8 ADDICTION AND PAIN
The brain’s capacity for neuroplasticity underlies our ability to learn from experience in order to form memories and acquire new skills, and also our ability to adapt and recover from brain injury, or at least to compensate for and work around any damage that has occurred. But the relationship between brain and behavior is not one-sided. Our experiences and behaviors induce plastic changes in the brain, and these in turn can influence our future behavior and experiences. And the consequences of neuroplasticity are not always desirable.
Addiction and pain are the best understood examples of conditions involving maladaptive forms of neuroplasticity. Addictive drugs activate and hijack the brain’s reward system, and the resulting changes can remain long after the substance has been cleared from the brain, leading to cravings and to compulsive, drug-seeking behavior. Prolonged pain can induce reorganization of the spinal cord circuitry involved in processing and then transmitting painful stimuli up to the brain, and these changes can similarly persist long after the stimuli that initially caused the pain have been removed, resulting in chronic pain states that can persist for months or years.
Addictive drugs activate and hijack the brain’s reward system, and these changes remain in place long after the substance has been cleared from the brain, leading to cravings and to compulsive, drug-seeking behavior.
Reward, Motivation, and Addiction
Addictive narcotic and prescription drugs act on and modify brain systems involved in reward and motivation. The most important of these systems is the mesolimbic pathway, which begins in a small region of the midbrain called the ventral tegmentum. In the human brain, the ventral tegmentum contains approximately 400,000 neurons. These cells synthesize and release the neurotransmitter dopamine and project their long axonal fibers to the nucleus accumbens, part of a set of subcortical structures called the basal ganglia, which are involved in procedural learning, habit formation, and the control of voluntary movement. The nucleus accumbens in turn projects to numerous other brain regions, including parts of the cerebral cortex involved in memory and decision making and the amygdala, a small, almond-shaped structure involved in fear, anxiety, and assigning emotions to our experiences.
Normally, these structures cooperate to translate motivation into goal-directed actions in order to obtain natural rewards such as food, water, and sex. The nucleus accumbens plays a central role in these processes. Everything that we find pleasurable causes ventral tegmentum neurons to fire and release dopamine into the nucleus accumbens, which then evaluates how rewarding it is, according to the amount of dopamine released. For this reason the nucleus accumbens is popularly referred to as the brain’s “reward center,” and dopamine as “the pleasure molecule,” although they both serve numerous other functions as well.1
All addictive drugs target the ventral tegmentum and act on it in one way or another to enhance dopamine transmission, increasing the concentration of the neurotransmitter both there and in the nucleus accumbens and its other projection areas. Nicotine increases the firing rate of dopamine-producing ventral tegmentum neurons by acting on nicotinic receptors expressed on their surface; opioids, cannabinoids, and benzodiazepines increase their firing rate indirectly, by inhibiting the activity of GABA-producing interneurons in the ventral tegmentum; and psychostimulants such as cocaine, amphetamines, and ecstasy block the dopamine transporter, a membrane protein that normally reabsorbs dopamine once it has been released by neurons into the synaptic cleft.
Since all pleasurable activities enhance dopamine release in the nucleus accumbens, they, too, can become addictive, and there is now evidence that activities such as gambling, sex, and shopping can lead to similar brain changes, causing people to perform them compulsively.
Drugs hijack the reward pathway because they are more effective than natural rewards at enhancing dopamine release in the mesolimbic pathway. A single dose of cocaine, morphine, nicotine, alcohol, or benzodiazepines induces LTP in the ventral tegmentum (see chapter 3), which persists for up to a week. Addictive substances can also produce structural changes to nerve cells, too: administration of cocaine or a related stimulant increases, whereas chronic administration of morphine decreases, the density of dendritic spines in the ventral tegmentum. Most of these findings come from experiments performed in slices of midbrain tissue dissected from the mouse brain, but brain scanning studies in humans confirm that addictive drugs increase the concentration of dopamine in the nucleus accumbens and that the increase is closely associated with the pleasurable effects of the substances.2,3
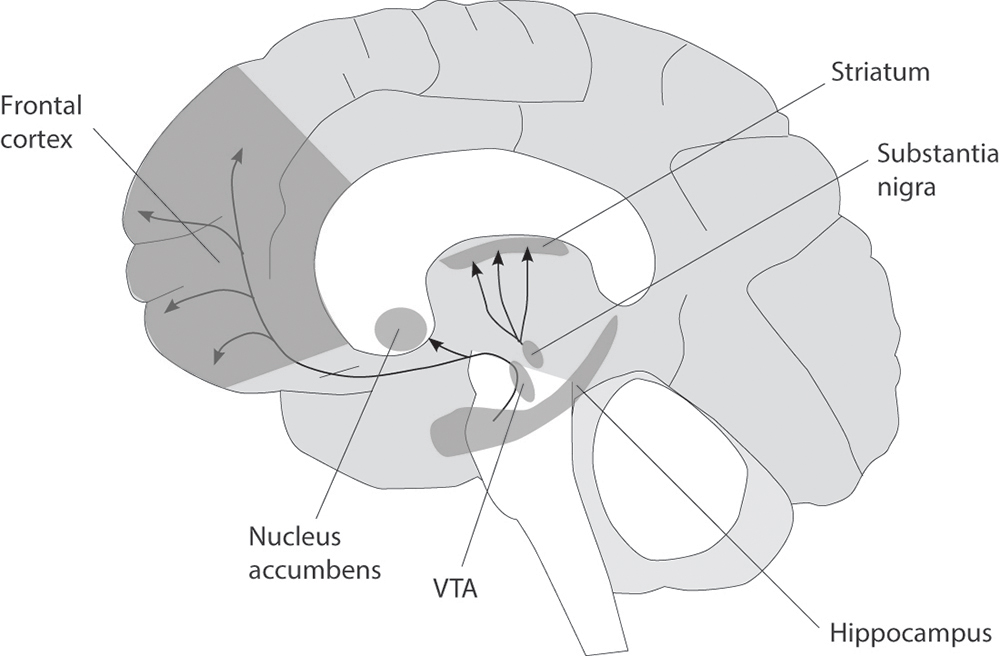
Figure 5 The human mesolimbic (reward) pathway.
