Discrete Practice Answers
- C
To determine the speed of the electrons ejected, we must first calculate their kinetic energy:
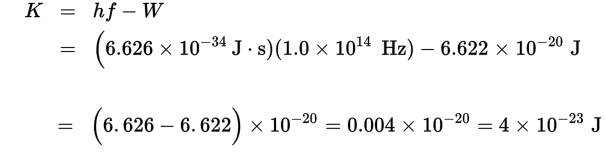
Now with a value for kinetic energy, we can calculate the speed of the ejected electrons:
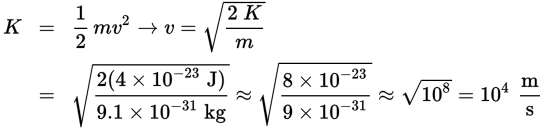
Notice the wide range in the exponents for the answer choices. While the math in this question may seem complex, this allows us to round significantly.
- B
To determine the wavelength of the light ray, first calculate its frequency from the photoelectric effect equation:

In this case, estimation of Planck's constant makes our calculation much simpler without leading us to a nonviable answer. It is worth attempting an estimation first to avoid doing more work than necessary. Now we can determine the wavelength of the incident ray of light by relating the frequency to the speed of light:

- D
The Bohr model is based on a set of postulates originally put forward to discuss the behavior of electrons in hydrogen. In summary, these postulates state that the energy levels of the electron are stable and discrete, and they correspond to specific orbits, eliminating (A). They also state that an electron emits or absorbs radiation only when making a transition from one energy level to another, eliminating (B). Specifically, when an electron jumps from a lower-energy orbit to a higher-energy one, it must absorb a photon of light of precisely the right frequency such that the photon’s energy equals the energy difference between the two orbits, eliminating (C). When falling from a higher-energy orbit to a lower-energy one, an electron emits a photon of light with a frequency that corresponds to the energy difference between the two orbits, This is the opposite of (D), which makes it the right answer.
- B
To solve this question correctly, one must be careful with the units. First, convert 10.2 eV to joules:

Next, to determine the wavelength of the radiation, we can combine the formulas E = hf and c = f λ:
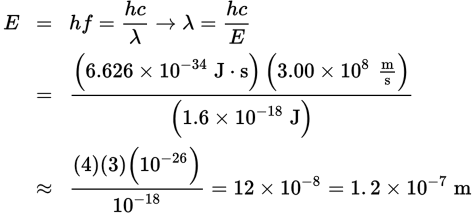
- B
The electron moves from a higher energy level to a lower energy level; this can only occur if the extra energy is dissipated through the emission of a photon. If the electron moved from B to A, it would absorb a photon and increase the atom’s total energy; however, the opposite is occurring, so (A) and (D) can be eliminated. The work function is the amount of energy required to eject an electron from a material; when moving from A to B, the electrical potential energy of the atom decreases, meaning that more energy will be required to free the electron from the atom, eliminating (C).
- D
The photoelectric effect occurs when a photon of sufficiently high energy strikes an atom with a sufficiently low work function. This means that a photon with higher energy is more likely to produce the effect. Because ultraviolet light has a higher frequency and lower wavelength than visible light, it also carries more energy according to the equation E = hf. All light travels at the speed of light, eliminating (B). As mentioned earlier, ultraviolet light has a shorter wavelength than visible light, eliminating (A). The visibility of a wave plays no role in its ability to cause the photoelectric effect, eliminating (C).
- A
The greater the intensity, the greater the number of incident photons and, therefore, the greater the number of electrons that will be ejected from the metal surface (provided that the frequency of the light remains above the threshold). This means a larger current. Remember that the frequency of the light (assuming it is above the threshold frequency) will determine the kinetic energy of the ejected electrons; the intensity of the light determines the number of electrons ejected per time (the current).
- C
To determine the binding energy, we must first determine the mass defect. The mass defect is simply the masses of each of the protons and neutrons in the unbound state added together minus the mass of the formed argon-40 nucleus (which contains 18 protons and 40 − 18 = 22 neutrons):

This math was difficult without a calculator, but by rounding one value down (proton) and one value up (neutron) by similar amounts we ended up very near the actual value. Calculating 18 x 7 = 126 and 22 x 9 = 198 for the decimal values is more manageable than the exact numbers and the spacing of the answer choices allows for our estimation. The binding energy can then be determined from this mass defect:

The closest answer is (C).
- C
This process can be described as electron capture. Certain unstable radionuclides are capable of capturing an inner electron that combines with a proton to form a neutron. The atomic number becomes one less than the original, but the mass number remains the same. Electron capture is a relatively rare process and can be thought of as the reverse of β− decay. Notice that the equation is similar to that of β+ decay but not identical because a particle is absorbed, not emitted.
- C
This problem presents a reaction and asks for the energy liberated due to transformation of mass into energy. To convert mass into energy, we are told that 1 amu can be converted into 932 MeV of energy. All we need to do now is calculate how much mass, in amu, is converted in the reaction. Because we are given the atomic mass for each of the elements in the reaction, this is simply a matter of balancing the equation:

Given both the small magnitude of this value and the small difference of the answer choices, it is best to not round at this point of the calculation. This is the amount of mass that has been converted into energy. To obtain energy from mass, we have to multiply by the conversion factor (1 amu = 932 MeV):
E = 0.003 × 932 ≈ 0.003 × 900 = 2.7 MeVAt this point we were able to round for an easier calculation that keeps us very near the correct answer choice.
- C
Because the half-life of element X is four days, 50 percent of an original sample remains after four days, 25% remains after eight days, and 12.5% remains after 12 days. Therefore, n = 12 days. Another approach is to set
 where x is the number of half-lives that have elapsed. Solving for x gives x = 3. Thus, 3 half-lives have elapsed, and because each half-life is four days, we know that n = 12 days.
where x is the number of half-lives that have elapsed. Solving for x gives x = 3. Thus, 3 half-lives have elapsed, and because each half-life is four days, we know that n = 12 days. - B
The expression n = n0e−λt is equivalent to
 Taking the natural logarithm of both sides, we get:
Taking the natural logarithm of both sides, we get:
From this expression, it is clear that plotting
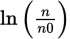 vs. t will give a straight line with a slope of −λ.
vs. t will give a straight line with a slope of −λ. - C
A typical carbon nucleus contains 6 protons and 6 neutrons. An α-particle contains 2 protons and 2 neutrons. Therefore, one carbon nucleus can dissociate into
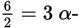 particles.
particles. - A
Because the half-life of carbon-12 is essentially infinite, a 25% decrease in the ratio of carbon-14 to carbon-12 means the same as a 25% decrease in the amount of carbon-14. If less than half of the carbon-14 has deteriorated, then less than one half-life has elapsed. Therefore, the sample is less than 5,730 years old. Be careful with the wording here—the question states that the ratio is 25% less than the ratio in nature, not 25% of the ratio in nature, which would correspond to (D).
- D
In alpha decay, an element loses two protons. In positron decay, a proton is converted into a neutron. Gamma decay has no impact on the atomic number of the nuclide. Therefore, two alpha decays and two positron decays will yield a daughter nuclide with six fewer protons than the parent nuclide.