, R, Downing, JR. Molecular diagnostics in pediatric acute lymphoblastic leukemia. Mol Diagn. 1996;1(2):139–151.
484. Patterson, JW, Blaylock, WK. Hodgkin’s disease. In: Demis DJ, Dobson RL, McGuire J, eds. Clinical Dermatology. Hagerstown, MD: Harper & Row; 1985:1–9. [unit 20-8].
485. Stein, H, Delsol, G, Pileri, S, et al. Classical Hodgkin lymphoma. In: Jaffe ES, Harris NL, Stein H, Vardiman JW, eds. Tumours of Haematopoietic and Lymphoid Tissues. Lyon: IARC Press; 2001:244–253.
486. Marafioti, T, Hummel, M, Foss, HD, et al. Hodgkin and Reed-Sternberg cells represent an expansion of a single clone originating from a germinal center B-cell with functional immunoglobulin gene rearrangements but defective immunoglobulin transcription. Blood. 2000;95(4):1443–1450.
487. Seitz, V, Hummel, M, Marafioti, T, et al. Detection of clonal T-cell receptor gamma-chain gene rearrangements in Reed-Sternberg cells of classic Hodgkin disease. Blood. 2000;95(10):3020–3024.
488. Benninghoff, DL, Medina, A, Alexander, LL, et al. The mode of spread of Hodgkin’s disease to the skin. Cancer. 1970;26(5):1135–1140.
489. White, RM, Patterson, JW. Cutaneous involvement in Hodgkin’s disease. Cancer. 1985;55(5):1136–1145.
490. Cerroni, L, Beham-Schmid, C, Kerl, H. Cutaneous Hodgkin’s disease: an immunohistochemical analysis. J Cutan Pathol. 1995;22(3):229–235.
491. Hanno, R, Bean, SF. Hodgkin’s disease with specific bullous lesions. Am J Dermatopathol. 1980;2(4):363–366.
492. Heyd, J, Weissberg, N, Gottschalk, S. Hodgkin’s disease of the skin. A case report. Cancer. 1989;63(5):924–929.
493. Torne, R, Umbert, P. Hodgkin’s disease presenting with superficial lymph nodes and tumors of the scalp. Dermatologica. 1986;172(4):225–228.
494. Zackheim, HS, LeBoit, PE, Gordon, BI, et al. Lymphomatoid papulosis followed by Hodgkin’s lymphoma. Differential response to therapy. Arch Dermatol. 1993;129(1):86–91.
495. Geldenhuys, L, Radhi, J, Hull, PR. Mycosis fungoides and cutaneous Hodgkin’s disease in the same patient: a case report. J Cutan Pathol. 1999;26(6):311–314.
496. Smith, JL, Jr., Butler, JJ. Skin involvement in Hodgkin’s disease. Cancer. 1980;45(2):354–361.
497. Guitart, J, Fretzin, D. Skin as the primary site of Hodgkin’s disease: a case report of primary cutaneous Hodgkin’s disease and review of its relationship with non-Hodgkin’s lymphoma. Am J Dermatopathol. 1998;20(2):218–222.
498. Silverman, CL, Strayer, DS, Wasserman, TH. Cutaneous Hodgkin’s disease. Arch Dermatol. 1982;118(11):918–921.
499. Kadin, ME, Muramoto, L, Said, J. Expression of T-cell antigens on Reed-Sternberg cells in a subset of patients with nodular sclerosing and mixed cellularity Hodgkin’s disease. Am J Pathol. 1988;130(2):345–353.
500. Foss, HD, Reusch, R, Demel, G, et al. Frequent expression of the B-cell-specific activator protein in Reed-Sternberg cells of classical Hodgkin’s disease provides further evidence for its B-cell origin. Blood. 1999;94(9):3108–3113.
501. Cho, RJ, McCalmont, TH, Ai, WZ, et al. Use of an expanded immunohistochemical panel to distinguish cutaneous Hodgkin lymphoma from histopathologic imitators. J Cutan Pathol. 2012;39(6):651–658.