Addiction can be thought of as a transition from recreational use, when the drug is taken voluntarily for its pleasurable effects, to habitual use, when control over intake is lost and the user becomes compelled to find and take the drug even though it might have adverse effects. Once the drug has taken hold, the addict enters a vicious cycle, bingeing on the drug to get high, but then beginning to experience withdrawal symptoms, which trigger craving that causes the user to seek out and take more of the drug.
It is currently thought that the progression from recreational user to addict is accompanied by a sequence of functional and structural brain changes within the reward pathway. Thus, initial use of an addictive substance induces LTP in the ventral tegmentum and nucleus accumbens, producing euphoric effects. With continued use, changes begin to occur in those parts of the pathway involved in memory and executive function. The user learns to associate drug use with certain environments, people, and paraphernalia, and every subsequent dose reinforces the behaviors that lead to drug taking. The brain adapts in such a way as to make the user overvalue the rewarding effects of the drug, and the use of it becomes habitual and compulsive.4
Since all pleasurable activities enhance dopamine release in the nucleus accumbens, they, too, can become addictive, and there is now evidence that activities such as gambling, sex, and shopping can lead to similar brain changes, causing people to perform them compulsively. We now know that prescription medications used to treat Parkinson’s disease can dramatically affect such behaviors. Parkinson’s is caused by the degeneration of dopamine-producing cells in another midbrain area called the substantia nigra, leading to movement deficits and cognitive problems. Some of these symptoms can be alleviated by drugs that increase dopamine levels in the brain, but because these drugs also act on the mesolimbic pathway, they can, in rare cases, lead to pathological gambling, hypersexuality, and other compulsive behaviors.5
The Pain Pathway
Physical pain serves the evolutionarily ancient and important function of alerting us to potentially life-threatening injuries. But it too can produce long-lasting adaptations in the nervous system—changes that may contribute to various forms of persistent, pathological pain.
Our ability to perceive noxious stimuli is mediated by primary sensory neurons of the peripheral nervous system. These pain-sensing neurons have their cell bodies clustered in the dorsal root ganglia, which lie just outside the spinal cord. They have a single fiber that splits in two close to the cell body. One branch extends out to a specific patch just beneath the skin surface; it contains various receptors that are sensitive to specific kinds of painful stimuli such as excessive mechanical pressure, noxious hot and cold temperatures, and certain ingredients of the chemical cocktail that spills out of damaged cells. The other extends a much shorter distance into the back of the spinal cord, where it forms synapses with the second-order sensory neurons that project up into the brain.6
When these pain-sensing neurons are activated, they produce nervous impulses that travel up into the spinal cord and are transmitted to the second-order sensory neurons in the spinal cord, which relay the signals up to the somatosensory cortex. Only when these signals have been processed do we become aware of the pain, and then act to stop it in order to prevent any further damage being done.
Plastic changes can occur at the peripheral end of pain-sensing neurons beneath the skin, as well as at the synapses they form with second-order sensory neurons in the spinal cord. Activation of the protein sensors rapidly redistributes them in the nerve terminal and alters their functional properties to lower their activation threshold. This hypersensitizes the damaged tissue, so that otherwise innocuous stimuli are perceived to be painful, which aids repair by minimizing contact with the damaged tissue. It also increases the firing rate of the pain-sensing neurons, and increases the probability of neurotransmitter release from their nerve terminals in the spinal cord.
These short-term changes are usually reversible. Under some circumstances, however, there can be longer-lasting modifications to the pain system. During inflammation, growth factors released from damaged cells can trigger the synthesis and trafficking of pain receptors and their related signaling molecules in pain-sensing neurons, sensitizing the cells to painful stimuli. Trains of impulses generated by these cells can then induce LTP at synapses in the spinal cord. This amplifies the response of the secondary sensory neurons to incoming pain signals, so that repetitive, low-frequency signals produce a progressively larger output—a process called wind-up.7,8
Chronic or persistent pain is also associated with functional and structural changes in the primary somatosensory cortex, but different kinds of pain and injuries effect these changes in different ways. For example, cortical representation of the painful fingers expands in carpel tunnel syndrome, perhaps exacerbating the pain felt by sufferers, while the representation of affected body part shrinks in complex regional pain syndrome, possibly through disuse. Cortical reorganization occurs in several steps: within minutes of the initial injury, previously inhibited connections are “unmasked”; later on, axonal sprouting may occur within the tissue being reorganized.9