Hardly anyone, and that included me for the first ten years after the concept was born, seems to know what Gaia is. Most scientists, when they think and talk about the living part of the Earth, call it the biosphere,† although strictly speaking the biosphere is no more than the geographical region where life exists, the thin spherical bubble at the Earth’s surface. They have unconsciously expanded the definition of the biosphere into something larger than a geographical region but seem vague about where it starts and ends geographically and what it does.
Going outwards from the centre, the Earth is almost entirely made of hot or molten rock and metal. Gaia is a thin spherical shell of matter that surrounds the incandescent interior; it begins where the crustal rocks meet the magma of the Earth’s hot interior, about 100 miles below the surface, and proceeds another 100 miles outwards through the ocean and air to the even hotter thermosphere at the edge of space. It includes the biosphere and is a dynamic physiological system that has kept our planet fit for life for over three billion years. I call Gaia a physiological system because it appears to have the unconscious goal of regulating the climate and the chemistry at a comfortable state for life. Its goals are not set points but adjustable for whatever is the current environment and adaptable to whatever forms of life it carries.
We have to think of Gaia as the whole system of animate and inanimate parts. The burgeoning growth of living things enabled by sunlight empowers Gaia, but this wild chaotic power is bridled by constraints which shape the goal-seeking entity that regulates itself on life’s behalf. I see the recognition of these constraints to growth as essential to the intuitive understanding of Gaia. Important to this understanding is that constraints affect not only the organisms or the biosphere but also the physical and chemical environment. It is obvious that it can be too hot or too cold for mainstream life, but not so obvious is the fact that the ocean becomes a desert when its surface temperature rises above about 12°C; when this happens, a stable surface layer of warm water forms that stays unmixed with the cooler, nutrient-rich waters below. This purely physical property of ocean water denies nutrients to the life in the warm layer, and soon the upper sunlit ocean water becomes a desert. This may be one of the reasons why Gaia’s goal appears to be to keep the Earth cool.
You will notice I am continuing to use the metaphor of ‘the living Earth’ for Gaia; but do not assume that I am thinking of the Earth as alive in a sentient way, or even alive like an animal or a bacterium. I think it is time we enlarged the somewhat dogmatic and limited definition of life as something that reproduces and corrects the errors of reproduction by natural selection among the progeny.
I have found it useful to imagine the Earth as like an animal, perhaps because my first experience of serious science as a graduate was in physiology. It has never been more than metaphor – an aide pensée, no more serious than the thoughts of a sailor who refers to his ship as ‘she’. Until recently no specific animal came into my mind, but always something large, like an elephant or a whale. Recently, on becoming aware of global heating, I have thought of the Earth more as a camel. Camels, unlike most animals, regulate their body temperatures at two different but stable states. During daytime in the desert, when it is unbearably hot, camels regulate close to 40°C, a close enough match to the air temperature to avoid having to cool by sweating precious water. At night the desert is cold, and even cold enough for frost; the camel would seriously lose heat if it tried to stay at 40°C, so it moves its regulation to a more suitable 34°C, which is warm enough. Gaia, like the camel, has several stable states so that it can accommodate to the changing internal and external environment. Most of the time things stay steady; as they were over the few thousand years before about 1900. When the forcing is too strong, either to the hot or the cold, Gaia, as a camel would, moves to a new stable state that is easier to maintain. She is about to move now.
Metaphor is important because to deal with, understand, and even ameliorate the fix we are now in over global change requires us to know the true nature of the Earth and imagine it as the largest living thing in the solar system, not something inanimate like that disreputable contraption ‘spaceship Earth’. Until this change of heart and mind happens we will not instinctively sense that we live on a live planet that can respond to the changes we make, either by cancelling the changes or by cancelling us. Unless we see the Earth as a planet that behaves as if it were alive, at least to the extent of regulating its climate and chemistry, we will lack the will to change our way of life and to understand that we have made it our greatest enemy. It is true that many scientists, especially climatologists, now see that our planet has the capacity to regulate its climate and chemistry, but this is still a long way from being the conventional wisdom. It is not easy to grasp the concept of Gaia, a planet able to keep itself fit for life for a third of the time the universe has existed, and until the IPCC sounded the alarm there was little inclination. I will try to provide an explanation that would satisfy a practical person like a physician. A complete explanation that would satisfy a scientist may be inaccessible, but the lack of it is no excuse for inaction.
