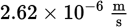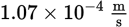Discrete Practice Questions
-
If the work function of a metal is 6.622 × 10–20 J and a ray of electromagnetic radiation with a frequency of 1.0 × 1014 Hz is incident on the metal, what will be the speed of the electrons ejected from the metal? (Note: h = 6.626 × 10−34 J·s and me− = 9.1 × 10−31 kg)
-
-
What is the wavelength of a photon that causes an electron to be emitted from a metal with a kinetic energy of 50 J? (Note: The work function of the metal is 16 J, and h = 6.626 × 10−34 J·s)
- 1.0 × 10−34 m
- 3.0 × 10−27 m
- 3.0 × 10−26 m
- 1.0 × 1035 m
-
Which of the following statements is inconsistent with the Bohr model of the atom?
- Energy levels of the electron are stable and discrete.
- An electron emits or absorbs radiation only when making a transition from one energy level to another.
- To jump from a lower energy to a higher energy orbit, an electron must absorb a photon of precisely the right frequency such that the photon’s energy equals the energy difference between the two orbits.
- To jump from a higher energy to a lower energy orbit, an electron absorbs a photon of a frequency such that the photon’s energy is exactly the energy difference between the two orbits.
-
When a hydrogen atom electron falls to the ground state from the n = 2 state, 10.2 eV of energy is emitted. What is the wavelength of this radiation? (Note: 1 eV = 1.60 × 10−19 J, and h = 6.626 × 10−34 J·s)
- 5.76 × 10−9 m
- 1.22 × 10−7 m
- 3.45 × 10−7 m
- 2.5 × 1015 m
-
The figure below illustrates an electron with initial energy of –10 eV moving from point A to point B. What change accompanies the movement of the electron?
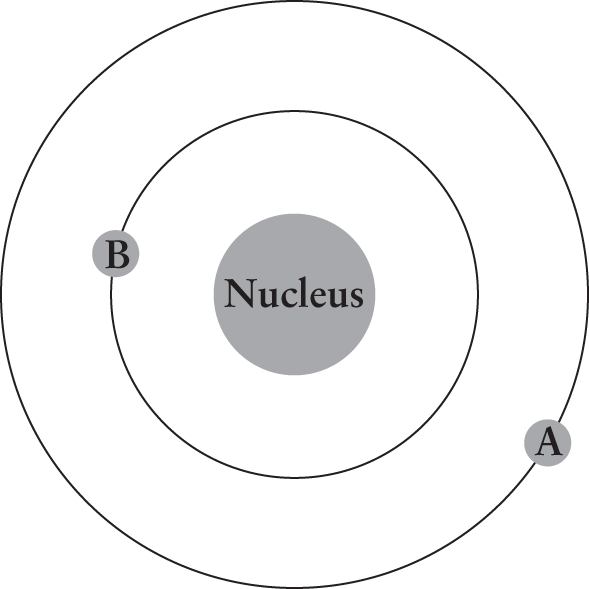
- Absorption of a photon
- Emission of a photon
- Decrease in the atom’s work function
- Increase in the atom’s total energy
-
Ultraviolet light is more likely to induce a current in a metal than visible light. This is because photons of ultraviolet light:
- have a longer wavelength.
- have a higher velocity.
- are not visible.
- have a higher energy.
-
All of the following statements about the photoelectric effect are true EXCEPT:
- the intensity of the light beam does not affect the photocurrent.
- the kinetic energies of the emitted electrons do not depend on the light intensity.
- a weak beam of light of frequency greater than the threshold frequency yields more current than an intense beam of light of frequency lower than the threshold frequency.
- for light of a given frequency, the kinetic energy of emitted electrons increases as the value of the work function decreases.
-
What is the binding energy of the argon-40 isotope in MeV? (Note: mproton = 1.0073 amu, mneutron = 1.0087 amu, mAr-40 nucleus = 39.9132 amu,
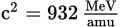 )
)- 0.4096 MeV
- 40.3228 MeV
- 381.7 MeV
- 643.8 MeV
-
Which of the following correctly identifies the following process?
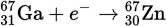
- β− decay
- β+ decay
- e− capture
- γ decay
-
Consider the following fission reaction.

The masses of the species involved are given in atomic mass units below each species, and 1 amu can create 932 MeV of energy. What is the energy liberated due to transformation of mass into energy during this reaction?
- 0.003 MeV
- 1.4 MeV
- 2.8 MeV
- 5.6 MeV
-
Element X is radioactive and decays via α decay with a half-life of four days. If 12.5 percent of an original sample of element X remains after n days, what is the value of n?
- 4
- 8
- 12
- 16
-
A graph of an exponential decay process is created. The y-axis is the natural logarithm of the ratio of the number of intact nuclei at a given time to the number of intact nuclei at time t = 0. The x-axis is time. What does the slope of such a graph represent?
- λ
- − λ
- e−λt
-

-
A certain carbon nucleus dissociates completely into α-particles. How many particles are formed?
- 1
- 2
- 3
- 4
-
The half-life of carbon-14 is approximately 5,730 years, while the half-life of carbon-12 is essentially infinite. If the ratio of carbon-14 to carbon-12 in a certain sample is 25% less than the normal ratio in nature, how old is the sample?
- Less than 5,730 years
- Approximately 5,730 years
- Significantly greater than 5,730 years, but less than 11,460 years
- Approximately 11,460 years
-
A nuclide undergoes two alpha decays, two positron decays, and two gamma decays. What is the difference between the atomic number of the parent nuclide and the atomic number of the daughter nuclide?
- 0
- 2
- 4
- 6