Discrete Practice Answers
- B
This question, while overtly testing the ability to use scientific notation, is also checking on the appropriate use of significant digits. Because there is no decimal point, the last zero is not significant and should not be used in scientific notation. The significand in scientific notation should always be between one and ten. - C
Significant digits include all nonzero digits, all zeroes that are between nonzero digits, and trailing zeroes in any number with a decimal point. In 14,320,010 there is no decimal point; thus, the last zero is insignificant and there are seven significant digits. In 3.618000, all of the digits are significant; thus there are also seven significant digits. - B
While all digits are preserved during calculations, the final determination of the number of digits is made by both significant figures and decimal places. During multiplication, the answer is maintained to the smallest number of significant digits. During addition, it is maintained to the smallest number of decimal places. By following the order of operations, addition is the last operation; thus we cannot have a decimal in our answer choice. Because multiplication occurred earlier, the result of that multiplication may be shortened according to the two significant figures in 4.10, but not the entire answer. - A
When estimating the product of two numbers, it is best to round one up while rounding the other down, as in (A). (B) and (D) each round both numbers in the same direction, which would increase the amount of error in the answer. (C) rounds the numbers in opposite directions, but the degree of rounding is significantly larger than in (A) and too extreme for answer choices that differ by small amounts. - A
The fourth root of a number, or a number raised to the one-quarter power, is the square root of the square root of that number:

The square root of 200 should be a bit larger than 14 (142 = 196); therefore, the fourth root of 200 should be a bit less than 4.
- D
Raising an exponent to another exponent requires multiplying the exponents. Thus, (A3)2 = A6. - A
The relationship between the natural logarithm of a number and the common logarithm of a number is  Therefore, the natural logarithm of a number must be divided by the constant 2.303
to obtain the common logarithm of the same number.
Therefore, the natural logarithm of a number must be divided by the constant 2.303
to obtain the common logarithm of the same number.
- A
The minimum value of the cosine function is −1 (cos 180° = −1). Therefore, the minimum value of 2 cos θ – 1 is 2 × (−1) −1 = −3. - D
sin θ ≠ sin (90° − θ), although sin θ = cos (90° − θ). The other statements must all be true. Because sine and cosine values are always between −1 and 1, the product of sine and cosine will always have a magnitude less than 1. The sum of the absolute value of sine and the absolute value of cosine, on the other hand, will always be greater than 1. Therefore, (A) can be eliminated. Because sine is the ratio of opposite to hypotenuse and cosine is the ratio of adjacent to hypotenuse, the quotient between the two is the ratio of opposite to adjacent, or the tangent of the angle. Therefore, (B) can be eliminated. By the same logic, because sin 90° = 1 and cos 90° = 0, tan 90° is undefined, eliminating (C). - B
This question involves both a unit conversion between millimolar values and molar values, and calculation of a logarithm. The relationship between pH and pKa is described by the Henderson–Hasselbalch equation given in the question stem. 100 mM = 0.1 M, so

- B
This question requires not only unit conversions, but algebra as well. Given that
 the temperature T can calculated as:
the temperature T can calculated as:

However, the answers are given in kelvin. −40°C + 273 = 233 K.
- D
In a direct relationship, a change in one of the variables will be associated with a proportional change in the other. Because the pressure was multiplied by  the temperature must also be multiplied by
the temperature must also be multiplied by
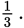 Note that the fractional relationships can only be used with temperatures in kelvins.
Note that the fractional relationships can only be used with temperatures in kelvins.
- C
Because grams are a unit of mass and pounds are a unit of force, we must first convert pounds to newtons, and then divide by the acceleration due to gravity to find kilograms. The weight of the person in newtons is

This corresponds to a mass of

Now, we can determine the dose:
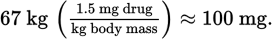
- C
According to the question stem, the rate of a reaction is measured as a change in concentration over time, and thus has the units
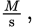 where M (molarity) is measured in moles per liter. However, the rate of the reaction is equal
to a rate constant times the concentrations of certain reactants squared. In this
case, we know the units of everything except the rate constant and must solve for
its units:
where M (molarity) is measured in moles per liter. However, the rate of the reaction is equal
to a rate constant times the concentrations of certain reactants squared. In this
case, we know the units of everything except the rate constant and must solve for
its units:

- C
This is a system of equations couched in data. From this information, we can construct two equations:

These equations can be solved by setting them equal:
