Titusville and Tucson
We took our required course in Pennsylvania history in the ninth grade. I remember very little about it beyond the names Joshua Tittery, who was a seventeenth-century potter, and John Wanamaker, the Philadelphia merchant who more or less invented the department store. I remember that we discussed a small town that lies north of Pittsburgh called Titusville, where the first oil well was drilled in 1859. At the time, this hardly seemed like an epochal event to me, and I am sure I went home that afternoon looking forward to a game of touch football or some other activity of true importance.
Much later, I learned that the history of Titusville included a little-known visitor. His name was Dmitri Mendeleev, inventor of the modern periodic table of the elements. In 1876, he was sent by Czar Alexander II to gather information on the flourishing oil industry in the state of Pennsylvania. A record of his experiences in Titusville and elsewhere is given in a document recently acquired by the Chemical Heritage Foundation and still not translated from the original Russian. When I asked about Mendeleev’s visit to her city, she told me that only one other person in the last five years had inquired about him.
Mendeleev’s connection to oil and the oil industry is not well known, even among chemists. When I taught geochemistry, I would spend a few minutes discussing his abiotic theory of oil formation, which claimed that water deep in the earth might be reacting with iron carbides to form hydrocarbons, and that these, through time, might polymerize to generate the many organic compounds found in oil. This was an ingenious idea designed to explain the many mysterious black oozes that have been known for centuries throughout the world and that have been used for medicinal purposes, for lighting lamps, lubricating machinery, and even in the early art of mummification. Now, however, it is well known through the study of biomarkers that oil is a product of the transformation of once-living matter.
But when I think of Mendeleev, it is in terms of the periodic table and the great city of Saint Petersburg. In my sophomore year, that city and the elements came to be joined in my mind. I was taking a course in qualitative analysis and running through the infamous “qual scheme,” with its blue lakes and flocculent precipitates and foul-smelling sulfides that eventually I came to like as though I were some kind of sediment-dwelling anerobe. The professor was particularly keen to point out how the reactions we were witnessing in the laboratory—the rain of calcium, strontium, and barium as white solids out of a pure clear liquid solution—made sense when you thought about their location in Mendeleev’s table.
At the same time, in the gothic lecture halls of Pitt’s Cathedral of Learning, I was enrolled in a literature course on tragedy. The professor, a distinguished Virginia poet by the name of Lawrence Lee, assigned the class some twenty novels, among them Camus’s The Stranger, Stendahl’s The Red and the Black, and an assortment of Russian classics, including Crime and Punishment and Anna Karenina. I had protested to my advisor that I had not wanted to take this requirement or any literature course. I was a chemistry major, after all, so why would I ever need it? Soon, though, I came to love the rich language and the images that were called to mind. Anna Karenina was begun only four years after the periodic law was announced—and when I thought of Mendeleev, I thought always of snow as it fell in St. Petersburg, while it fell just beyond the trolley windows where I read, on the streets of Pittsburgh.
Mendeleev’s early version of the periodic table
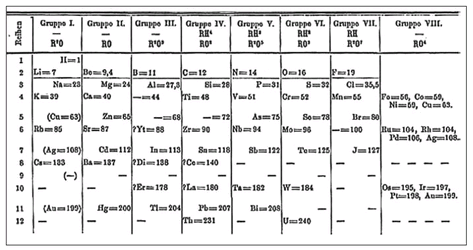
After Lavoisier, there had been a great loosening up of chemistry. The science of matter moved away from its emphasis on the forces existing between atoms and focused instead on something that could actually be measured: weight. The English meteorologist John Dalton provided clear definitions of atom and element and described a method for determining the relative weights of atoms. Careful measurements followed and there were breakthroughs in the isolation of new elements, especially with the development of spectroscopy and electrolysis. By Mendeleyev’s time there were sixty-three known elements and they displayed an array of properties that must have seemed to the casual observer stupefying at best. Some were gases, like oxygen and hydrogen; others were dense hard metals, like iron and nickel; still others, like lithium, floated like matchsticks on water. There was silvery mercury, a metal that pooled and skittered along the floor and whose very nature, to the Chinese emperors and the European alchemists, was a mystery. Some elements were highly reactive, like fluorine, which could etch glass and burn skin down to the bone, and others, like gold, were famous for their glittering inertness. There was, moreover, a long range of atomic weights that stretched from hydrogen, with a relative weight of 1, to uranium, whose atomic weight had been measured at 238.