I find explaining Gaia is like teaching someone how to swim or to ride a bicycle: there is much that cannot be put into words. To make it easier I will start at the shallow end with a simple question that illustrates the mind-wrenching difference between two equally important ways of thinking about the world. The first is systems science, which is about anything alive, whether an organism or an engineering mechanism while it is working; the second is reductionist science, the cause-and-effect thinking that has dominated the last two centuries of science. The question is: what has peeing to do with the selfish gene?
When I was a young man I was amazed by the number of euphemisms that existed for the simple but essential practice of passing urine. Doctors and nurses would ask you to ‘produce a specimen’ or ‘pass some water’ and often hand out a small container to make their request clear. In everyday speech we ‘pumped the ship’, ‘sprung a leak’ or ‘shed the load’ and we did it in ‘the little boys’ room’ or the ‘bathroom’. Sometimes we just ‘spent a penny’.
Perhaps it was all a hangover from the nineteenth-century confusion over sex. It was not only impossible in polite speech to mention the genitals; the taboo applied also to their alternative uses. But as the outstanding American biologist George Williams observed in 1996, what an odd evolutionary economy to use the same organ for pleasure, reproduction and waste disposal. It was not until quite recently that I began to wonder if there might not be something deeper lurking behind this minor mystery. Why do we pee? Not so silly a question as it might seem. The need to rid oneself of waste products like excess salt, urea, creatinine and numerous other scraps of metabolism is obvious but only part of the answer. Perhaps we pee for altruistic reasons. If we and other animals did not pass urine some of the vegetable life of the Earth might be starved of nitrogen.
Is it possible that in the evolution of Gaia, the great Earth system, animals have evolved to excrete nitrogen as urea or uricacid instead of gaseous nitrogen? For us the excretion of urea represents a significant waste of energy and of water. Why should we evolve something to our disadvantage unless it was for altruistic reasons? Urea is the waste product of the metabolism of the meat, the fish, the cheese and the beans we eat; all are rich in protein, the stuff of life. We digest what we eat and break it down to its component chemicals; we do not take beef-muscle protein and use it in our own muscles. We build or replace our muscles and other tissue by assembling the component parts, the amino acids of the proteins, into fresh protein according to the plan in our DNA. To use the protein from beef directly to make our muscles would be like taking the parts of a tractor to repair a washing machine. The waste left over from this busy construction and deconstruction ultimately becomes urea, and we seem to have no option but to get rid of it as a dilute solution in water, urine.
Urea is a simple chemical, a combination of ammonia and carbon dioxide, or as an organic chemist would say, the diamide of carbonic acid, NH2CONH2. Why did we and other mammals evolve to excrete our nitrogen in this form? Why not break down the urea into carbon dioxide, water and nitrogen gas? Much easier to excrete nitrogen by breathing it out, and it would save the water needed for excreting urea; oxidizing the urea would even add a little water, to say nothing of providing more energy.
Let us look at the figures: 100 grams of urea is metabolically worth 90 kilocalories or, if you prefer, 379 kilojoules. But if instead of being consumed it is passed in urine, more than four litres of water are needed to excrete the 100 grams of urea at a non-toxic dilution. Normally we excrete about 40 grams of urea daily in about 1.5 litres of water. Not much of a problem, you might think, but just consider animals living in a desert region short of food and water. If a mutant appeared that was able to metabolize urea to nitrogen, carbon dioxide and water, it would be at a considerable advantage and probably be able to leave more progeny than its urea–excreting competitors. According to a simplistic interpretation of Darwinian theory, selection would favour this mutant trait and it would spread rapidly and become the norm.
At this point a sceptical biochemist will say, ‘Don’t you realize that the products of ammonia or urea oxidation are all poisonous, and that is why we excrete nitrogen as urea?’ My reply would be, ‘Tell that to the bacteria that change nitrogen compounds into nitrogen gas and which are abundant in the soil and ocean.’ More than this, a symbiosis with denitrifying organisms might be as good as or better than trying to metabolize urea ourselves.
So you see, urea is waste for us and wasting it loses valuable water and energy. But if we and other animals did not pee and breathed out nitrogen instead, there might be fewer plants and later we would be hungry. How on Earth did we evolve to be so altruistic and have such enlightened self-interest? Perhaps there is wisdom in the workings of Gaia and the way she interprets the selfish gene.