502. Browne, P, Petrosyan, K, Hernandez, A, et al. The B-cell transcription factors BSAP, Oct-2, and BOB.1 and the pan-B-cell markers CD20, CD22, and CD79a are useful in the differential diagnosis of classic Hodgkin lymphoma. Am J Clin Pathol. 2003;120(5):767–777.
503. Garcia-Cosio, M, Santon, A, Martin, P, et al. Analysis of transcription factor OCT.1, OCT.2 and BOB.1 expression using tissue arrays in classical Hodgkin’s lymphoma. Mod Pathol. 2004;17(12):1531–1538.
504. Caughman, W, Stern, R, Haynes, H. Neutrophilic dermatosis of myeloproliferative disorders. Atypical forms of pyoderma gangrenosum and Sweet’s syndrome associated with myeloproliferative disorders. J Am Acad Dermatol. 1983;9(5):751–758.
505. Verhagen, C, Stalpers, LJ, de Pauw, BE, et al. Drug-induced skin reactions in patients with acute non-lymphocytic leukaemia. Eur J Haematol. 1987;38(3):225–230.
506. Barzilai, A, Shpiro, D, Goldberg, I, et al. Insect bite-like reaction in patients with hematologic malignant neoplasms. Arch Dermatol. 1999;135(12):1503–1507.
507. Ratnam, KV, Khor, CJ, Su, WP. Leukemia cutis. Dermatol Clin. 1994;12(2):419–431.
508. Chan, JK. Natural killer cell neoplasms. Anat Pathol. 1998;3:77–145.
509. Braverman, IM. Leukemia and Allied Disorders. Skin Signs of Systemic Disease. Philadelphia: WB Saunders; 1998.
510. Asada, H, Okada, N, Tei, H, et al. Epstein-Barr virus-associated large granular lymphocyte leukemia with cutaneous infiltration. J Am Acad Dermatol. 1994;31(2 Pt 1):251–255.
511. Helm, KF, Peters, MS, Tefferi, A, et al. Pyoderma gangrenosum-like ulcer in a patient with large granular lymphocytic leukemia. J Am Acad Dermatol. 1992;27(5 Pt 2):868–871.
512. Arai, E, Ikeda, S, Itoh, S, Katayama, I, et al. Specific skin lesions as the presenting symptom of hairy cell leukemia. Am J Clin Pathol. 1988;90(4):459–464.
513. Lawrence, DM, Sun, NC, Mena, R, et al. Cutaneous lesions in hairy-cell leukemia. Case report and review of the literature. Arch Dermatol. 1983;119(4):322–325.
514. Stawiski, MA. Skin manifestations of leukemias and lymphomas. Cutis. 1978;21(6):814–818.
515. Su, WP, Buechner, SA, Li, CY. Clinicopathologic correlations in leukemia cutis. J Am Acad Dermatol. 1984;11(1):121–128.
516. De Coninck, A, De Hou, MF, Peters, O, et al. Aleukemic leukemia cutis. An unusual presentation of acute myelomonocytic leukemia. Dermatologica. 1986;172(5):272–275.
517. Angulo, J, Haro, R, Gonzalez-Guerra, E, et al. Leukemia cutis presenting as localized cutaneous hyperpigmentation. J Cutan Pathol. 2008;35(7):662–665.
518. Newman, MD, Milgraum, S. Leukemia cutis masquerading as vitiligo. Cutis. 2008;81(2):163–165.
519. Sanz, MA, Larrea, L, Sanz, G, et al. Cutaneous promyelocytic sarcoma at sites of vascular access and marrow aspiration. A characteristic localization of chloromas in acute promyelocytic leukemia? Haematologica. 2000;85(7):758–762.
520. Baer, MR, Barcos, M, Farrell, H, et al. Acute myelogenous leukemia with leukemia cutis. Eighteen cases seen between 1969 and 1986. Cancer. 1989;63(11):2192–2200.