How was it possible, in 1869, to make sense of all of this? Were there only sixty-three elements? Were there thousands more that no one knew about? Was the number of elements that made up the universe infinite? I have always found this the most vexing problem, the question of knowing whether there was any limit to the variety of particles that constitute the physical world or whether a little more probing might not just bring to light a snowstorm’s abundance of unique atomic forms, numerous as the shapes of crystals that fall from the winter skies. Lavoisier, as he often did, summarized the situation as it was in his day: “All that can be said upon the nature and number of the elements is confined to discussions of an entirely metaphysical nature. The subject only furnishes us with indefinite problems.” If the elements had given Lavoisier a headache, they must have given Mendeleev a migraine.
In West Lafayette, my friend Harv, with whom I was taking inorganic chemistry, would say, “It’s all a bunch of memorization, a bunch of rules. Nothing ever gets explained in any deep, satisfying way until physical chemistry comes along.” In part he was right, but I never thought of the periodic table in this way. To me it was a great plateau in chemistry’s long ascent, a place that when you finally reached it you could see order in the bare landscape over which you had been struggling. It was so much more than just a chart on a wall, more than a thing to be sung out with mnemonics like the scales you had learned as a kid. I would tell Harv, “There’s real beauty here. There’s no way to overemphasize what Mendeleev gave us.” And he would tell me, “If you want real beauty, real creativity, look to Schrödinger or to Pauli or Boltzmann.” He scoffed at anything that had to be memorized.
But what so impressed me about Mendeleev was the apparent simplicity of his approach. It was like Kepler seeing into the heart of the solar system, finding the rules that governed the planets, the elliptical paths from which so much that was baroque and unnecessary in the Copernican system just fell away. Or Newton condensing much of physics into the crafted parsimony of the Principia. Mendeleev arranged his elements in order of their atomic number, but instead of stretching them out in a long sequence from 1 to 238, he noted a tendency for repetition. For example, the element that follows fluorine in order of atomic weights is sodium (neon would not be discovered until 1898), and Mendeleev placed it in a column beneath lithium. The two have similar physical and chemical properties and are rightly placed in a common family. The next element, magnesium, shares characteristics with beryllium and so it was positioned below it in the table.
This use of themes and repetition may well have been inspired by the music of Robert Schumann. By his own account, Mendeleev and his wife and children occasionally gathered to play music at the family home. One evening they performed the Piano Quintet in E flat major composed by Schumann in 1842. The abrupt stops and the frequent repetitions associated with that piece were precisely the ethereal goad that Mendeleev needed to set down onto paper the rough sketch of his table. Hearing this reminded me of August Kekulé’s famous dream of whirling snakes with tails in their mouths, a self-described dream from which came the ring structure of benzene.
A deep unfathomable creativity lies in the images that come to the prepared and waiting mind. Ideas, theories, and hypotheses in science derive from strange places: dreams and shifting ice flows, symphonies, the pure luck of a conversation in the park or in some stuffy office, Platonic musings on ideal worlds, chandeliers moving on an autumn breeze.
But beyond the image, as always in science, there must be tests, the hard stones of fact, proof, logic, and sometimes mockery and bitter debate. And it was like this for Mendeleev. But his genius lay not only in the conception but in the argument that would win him international fame even by the time he had arrived in Titusville.
His strategy, one that no one before him had employed, was to leave gaps in the table and to predict the existence of elements as yet undiscovered, and then to go even further and predict their properties. It has always sounded crazy to me, like walking off into thin air with the hope that some jutting rock will project out beneath your feet. But a great theory takes chances. If you had lined up the elements by atomic weight in Mendeleev’s day, you would have found that beyond zinc (atomic weight = 65.37) lay arsenic (atomic weight = 74.92). Mendeleev could have chosen to place this in his table just below aluminum. But realizing that aluminum and arsenic were just too different in their properties to share a common family, he decided that there must be another element that would fit beneath aluminum, an element which would in many ways resemble it more closely. He gave this unknown entity a name: eka-aluminum. As far as anyone knew, it did not exist.