When I started working on Gaia forty years ago, science was not as now a highly organized and often corporate enterprise. There was almost no forward planning or status reports, and there were almost never meetings to plan what to do next. There was no health and safety bureaucracy – we were expected to be, as qualified scientists, responsible for our own and our colleagues’ safety. Most differently, science was done hands–on in the laboratory, not simulated on a computer screen in an office or a cubicle. In this idyllic environment it was possible to do an experiment to confirm or deny an idea. Sometimes the answer was a simple right or wrong, but on other occasions something equivocal. These ‘don’t knows’ were what was led by serendipity to the revelation of something wholly unexpected, a real discovery.
So it might be with the idea of urea excretion. Thinking about nitrogen this way led me to wonder about the vexing problem of oxygen in the Carboniferous period some 300 million years ago. An important part of the evidence for Gaia comes from the abundance of atmospheric gases, such as oxygen and carbon dioxide; these are regulated at a level comfortable for whatever happens to be the current form of life. There are good experimental as well as theoretical grounds for thinking that the present percentage of oxygen in the atmosphere is about right. More than 21 per cent carries an increasing fire risk; at 25 per cent the probability of a blaze from a spark increases about tenfold. Andrew Watson and Tim Lenton have modelled the regulation of oxygen and have found the fire risk of dry vegetation to play an important part in the mechanism of oxygen regulation. Below 13 per cent there are no fires, and above 25 per cent they are so fierce that it seems impossible that forests could reach maturity. Imagine our surprise when the eminent geochemist Robert Berner proposed that in the Carboniferous period, about 300 million years ago, oxygen was 35 per cent of the atmosphere. His conclusion came from a model based on a thorough analysis of the composition of carboniferous rocks. He argued that at that time so much carbon was being buried, much of which we now see as the coal measures, that there had to be much more oxygen in the air to balance this greater rate of carbon burial.
My first reaction was that Berner must be wrong; I knew from the careful experiments made by my colleague Andrew Watson in the 1970s that fires in 35 per cent oxygen are almost as fierce as in pure oxygen. I was not impressed by laboratory experiments that suggested that twigs from trees did not readily inflame in 35 per cent oxygen; there is a world of difference between a laboratory simulation and a real forest fire, where its intense radiation dries out the wood in the path of the fire and where the winds drawn by the fire bring in fresh oxygen-rich air. Nor was I impressed by arguments that the huge dragonflies that existed at that time could not have flown without 35 per cent oxygen in the air. It is now realized that insects are unusually vulnerable to oxygen poisoning and that the Cretaceous dragonflies would have had no difficulty flying at our present oxygen levels. The argument went on until a friend, Andrew Thomas, an acoustic scientist and also a diver, suggested that maybe we were both right. Berner was right to claim that there was more oxygen and I was right to say it could not have been present at much over 25 per cent. All that was required was more nitrogen in the air. It is not the amount of oxygen that determines flammability, but its proportion in the mixture with nitrogen.
About 40 per cent of the nitrogen on Earth is now buried in the crust; perhaps in the Cretaceous that nitrogen had not yet been buried and existed in the air and so kept the proportion of oxygen safer for trees. We might also speculate that the microbial life of the Precambrian that preceded the appearance of trees and animals did not conserve nitrogen, so that it would have been present mainly as gas in the air.
These thoughts about nitrogen are wholly speculative, but I include them to illustrate the way that Gaia Theory† has developed from ideas that were at first vague or from fruitful errors that were the seeds from which a truer account has emerged.
So let us go further now and try to sense Gaia by looking at the Earth from outside as a whole planet. Imagine a spacecraft manned by intelligent aliens who are looking at the solar system from space. They would have aboard their ship instruments powerful enough to show the travellers the chemical composition of every planet’s atmosphere. From this analysis and nothing more, their automated instruments would tell them that the only planet with abundant life was the Earth; more than that, they would say that the life form was carbon-based and was sufficiently advanced to have an industrial civilization. There is nothing science fictional about the instrument itself; a small telescope with an infra-red spectrometer and a computer to control them and analyse their observations would do. They would see methane and oxygen coexisting in the upper air of the Earth, and the ship’s scientist would know that these gases were reacting in the bright sunlight and that therefore something on the ground must be making large quantities of them both. The odds against this happening by chance inorganic chemistry are near infinity. They would conclude that our planet is a rich habitat for life, and the presence of CFCs would suggest a civilization unwise enough to have allowed their escape.