521. Shaikh, BS, Frantz, E, Lookingbill, DP. Histologically proven leukemia cutis carries a poor prognosis in acute nonlymphocytic leukemia. Cutis. 1987;39(1):57–60.
522. Blaustein, JC, Narang, S, Palutke, M, et al. Extramedullary (skin) presentation of acute monocytic leukemia resembling cutaneous lymphoma: morphological and immunological features. J Cutan Pathol. 1987;14(4):232–237.
523. Gil-Mateo, MP, Miquel, FJ, Piris, MA, et al. Aleukemic “leukemia cutis” of monocytic lineage. J Am Acad Dermatol. 1997;36(5 Pt 2):837–840.
524. Ohno, S, Yokoo, T, Ohta, M, et al. Aleukemic leukemia cutis. J Am Acad Dermatol. 1990;22(2 Pt 2):374–377.
525. Taniguchi, S, Hamada, T, Kutsuna, H, et al. Lymphocytic aleukemic leukemia cutis. J Am Acad Dermatol. 1996;35(5 Pt 2):849–850.
526. Buechner, SA, Li, CY, Su, WP. Leukemia cutis. A histopathologic study of 42 cases. Am J Dermatopathol. 1985;7(2):109–119.
527. Wong, TY, Suster, S, Bouffard, D, et al. Histologic spectrum of cutaneous involvement in patients with myelogenous leukemia including the neutrophilic dermatoses. Int J Dermatol. 1995;34(5):323–329.
528. Jones, D, Dorfman, DM, Barnhill, RL, et al. Leukemic vasculitis: a feature of leukemia cutis in some patients. Am J Clin Pathol. 1997;107(6):637–642.
529. Canueto, J, Meseguer-Yebra, C, Roman-Curto, C, et al. Leukemic vasculitis: a rare pattern of leukemia cutis. J Cutan Pathol. 2011;38(4):360–364.
530. Seckin, D, Senol, A, Gurbuz, O, et al. Leukemic vasculitis: an unusual manifestation of leukemia cutis. J Am Acad Dermatol. 2009;61(3):519–521.
531. Bonvalet, D, Foldes, C, Civatte, J. Cutaneous manifestations in chronic lymphocytic leukemia. J Dermatol Surg Oncol. 1984;10(4):278–282.
531a. Magro, CM, Morrison, CD, Heerema, N, et al. T-cell prolymphocytic leukemia: an aggressive T cell malignancy with frequent cutaneous tropism. J Am Acad Dermatol. 2006;55:467–477.
532. Finan, MC, Su, WP, Li, CY. Cutaneous findings in hairy cell leukemia. J Am Acad Dermatol. 1984;11(5 Pt 1):788–797.
533. Cronin, DM, George, TI, Sundram, UN. An updated approach to the diagnosis of myeloid leukemia cutis. Am J Clin Pathol. 2009;132(1):101–110.
534. Canioni, D, Fraitag, S, Thomas, C, et al. Skin lesions revealing neonatal acute leukemias with monocytic differentiation. A report of 3 cases. J Cutan Pathol. 1996;23(3):254–258.
535. Daoud, MS, Snow, JL, Gibson, LE, et al. Aleukemic monocytic leukemia cutis. Mayo Clin Proc. 1996;71(2):166–168.
536. Sires, UI, Mallory, SB, Hess, JL, et al. Cutaneous presentation of juvenile chronic myelogenous leukemia: a diagnostic and therapeutic dilemma. Pediatr Dermatol. 1995;12(4):364–368.
537. Park, YS, Park, SH, Park, SJ, et al. Expression of JL1 is an effective adjunctive marker of leukemia cutis. Arch Pathol Lab Med. 2010;134(1):95–102.
538. Dorfman, DM, Kraus, M, Perez-Atayde, AR, et al. CD99 (p30/32MIC2) immunoreactivity in the diagnosis of leukemia cutis. Mod Pathol. 1997;10(4):283–288.