He went even further. He assigned eka-aluminum a set of properties. He predicted it would have an atomic weight of 68, a density of 5.9, and a low boiling point. He described the kind of compounds it should form with oxygen. The confirmation took a few years, but in 1875, Lecoq de Boisbaudran came upon a new element in the midst of a zinc deposit in the Pyrenees. He called it gallium, after the Latin name for France, and his careful measurements showed that it had an atomic weight of 69.9, a density of 5.93, and a melting point of 30.1 degrees Celsius. It was indeed the eka-aluminum that Mendeleev had predicted.
There were other successes along these lines. Mendeleev knew there should be, somewhere in the world, an element to fill in the blank space he had left beneath silicon. He called it eka-silicon, predicted its properties, including its detailed chemistry, and in 1887, Clemens Winckler, in Freiburg, discovered an element that had nearly the properties Mendeleev had foretold. He called it germanium. These and other predictions were convincing evidence that there was indeed an order to the building elements of the physical world, and that the fears of Lavoisier, that this misguided quest would result only in an “indefinite confusion,” were happily far from the mark.
Exactly where all of these elements had come from, why there were so many when one—a single primordial hydrogen atom, round and small and perfectly made—would have easily sufficed, and what deeper underlying processes were at work to insure the structure of Mendeleev’s table—these were questions whose answers would come much later, in our own time.
The road west of Tucson takes you out past the Saguraro National Park, with its stately exotic cactus, toward tall mountains that in November are brown and barren. Against snow high above the road, the white domes of the telescopes emerge like a string of coral stretched across the highest ridge.
The ascent is steep and edged by deep valleys whose desert floors would take only one mistaken turn to arrive at with ease. There are switchbacks and scenic overlooks with signs that tell you the top is only five miles away. The road is just twelve miles long, but Wanda is looking grim, with one of those expressions that says: “Remind me again‚why are we doing this?”
The sign near the parking lot reads:
Welcome
Kitt Peak National Laboratory
Operated by the
Association of Universities
for
Research in Astronomy
Under Cooperative Agreement with
National Science Foundation
The names of the twenty-two participating universities follow.
By the visitor’s center, there is a large, colorful mural done in bright oranges and blues and yellows, a representation of the heavens as envisioned by the Mayans. Wanda is feeling in her element now and may not even be regretting the trip up here.
Kitt Peak Observatory is located on the Tohono O’odham Reservation, fifty-eight miles to the southwest of Tucson. At an elevation of roughly seven thousand feet, it rises high above the Sonoran Desert and is considered a sacred mountain by the Tohono people. To even build this facility required delicate negotiations, which included a telescopic viewing of the moon by the tribal elders, who eventually gave their consent but placed constraints upon the use of their revered mountain. The facility includes twenty-four optical and two radio telescopes and the world’s largest solar telescope. This last instrument was dedicated in 1962 by President John Kennedy, who called it “a source of pride to the nation.”
I went with a small group to view this telescope and to hear about the kind of research that was being conducted here. The McMath-Pierce telescope is a huge piece of equipment. The main tower, on which the heliostat is located, is one hundred feet tall. The mirror atop this tower tracks the movement of the sun throughout the day and focuses a beam of sunlight down a two-hundred-foot shaft, which extends deep into the mountain itself. The light is eventually focused into a viewing lab beneath ground, where, through the use of spectroscopes, it can be used to examine the structure of sunspots, which may be thousands of miles in diameter, or to determine the spectra of various elements. The McMath-Pierce was the first telescope to detect the presence of water vapor in the sun and to discover isotopic helium.