In the 1960s I was a contractor designing instruments for NASA’s planetary exploration team, and thoughts like these led me to propose planetary atmospheric analysis for the detection of life on Mars. I argued that if there was life on Mars it would have to use the atmosphere as a source of raw materials and as somewhere to deposit its wastes; this would change the atmospheric composition and make it recognizably different from that of a dead planet. I saw the Earth, rich with life, as the contrasting planet, and I used the eminent scientist G. E. Hutchinson’s authoritative review of biogeochemistry as my source of information on the sources and sinks for the gases of the air. He reported methane and nitrous oxide as biological products, and nitrogen, oxygen and carbon dioxide as massively changed in abundance by organisms. At the time, none of us knew much about the composition of Mars’s atmosphere, but in 1965 Earth-based infra-red astronomy revealed the Mars atmosphere to be composed almost entirely of carbon dioxide and close to chemical equilibrium; according to my proposal it was therefore probably lifeless – not a popular conclusion to give my sponsors. Turning aside from life detection, I wondered what could be keeping our chemically unstable atmosphere in a dynamicsteady state and the Earth always apparently habitable. Moreover, the continuity of life requires a tolerable climate despite a 37 per cent increase of solar luminosity since the Earth formed. Together, these thoughts led me to the hypothesis that living organisms regulate the climate and the chemistry of the atmosphere in their own interest, and in 1969 the novelist William Golding proposed Gaia as its name. A few years later, I started collaborating with the eminent American biologist Lynn Margulis, and in our first joint paper we stated: the Gaia Hypothesis views the biosphere as an active, adaptive control system able to maintain the Earth in homeostasis.
From its beginning in the 1960s, the idea of the global self-regulation of climate and chemistry was unpopular with both Earth scientists and life scientists. At best, they found it unnecessary as an explanation of the facts of life and the Earth; at worst, they condemned it outright in scathing terms. The only scientists who welcomed the idea were a few meteorologists and climatologists. Some biologists soon challenged the hypothesis, arguing that a self-regulating biosphere could never have evolved, since the organism was the unit of selection, not the biosphere. I was fortunate to have that fine and clear author Richard Dawkins as the advocate for the Darwinian opposition to Gaia; it was painful but in time I found myself agreeing with him that Darwinian evolution, as it was then understood, was incompatible with the Gaia Hypothesis.† I did not then doubt Darwin, so what was wrong with the Gaia hypothesis? I knew that the constancy of climate and of the chemical composition of the air were good evidence for a self-regulating planet. Moreover, the concept of Gaia is fruitful, and it led me to discover the natural molecular carriers of the elements sulphur and iodine: dimethyl sulphide (DMS) and methyl iodide. Several years later in 1986, while collaborating with colleagues in Seattle, we made the awesome discovery that DMS from ocean algae† was connected with the formation of clouds and with climate. We were moved to catch a glimpse of one of Gaia’s climate-regulation mechanisms, and we were indebted to the climate-science community who took us seriously enough to award to the four of us, Robert Charlson, M. O. Andreae, Steven Warren and me, their Norbert Gerbier Prize in 1988.
To return to the arguments with the Darwinists, it occurred to me in 1981 that Gaia was the whole system – organisms and material environment coupled together – and it was this huge Earth system that evolved self-regulation, not life or the biosphere alone. To test this idea I composed a computer model of dark- and light-coloured plants competing for growth on a planet in progressively increasing sunlight. It was no more than a simulation of the world, but the running program showed the imaginary world regulating its temperature close to the optimum for daisy growth and over a wide range of heat outputs from its star. This model, which I called Daisyworld, was unusual for an evolutionary model made from coupled differential equations; it was stable, insensitive to initial conditions and resistant to perturbation.
Daisyworld models a planet like the Earth, orbiting a star like our sun. On Daisyworld there are only the two plant species, and they both compete for living space as any plants would do. When the sun is younger and cooler, so is the model planet, and at that time the dark daisies flourish. Only at the hottest places near the equator are light daisies found. This is because dark daisies absorb sunlight and keep themselves, their region and the whole planet warm. As the star heats up, the dark daisies living in the tropics are displaced by light daisies, because the light ones reflect sunlight and so are cooler; they also cool their region and the whole planet. As the star continues to warm, the light daisies displace the dark, and through their competition for space the planet always stays near to the ideal temperature for life. Eventually, the star grows so hot that even light daisies can no longer survive and the planet becomes a lifeless ball of rock.
