5 ADULT NEUROGENESIS
The fine structure of the nervous system was a subject of heated debate throughout the nineteenth century. In the late 1830s, after viewing plant and animal tissues under the microscope, the German scientists Theodor Schwann and Matthias Schleiden proposed that cells are the basic building blocks of all living things, a view that came to be called the cell theory. But the microscopes available at the time were not powerful enough to resolve synapses, which measure approximately 20 to 40 nanometers (nm, or billionths of a meter), and so it remained unclear whether this also applied to the nervous system.
Investigators were split into two camps. Some believed the brain and spinal cord consisted of a reticulum, or a continuous network of tissue, while others argued that the nervous system, like all other living things, must be made up of cells. With improvements in microscopy and the methods for staining and visualizing their samples, investigators could view nervous tissue in increasing detail, and by the turn of the twentieth century, the long-standing debate was finally settled.1
Thanks largely to the work of Cajal, researchers came to accept the so-called neuron doctrine, which stated that specialized cells called neurons are the basic structural and functional units of the brain and spinal cord. Cajal and others had studied how the nervous system of man and other animals develops, and described the various stages through which neurons pass as they are maturing: birth by cell division, followed by migration of the daughter cells, growth and extension of their fibers, and, finally, the precise formation of synaptic connections. Because they never saw immature neurons in adults, they concluded that brain structure becomes fixed soon after birth.
In his 1913 book, Degeneration and Regeneration of the Nervous System, Cajal stated that the neural pathways in the adult brain and spinal cord are “something fixed, ended, and immutable.” This conclusion came to be widely accepted, and before long, the idea that the adult mammalian brain does not create new cells became a central dogma of modern neuroscience. Most researchers agreed that while vast amounts of neurons and glial cells are generated during development, this process ends in the period just after birth. Thus, it followed that we are born with all the brain cells we will ever have, and that those that are lost through injury or disease can never be replaced.
This dogma persisted for the best part of a century, even though evidence challenging the idea began to emerge in the early 1960s, following the introduction of a technique called [3H]-thymidine (or tritiated thymidine autoradiography. In this process animals are injected with radioactive thymidine, which is taken up by cells and incorporated into the newly synthesized DNA found in newborn cells. Their brains are then dissected, and X-rays are used to detect any radioactivity.2
Joseph Altman and Gopal Das of the Massachusetts Institute of Technology began using this technique to examine various animal species, and they soon published evidence of the growth of new brain cells in the dentate gyrus, olfactory bulb, and cerebral cortex of the rat, and also in the cortex of the cat.3 These initial findings were independently replicated and confirmed by others in the early 1980s, but they were met with skepticism by the scientific community, and largely ignored. 4,5
Soon, more evidence came from the brains of songbirds. Sexually mature male canaries learn a new song every year, in order to serenade potential mates, and learning and production of their songs are controlled by two brain nuclei. Fernando Nottebohm of Rockefeller University performed a series of experiments which showed that the size of these brain nuclei fluctuate with the seasons: both were found to be substantially larger in the spring than in the fall.
Nottebohm hypothesized that these fluctuations were due to an increase and then a reduction in the number of synapses and neurons within the song-producing nuclei. When mating season ends, large numbers of neurons die off, causing the nuclei to shrink; in the spring, however, the nuclei are regenerated by the production of new neurons, so that the bird can learn to sing once again. Nottebohm had not only discovered a clear and direct link between brain and behavior, but his results also “showed beyond reasonable doubt that neurons are born in adulthood and incorporated into existing circuits.”6,7
A series of advances and discoveries finally broke the long-standing conviction that the mammalian brain lacks the ability to regenerate itself. In the late 1980s, Elizabeth Gould and her colleagues at Princeton University began to publish evidence of newborn neurons in the hippocampus of adult rats and then, some time later, in both the hippocampus and cerebral cortex of macaque monkeys. Evolutionarily, monkeys are far more closely related to humans than rats are, and so this raised hopes that the human brain may also continue to form new cells throughout life.8
The development of new techniques using fluorescently labeled antibodies that bind to specific cellular proteins enabled researchers to distinguish between the neurons and glial cells in their tissue samples. In 1992, a pair of researchers at the University of Calgary in Alberta, Canada used these methods to identify and then isolate neural stem cells from the brains of adult mice.9 Neural stem cells are said to be “multipotent” because they retain their undifferentiated, embryonic state and can go on to form any type of cell found in the brain. But they divide asymmetrically, so, while they give rise to new neurons and glial cells, they can also renew themselves indefinitely.