In the viewing room, I was surprised to learn that seventy elements have been identified in the sun. Of course, hydrogen (91.2 percent) and helium (8.7 percent) account for most of the sun’s mass, but there are also significant quantities of oxygen, carbon, nitrogen, sulfur, magnesium, and iron present, in addition to a host of lesser elements. The day I visited, the two scientists there were looking at the spectrum of helium in the sun’s photosphere. The photosphere is the outermost region of the sun. It is where our light and heat come from. It is what sustains us, nurtures us, gives us warmth.
I have always thought it interesting that we found helium on the Sun before we found it on Earth. Using the newly invented spectroscope of Bunsen and Kirchhoff, Norman Lockyer found two absorption lines in 1868 that did not correspond to any known element. He knew he had a new element and he named it after the Greek sun god, Helios. Twenty-five years later the element was found on earth by Sir William Ramsay, in the mineral cleveite. On earth, most of this rare inert gas is produced from the alpha-decay of uranium and thorium.
Two of the telescopes at the Kitt Peak National Observatory
On the sacred mountain high above the desert, above the tall saguaro, among the white domed scopes of Kitt Peak, your thoughts turn naturally to the vast universe whose very nature is the subject of all that is here. All the negotiations, the hard sweat and labor of construction, the building of the sinuous road we had just ascended, the instruments of discovery—all of these are here to serve a single end. So in this setting it is easy to think about large questions, to wonder about beginnings and ends, births and deaths, about origins and the way things are put together.
There are 70 elements in the sun; 114 elements are known to science, and all have been placed in the table. Mendeleev knew of only 63. Where all of these came from is a story that joins the chemistry of matter to the behavior of the distant stars.
From the mountain, you look south toward Mexico, west toward California. Below is the desert. All around are the domes of the telescopes, the chute that guides the sun’s light into the earth and the spectrographs. Everywhere there is matter piled upon matter, element upon element, the rock stability of the silicates anchoring the carbon spines of saguaro in the distance below. The fugacious atmosphere, rich with its gases, moves the labeled pines, and the chromium and cobalt of the Mayan mural catches the light of the sun. In the beginning, a few short minutes after the beginning, there were only two elements, hydrogen and helium, and these alone swirled and twisted in streamers and clouds through the newly minted universe. From these, everything that we see up here came and made possible the habitable and uninhabitable worlds, the ringed and jeweled and moon-filled worlds whose presence is a thing of wonder.
There is a story that has passed many tests, that shades into truth and has become truth, that all of this was born in the forges and furnaces of the stars, in the pulse of collapse and expansion, and in the unimaginable violence of explosion. It is this explosion that scatters the wealth of elements, as a beneficent hand might scatter gold coins.
The process by which new elements are made, brought into being in the dust storm of creation, is called nucleosynthesis. We know now that stars—like the out-rushing, fourteen-billion-year-old universe itself, the expanding red-shifted universe of Edwin’s Hubble’s observations on Mount Wilson—evolve.
A star forms from the tenuous clouds of hydrogen and helium that comprise a nebula. A nebular cloud is, in effect, the birthplace of stars and the locus of beauty to rival any landscape. The Eagle Nebula, with its three pillars, like the lonely tufas of Mono Lake, or the conch shells and rings of the Cat’s Eye Nebula, are reminders of nature’s boundless extent beyond the park boundaries and the scenic overlooks of our more common experience.
Through the Hubble, that masterpiece of engineering and craft, you can virtually see star birth occurring before your eyes. Here, dense regions of gas undergo collapse to form a rotating globule, and temperature and pressure increase. At the core of the rotating mass, temperatures soon reach the fusion point. At the periphery of the rotating disk, planets often form, as in our own solar system.
At the core of stars, temperatures are in the millions of degrees. The star’s core is a place of relentless burning, the fires and light of the heavens. But the fuel is not the ordinary oils of Mendeleev or the coke of steel. It is the simplest of all elements, a single proton endlessly circled by an electron, or, as we now know, by a tiny cloud, denser here, thinner there, a patchwork of probabilities. At the temperatures of the core, hydrogen can undergo fusion and become, through a chain of reactions, the element helium, which has two protons and two neutrons in its nucleus. It is this hydrogen burning that keeps the sun from collapse. Always, in any star, it is the balance of these two, the outward pressure from the burning and the inward force of gravity, that maintains a kind of equilibrium. Our own sun, which is five billion years old, has enough hydrogen to maintain this balance for another five billion years.