The model is no more than a caricature, but think of it like that splendid map of the London Tube system – not good as a guide to the streets of London, but ideal for finding your way around the tube system of that bustling city. Daisyworld was invented to show that Darwin’s theory of evolution from natural selection is not contrary to Gaia theory, but part of it.
The main reaction of biologists and geologists to Daisyworld was, as good scientists, to try to falsify it, and this they did repeatedly, with increasing irritation, but none succeeded. To answer some of these critics I made models much richer in species than Daisyworld. They included many different types of plant, rabbits to graze them and foxes as predators. They were just as stable and self-regulating as Daisyworld. My friend Stephan Harding has made models of whole ecosystems complete with food webs and used them to enlighten our understanding of biodiversity. The persistence of the critics made me realize that Gaia would not be taken as serious science until eminent scientists approved of it in public. In 1995 I started dialogues with John Maynard Smith and William Hamilton, both of whom were prepared to discuss Gaia as a scientific topic but neither of whom could see how planetary self-regulation could evolve through natural selection. Even so, Maynard Smith gave unstinting support to my friend and colleague Tim Lenton, when the latter wrote a seminal article in Nature called ‘Gaia and Natural Selection’. In it he described the several ways that the Earth keeps to its goal of sustaining habitability for whatever life forms happen to be its inhabitants. Hamilton wondered in a joint paper with Lenton, with the provocative title ‘Spora and Gaia’, if the need for organisms to disperse was the link that connected ocean algae with climate. In 1999 Hamilton said in a television programme, ‘Just as the observations of Copernicus needed a Newton to explain them, we need another Newton to explain how Darwinian evolution leads to a habitable planet.’
Then, at least in Europe, the ice began to melt, and at a meeting in Amsterdam in 2001 – at which four principal global-change organizations were represented – more than a thousand delegates signed a declaration that had as its first main statement: ‘The Earth System behaves as a single, self-regulating system comprised of physical, chemical, biological and human components.’
These words marked an abrupt transition from a previously solid conventional wisdom in which biologists held that organisms adapt to, but do not change, their environments and in which Earth scientists held that geological forces alone could explain the evolution of the atmosphere, crust and oceans. We should recall at this point the trials of that eminent biologist Eugene Odum, who in the 1960s saw an ecosystem as an entity like Gaia. So far as I am aware, none of the biologists who stridently rejected Odum’s concept have admitted that they were wrong.
The Amsterdam Declaration was an important step towards the adoption of Gaia Theory as a working model for the Earth; however, territorial divisions and lingering doubts kept the declaring scientists from stating the goal of the self-regulating Earth, which is, according to my theory, to sustain habitability. This omission allows scientists to pay lip service to Earth System Science (ESS)†, or Gaia, but continue to model and research in isolation as before. This natural and human tendency of scientists to resist change would not ordinarily have mattered: eventually the strings of habit would have broken and geo-chemists would have started to think of the biota as an evolving and responding part of the Earth, not as if life were merely a passive reservoir like the sediments or the oceans. Eventually also biologists would have thought of the environment as something that organisms actively changed and not as something fixed to which they adapted. But unfortunately, while scientists are slowly changing their minds, we of the industrial world have been busy changing the surface and atmosphere. Now humanity and the Earth face a deadly peril, with little time left to escape. If the middle management of science had been somewhat less reactionary about Gaia, we might have had twenty more years in which to resolve the much more difficult human and political decisions about our future.
The key to understanding Gaia is to remember that it operates within a set of bounds or constraints. All life is urged by its selfish genes to reproduce, and if the only constraints are competition and predation, the result is a chaotic fluctuation of populations. Attempts to model natural ecosystems that do not include environmental constraints, from the famous rabbits and foxes model of the biophysicist Alfred Lotka and his colleague Vito Volterra, to the latest attempts using complexity theory, all fail to produce the robust stability of a natural ecosystem. Lotka warned as long ago as 1925 that the equations of these too-simple models lacked a constraining physical environment and would be difficult to solve.
In spite of this warning, the abstract mathematics of population biology has fascinated academic biologists for at least seventy years, but it hardly represents the real world, or satisfies their down-to-earth colleagues, the muddy-boots ecologists. Examine any long-term natural ecosystem in one of the few remaining untouched places of the Earth, and you will find it is dynamically stable, just like your own body.