Subsequent research revealed that the brains of adult mice and rats contain two discrete populations of neural stem cells. During early development, the nervous system consists of a hollow tube running along the back of the embryo, and the inner lining of this neural tube is packed with stem cells, which divide to produce immature neurons that migrate through the thickness of the tube. At the front end of the tube, successive waves of migrating cells jostle past each other to form the layers of the cerebral cortex, one after the other, from the inside out. Further back, smaller numbers of cells migrate outward to form the spinal cord.
In adults, neural stem cells are restricted to two discrete niches within the walls of the lateral ventricles: the subventricular zone, which creates cells that migrate through the rostral migratory stream to the tip of the olfactory bulb, and the dentate gyrus of the hippocampus, whose new cells stay near their birthplace and differentiate into granule neurons.10
The neurons formed in these niches appear to be critical for brain function and behavior. Experiments using genetic engineering to kill off newly generated cells as soon as they are born, or at a specific time point in the animals’ lives, show that the addition of new neurons to the olfactory bulb is essential for the formation of new smell memories, while those added to the hippocampus contribute to spatial memory, object recognition, and pattern separation, the process by which the brain distinguishes between similar patterns of neural activity.11
Certain environmental factors can regulate the process to dramatically affect the rate at which new neurons are produced. For example, physical activity, environmental enrichment, and learning tasks enhance the proliferation of neural stem cells and, in some cases, promote the survival of newborn neurons, whereas stress, certain types of inflammation, and sensory deprivation have the opposite effect.12
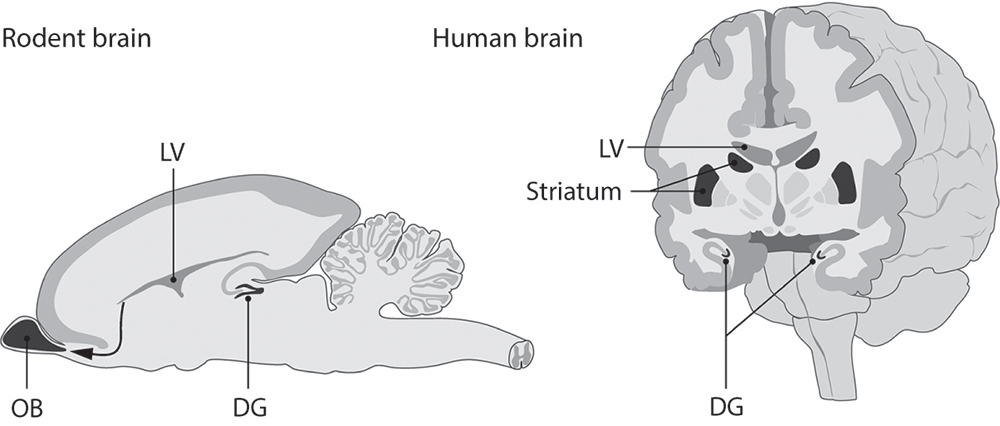
Figure 4 Neurogenic niches in the rodent and human brains.
Physical activity, environmental enrichment, and learning tasks enhance the proliferation of neural stem cells and, in some cases, promote the survival of newborn neurons, whereas stress, certain types of inflammation, and sensory deprivation have the opposite effect.
Another major breakthrough came in 1998 with the publication of a landmark study that provided the very first evidence that the human brain also forms new cells throughout life. The late Peter Eriksson and his colleagues realized that doctors were injecting cancer patients with bromodeoxyuridine (BrdU) in order to visualize and monitor the growth of their tumors. BrdU, like tritiated thymidine, is an analog of one of the four chemical bases found in DNA; as such it is incorporated into the newly synthesized DNA of newborn neurons. Eriksson and his colleagues got permission to examine the brains of five such patients after they had died. They treated samples of hippocampal tissue with different fluorescently labeled antibodies that bind to BrdU and to proteins expressed by neurons but not by glial cells, and detected newborn neurons in all five samples.13
Eventually, neural stem cells were isolated from the human brain, too. In rodents, these two populations of neural stem cells continue to generate new cells throughout life but the rate at which new neurons are produced decreases with age; the same pattern is also seen in the human hippocampus.14
There are important differences, however. The rostral migratory stream is found in the human brain, and even has a unique “ribbon” that branches off toward the frontal cortex. But evidently this pathway is active only up to early childhood. Extensive migration takes place until about 18 months of age, but subsides in older children and is almost completely absent in adults. In this respect, humans appear to be unique among the mammals.15,16
By contrast, a 2013 study by researchers in Sweden shows that the human hippocampus produces about 700 cells per day—which corresponds to an annual turnover of about 1.75% of the total number of cells in that part of the brain—and that the rate decreases only slightly with age.17 More recently, the same group published evidence of adult neurogenesis in the human striatum, a subcortical structure involved in motor control, reward, and motivation. These cells apparently originate in the subventricular zone and go on to form interneurons, whose fibers are restricted to the immediate area and whose inhibitory signals are vital for circuit function.18
The vital question is, does adult neurogenesis in the human brain serve any purpose, as it does in birds and rodents? The extent of adult neurogenesis in the human hippocampus is comparable to that seen in rodents, so it’s certainly possible that the new neurons contribute to brain function, but there is as yet no direct evidence for this.