539. Takai, K, Sanada, M. [Aggressive NK cell leukemia/lymphoma: an autopsy case]. Rinsho Ketsueki. 2001;42(8):621–626.
540. Sundram, UN, Natkunam, Y. Mast cell tryptase and microphthalmia transcription factor effectively discriminate cutaneous mast cell disease from myeloid leukemia cutis. J Cutan Pathol. 2007;34(4):289–295.
541. Thomas, CG, Patel, RM, Bergfeld, WF. Cytophagic and S-100 protein immunoreactive myeloid leukemia cutis. J Cutan Pathol. 2010;37(3):390–395.
542. Bowden, JB, Hebert, AA, Rapini, RP. Dermal hematopoiesis in neonates: report of five cases. J Am Acad Dermatol. 1989;20(6):1104–1110.
543. Hocking, WG, Lazar, GS, Lipsett, JA, Busuttil, RW. Cutaneous extramedullary hematopoiesis following splenectomy for idiopathic myelofibrosis. Am J Med. 1984;76(5):956–968.
544. Argyle, JC, Zone, JJ. Dermal erythropoiesis in a neonate. Arch Dermatol. 1981;117(8):492–494.
545. Hebert, AA, Esterly, NB, Gardner, TH. Dermal erythropoiesis in Rh hemolytic disease of the newborn. J Pediatr. 1985;107(5):799–801.
546. Schwartz, JL, Maniscalco, WM, Lane, AT, et al. Twin transfusion syndrome causing cutaneous erythropoiesis. Pediatrics. 1984;74(4):527–529.
547. Evole-Buselli, M, Hernandez-Marti, MJ, Gasco-Lacalle, B, et al. Neonatal dermal hematopoiesis associated with diffuse neonatal hemangiomatosis. Pediatr Dermatol. 1997;14(5):383–386.
548. Hodl, S, Aubock, L, Reiterer, F, et al. [Blueberry muffin baby: the pathogenesis of cutaneous extramedullary hematopoiesis]. Hautarzt. 2001;52(11):1035–1042.
549. Kwon, KS, Lee, JB, Jang, HS, et al. A case of cutaneous extramedullary hematopoiesis in myelofibrosis with a preponderance of eosinophilic precursor cells. J Dermatol. 1999;26(6):379–384.
550. Tagami, H, Tashima, M, Uehara, N. Myelofibrosis with skin lesions. Br J Dermatol. 1980;102(1):109–112.
551. Green, LK, Klima, M, Burns, TR. Extramedullary hematopoiesis occurring in a hemangioma of the skin. Arch Dermatol. 1988;124(11):1720–1721.
552. Hoss, DM, McNutt, NS. Cutaneous myelofibrosis. J Cutan Pathol. 1992;19(3):221–225.
553. Mizoguchi, M, Kawa, Y, Minami, T, et al. Cutaneous extramedullary hematopoiesis in myelofibrosis. J Am Acad Dermatol. 1990;22(2 Pt 2):351–355.
554. Kuo, T. Cutaneous extramedullary hematopoiesis presenting as leg ulcers. J Am Acad Dermatol. 1981;4(5):592–596.
555. Schofield, JK, Shun, JL, Cerio, R, et al. Cutaneous extramedullary hematopoiesis with a preponderance of atypical megakaryocytes in myelofibrosis. J Am Acad Dermatol. 1990;22(2 Pt 2):334–337.
556. Fraga, GR, Caughron, SK. Cutaneous myelofibrosis with JAK2 V617F mutation: metastasis, not merely extramedullary hematopoiesis!. Am J Dermatopathol. 2010;32(7):727–730.
557. Kawakami, T, Kimura, S, Kato, M, et al. Transforming growth factor-beta overexpression in cutaneous extramedullary hematopoiesis of a patient with myelodysplastic syndrome associated with myelofibrosis. J Am Acad Dermatol. 2008;58(4):703–706.