Many twentieth-century biologists approached their science with a faith in the infallibility of a genetic description of life. Their faith was so strong that they could not envisage the evolution of an ecosystem happening independently of the genes of its constituent organisms. In fact, the epigenetic evolution of ecosystems and Gaia can take place simply by the selection of existing species. When an ecosystem experiences continued disturbance, such as excessive heat or drought, those species that are tolerant are selected from the ensemble of existing genotypes and they may grow until they dominate; the fine tuning of genetic evolution completes the process of adaptation. The evolution of ecosystems and of Gaia involves more than the selfish gene.
The unstable mathematics of unconstrained competition and predation among living organisms is not unlike the behaviour of the unruly, often drunken, mobs that gather in the city centres at night. The constraint of a strong community confident in its power and backed up by an effective police force once gave quiet and stability, but it has gone and often chaos rules. Gaia itself is firmly constrained by feedback from the non-living environment. Darwinists are right to say that selection favours the organisms that leave alive the most progeny, but vigorous growth takes place within a constrained space where feedback from the environment allows the emergence of natural self-regulation.
The consequences of unconstrained exponential growth have often been calculated and used as examples of the vigour of life. If a single bacteria divided and repeated that division every twenty minutes, provided that there were no constraints to growth and the food supply was unlimited, in just over two days the total progeny would weigh as much as the Earth. Predation and limits to the supply of nutrients are the local constraints, and pre-Gaia these were all that biologists considered. Now we know that such global properties as atmospheric and oceanic composition and climate set the constraints that bring stability.
So how do these environmental constraints work? They depend upon the tolerances of the organisms themselves. All life forms have a lower, an upper and an optimum temperature for growth, and the same is true for acidity, salinity and the abundance of oxygen in air and water. Consequently, organisms have to live within the bounds of these properties of their environment.
Apart from a few highly adapted organisms, the extremophiles, which live in hot springs near to the boiling point or in the saturated brine of salt lakes or even in the strong acid of our stomachs, almost all life forms are quite fussy about their living conditions. The individual cells that constitute life demand exactly the right mix of salts and nutrients in their internal environment and will tolerate only small changes in the composition of the world around them. When these cells aggregate in their billions to form large animals and plants they can regulate their internal milieu independently of environmental change; we are not harmed by swimming in salt water or by taking a sauna. But bacteria, algae and other single-cell organisms have no choice but to live at whatever temperature and other conditions they find themselves in, and consequently they have adapted to a considerable range of temperature, salinity and acidity. But even for them the temperature range is limited to between −1.6°C, when sea water freezes, to 50°C. We humans and most mammals and birds choose to regulate ourselves close to 37°C and are called homeotherms. The less fussy reptiles and invertebrates are called that curious word poikilotherms or, as we would say, cold blooded. Our own bodies can withstand an internal temperature of 34 or 41°C for short periods, but we are definitely unwell if below 36 or above 39°C. Whether we live as Inuits in the Arctic or as Bushmen in the heat of the Kalahari Desert, those are our internal limits.
Mainstream life flourishes best between 25 and 35°C, but this is only the physiological part of regulation; life is also influenced by the physical properties of the material parts of the Earth. Above 4°C water expands as it warms, and if the ocean surface is warmed from above by sunlight, the top layer absorbs most of the sun’s heat and expands to become lighter than the still colder waters beneath. This warmer surface layer has a depth of between 30 and 100 metres. It forms when the sunlight is strong enough to raise the surface temperature above about 10°C.
The warm surface layer is stable, and except in fierce storms, like hurricanes, it stays intact and the cooler waters below do not mix with it. The formation of the surface layer exerts a powerful constraint on ocean life; primary producers that seed the newly formed warm layer in early spring soon go through a succession that uses up nearly all the nutrients of the layer. The dead bodies of this spring bloom sink to the ocean floor, and soon the surface layer is empty of all but a limited and starving population of algae. This is why warm and tropical waters are so clear and blue; they are the deserts of the ocean, and just now they occupy 80 per cent of the world’s water surface. In the Arctic and Antarctic, the surface waters remain below 10°C and so are well mixed from the bottom to the surface and nutrients are available everywhere.