Most of a star’s lifetime—an estimated 99 percent—is spent in the conversion of hydrogen to helium at about ten million degrees Kelvin. But as the star evolves, higher elements are created in an environment no Cotton Mather of hell and brimstone fame could have ever envisioned. The making of higher elements requires more extreme temperatures because fusion involves bringing together nuclei of ever greater positive charge. When hydrogen at the core is finally exhausted, converted into the invisible ash of helium, the star begins to collapse under its own gravitational mass. This process generates enormous heat and enormous nuclear velocities, which are sufficient to overcome the electrostatic repulsion and to allow for the fusion of helium into carbon. This process requires about one hundred million degrees. Heavier elements—oxygen, for example—are created in a similar way, but at still higher temperatures.
The nuclear reactions needed to build the bricks and mortar of the material world up to uranium are far too numerous to list here. But it is interesting to note that nickel-56 is the heaviest element generated by the addition of a helium nucleus (alpha particle). This unstable form of nickel then eventually decays into iron, an element that is especially abundant in our solar system. On earth, it is the element of building and progress, of the ribbed steel and girders of New York and Chicago and Pittsburgh, stacked toward the sky.
Elements more massive than iron often form by the slow process of neutron capture (s-process) or by the more rapid capture process (r-process) frequently associated with a supernova. When the core of a star contains mostly iron and can no longer undergo significant fusion, the star collapses. It is estimated that such a collapse takes less than a second. The resulting explosion sends atoms in a great mist across the universe, and these atoms, in a generous act of cosmic recycling, become part of the nebular cloud from which new stars are born. Our sun is thought to be a third-generation star formed, in part, from the elemental debris of earlier starbursts. Some of the heavier elements from which our solar system was formed came from this violence of long ago.
Toward the end of our day, we stopped again at the visitor’s center. For sale at the counter was a small telescope, a replica of the one Galileo had used for his book The Starry Messenger.
Nearby, there was a framed quote from Johannes Kepler. It read:
We do not know for what purpose the birds do sing, for their song is pleasure since they were created for singing. Similarly, we ought not ask why the human mind troubles to fathom the secrets of the heavens. The diversity of the phenomena of Nature is so great and the treasures hidden in the heavens so rich, precisely in order that the human mind shall never be lacking in fresh nourishment.
At Kitt Peak, who could help but agree. For me, it was a pleasure to see the two great astronomers, who had had never met in life, side by side, honored here together on the mountain.
Shortly after Tucson, Wanda and I were in Titusville, Pennsylvania. We stayed for only a day. We walked around the grounds, explored the well that Colonel Edwin Drake had drilled. His crew struck oil in 1859 at a depth of 69.5 feet. There was great celebration at this find—the first oil well in the world. Titusville became a boomtown overnight. Wanda mentioned that the smell of oil was still in the air. There was even a faint taste of it on my tongue.
In the park, there were drilling rigs from different periods, showing the evolution of the industry. Huge flywheels, “dunking birds,” derricks, cast-iron boilers, spindly rods connected to pipes. There were photos of the men, long gone, who had worked the fields. The drillers and tool pressers, roughnecks and roustabouts—the standard crew. Amid the machines that had transformed the world, had changed for centuries to come the thin chemistry of the atmosphere, there was cropped green grass and broad-leafed rhododendron and black oak. The leaves of mid-November had all fallen. Wanda said, “It looks like a sculpture garden.”
Though there was no mention on any of the signs of Dmitri Mendeleev, you could imagine how the place looked when he came here. In time, it would be learned what oil contained. Mostly it was carbon and hydrogen and some oxygen arranged in thousands of compounds, but there were heavy metals, too, and many of them had been precipitated by sulfides from the waters of ancient seas.
It was a pleasant, overcast November day, and we walked with our coats opened. Before we left, I took a picture of Oil Creek. It was broad, smooth, and dark. Across its waters, a mountain of star-fused elements rose straight up from the shore.