In adult mice, fluoxetine (Prozac) and related antidepressants stimulate hippocampal neurogenesis. This finding led some researchers to speculate that neurogenesis may play a critical role in the development and treatment of depression. Animal experiments in which adult hippocampal neurogenesis is eliminated altogether have produced conflicting results: in some, the animals display an enhanced stress response and increased depression-like behaviors, but in others they do not.
In humans, depression is indeed associated with a reduction in hippocampal volume, but it is by no means clear that this reduction is due to impaired neurogenesis. It’s possible that impaired neurogenesis is one of many factors contributing to this complex disease, and is more important in some cases than in others. Likewise, the study showing that neurons are continuously added to the striatum also showed that adult-born cells are depleted in Parkinson’s disease, but it is still unclear whether or how this process is related to impaired neurogenesis.19,20 There is another possible downside to adult neurogenesis. Cancer arises when cells divide uncontrollably and spread through the body, and so it’s possible that the neural stem cell populations present in the adult human brain could contribute to the formation of brain tumors.21
Skeptics maintain that the numbers of cells produced by the adult human brain are too small to be of functional significance. They also argue that adding new cells could disrupt the stability of existing neuronal circuitry and, therefore, that the process is nothing more than a relic left over from our evolutionary ancestors.
Skeptics maintain that the numbers of cells produced by the adult human brain are too small to be of functional significance.
The most outspoken skeptic is the developmental neurobiologist Pasko Rakic, of Yale University. In the early 1970s Rakic performed a series of highly influential studies revealing how immature neurons migrate through the developing monkey brain, and he has worked on monkeys ever since. He has repeatedly failed to find any evidence of adult neurogenesis in the monkey cerebral cortex, and he is critical of the labeling methods used to identify newborn cells.
From his work in monkeys, Rakic estimates that neurons added to the adult human brain would probably take about a year to reach full maturity. This, he says, makes it highly unlikely that Prozac and related drugs work by stimulating neurogenesis, because they take just six weeks to exert their effects. There is some evidence that newborn neurons in the adult brain have enhanced synaptic plasticity, however, and so some argue that a year-long period of immaturity could actually make newborn neurons better able to contribute to brain function.22
Despite the controversy, the discovery of neurogenesis and neural stem cells in the adult human brain quickly raised hopes of stem cell–based therapies for neurological injury and disease, and also suggested two potential approaches for how such therapies might be developed. We know that neural stem cells can divide in response to brain injury, suggesting that this self-repair mechanism might one day be harnessed, by coaxing the brain’s endogenous stem cells to deploy new cells that would travel to an injury site and replace those that have been damaged or killed. An alternative strategy is to transplant stem cells into the brain and target them to the injury site.
Our understanding of neural stem cell biology is still far from complete, and researchers trying to develop such therapies face major technical challenges. Which types of stem cells are most appropriate for transplantation, and might different types be better suited to a given disease or type of injury? What is the optimum number of cells to be transplanted? And how can we be sure that transplanted cells will survive for long enough to integrate and aid in the recovery of neurological function?
Because of these difficulties, stem cell–based therapies for neurological disease and injury are still far from achieving their full potential, and in fact all of the clinical trials conducted so far have failed.23 Regardless, public awareness of these issues has led to a dramatic increase in stem-cell tourism to countries whose lax regulations enable unscrupulous vendors to sell unapproved—and possibly dangerous—therapies to desperate patients.