In the early part of the twentieth century intercontinental travellers went by sea. Those on a ship travelling to Europe from New York would first see the clear blue warm waters of the Gulf Stream, and then quite suddenly, as they sailed north and east past Cape Cod and entered the Labrador cold current, the water would turn dark and soupy. Ocean life may like to be warm, but the properties of water prevent them from enjoying warmth much above 10°C, unless they are prepared to stay at small numbers and near starvation. This is an important global constraint to growth and is why Gaia does better when cool.
There are oases in the vast deserts of the present world oceans, and these are found at the edges of continents where cold nutrient-rich water wells up from the depths. The seas beyond the estuaries of large rivers like the Mississippi, the Rhine, the Indus and the Yangtze are artificial oases, rich in nutrients, the run-off from intensive agriculture on the land. But these oases, natural and artificial, play only a small part.
A similar and equally important constraint to growth operates on the land surface. Living organisms flourish as it grows warmer up to nearly 40°C, but in the natural world the water they need for life becomes difficult to access once the temperature is much above 20°C. In wintertime when it rains and temperatures are below 10°C, the water stays around for quite a while and the soil stays moist and suitable for growth. In summertime, with average temperatures near 20°C, newly deposited rain soon evaporates and leaves the surface dry; soil loses moisture unless the rain is repeated frequently. Somewhere above 25°C evaporation is so rapid that without continuous rain the soil dries out and the land becomes a desert. Just as in the surface layer of the ocean, organisms may like it warm but the properties of water set a limit to growth.
Richard Betts of the Hadley Centre has shown how the great tropical rainforests have to some extent overcome this limitation by adapting to their warm environment so as to be able to recycle water. The ecosystem does it by sustaining the clouds and rain above the forest canopy, but this ability has its limits. He and Peter Cox suggest a 4°C rise in temperature would be enough to disable the Amazon forest and turn it into scrub or desert, and it would happen partly from the local consequences of a faster evaporation of rain but also from global changes in wind patterns in a 4°C warmer world.
Pure water freezes at 0°C, while in the oceans the salt in the water lowers the freezing point to −1.6°C. Life can adapt to temperatures below freezing – fish swim in water still unfrozen but below 0°C – but active life is impossible in the frozen state. When Sandy and I visited the British Antarctic Survey’s labs at Cambridge we were enthralled to see a fish, in a tank held at −1.6°C, swim in a live and responsive way to our host, Lloyd Peck, in anticipation of food. For the fish this was obviously an acceptable temperature. When water is taken from an organism to form ice or as water vapour in drying, the dissolved salts in the organism are concentrated. If the concentration of salt rises above 8 per cent death is immediate. Organisms have adapted to some extent to this problem; sea water, for example, is 6 per cent salt and close to this lethal limit; selection has favoured those organisms that can make substances that neutralize the harmful consequences of increased salt. In the ocean they make large quantities of dimethyl sulphonio propionate (DMSP) for this purpose; on the land insects in the Arctic have evolved antifreeze compounds that prevent salt from accumulating to lethal levels when they freeze.
These physical constraints set by the properties of water feed back on growth and set the shape of the relationship between growth and temperature and the distribution of life on the Earth. From a purely human viewpoint the present interglacial, at least before we started to meddle with it, is a better state than a glaciation. This may be because the more influential humans live in northern hemispheric regions that were either covered in glaciers or tundra during the ice age. From Gaia’s viewpoint the glaciation was a desirable state, with much less warm surface water and therefore abundant ocean life; the water taken from the oceans to form the great glaciers would have lowered the sea level by 120 metres and this would have provided an area of land as large as Africa on which plants could grow. As we have seen, there was more life on the colder Earth, shown by the low abundance of carbon dioxide at that time; it takes a lot of life to pump it down to less than 200 parts per million (ppm). More than this, the ice-core evidence from Antarctica suggests that the output of dimethyl sulphide (DMS) was nearly five times greater in the ice age. This larger production of sulphur gas implies more marine algae, the source of DMS, in the oceans. In my view, if the Earth system, Gaia, could express a preference it would be for the cold of an ice age, not for today’s comparative warmth.
There is much more to Gaia than temperature regulation. The maintenance of a stable chemical composition is similarly vital. Andrew Watson and Tim Lenton have gone far towards discovering the mechanism by which atmosphericoxygen is regulated and the part played by that important but rare element phosphorus. Peter Liss has investigated the biological sources in the oceans of the essential elements sulphur, selenium and iodine. The intricate links between algae living in the oceans, sulphur gas production, atmospheric chemistry, cloud physics and climate are slowly being uncovered in dozens of laboratories around the world. Now that Gaian regulation is accepted, even if not understood, there is a worldwide effort to uncover the Earth’s vital statistics. Much of the detail is available in the book The Earth System by Kump, Kasting and Crane. It is well worth reading as a source, even if it is not as Gaian as it could be.
In 1994 one of the authors, my friend the American geochemist Lee Kump, and I published a paper in Nature that described a computer model of the Earth like Daisyworld but more realistic; instead of daisies, we had ocean algal ecosystems that affected climate by pumping down carbon dioxide and also by making white reflecting clouds. On the land masses we had forest ecosystems that also pumped down carbon dioxide and made clouds. The defining part of our model was the growth rate of organisms at different temperatures. We took the generally accepted values of the growth rates of algae and forest trees under ideal conditions where water and nutrients were unlimited. This data revealed that growth was best near 30°C and stopped below 0°C and above 45°C. We then took into account the real world constraints set by the physical properties of water. For the algae in the ocean the best temperature for growth would be close to 10°C, because above this the stable surface layer forms and shuts off the supply of nutrients. Similarly, on the land the upper limit of tree growth would be set by the rate of evaporation of water, and the optimum for trees was close to 20°C.
When we ran our model by either steadily increasing the input of heat from the sun or by keeping the sun constant but increasing the input of carbon dioxide, as we are now doing in the real world, the model showed good regulation, with both the ocean and land ecosystems playing their part. But as the carbon-dioxide abundance approached 500 ppm, regulation began to fail and there was a sudden upward jump in temperature. The cause was the failure of the ocean ecosystem. As the world grew warm, the algae were denied nutrients by the expanding warm surface of the oceans, until eventually they became extinct. As the area of ocean covered by algae grew smaller, their cooling effect diminished and the temperature surged upwards.
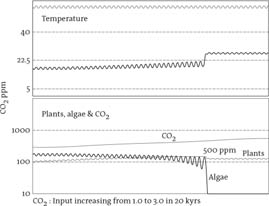
Figure 1 shows a run of this model with a steadily increasing input of CO2pollution going from the pre-industrial level to up to three times as much, which is less than we are now adding to the atmosphere. The upper panel of the chart shows temperature change, with the

Figure 1. Climate prediction according to the model described in the text
upper line the temperature expected for a dead planet and the lower line for our model Earth. A feature of the model is a simple device to indicate if feedback is positive or negative. We introduced a small periodic variation in the heat received from the sun. The amplitude of this fluctuation was kept constant and is reflected in the variations of the otherwise constant temperature of the control dead planet shown in the upper line on the figure. The lower panel of the chart shows the changes in the land vegetation, in the ocean algae and in the carbon-dioxide abundance. When regulation was working well, the abundance of the algae and plants and the temperature all show dampened fluctuations, but when the algal ecosystem became stressed the fluctuations grew large and showed amplification by positive feedback. The sudden jump in mean temperature from about 16 to 24°C followed the largest fluctuation and the extinction of the algae.
The model maps surprisingly well onto the observed and the predicted behaviour of the Earth. The turning point, 500 ppm of carbon dioxide, would, according to the IPCC, represent a temperature rise of about 3°C. This is close to the temperature rise of 2.7°C predicted by the climate modeller Jonathon Gregory as sufficient to start the irreversible melting of Greenland’s ice. Those respected professional scientists who monitor the oceans and atmosphere already report an acceleration of the rise of carbon-dioxide abundance and a decline in algae in the Atlantic and Pacific oceans as they warm.
I acknowledge that arguments from models like this one and from geophysiology are not by themselves strong enough to justify political action, but they become serious when taken in conjunction with the evidence from the Earth that nearly all the systems known to affect climate are now in positive feedback. Any addition of heat from any source will be amplified, not resisted, as would be expected on a healthy Earth. Of course, if we could manage to establish a net cooling trend the same positive feedback would work in our favour and accelerate cooling.
Some of these positive feedbacks are:
1) The ice albedo feedback first proposed by the Russian geophysicist M. I. Budyko (‘albedo’ refers to the reflectivity of an object or a surface). Ground covered by snow reflects almost all sunlight falling on it back into space and therefore stays cold. But once the snow at the edges begins to melt, dark ground emerges which absorbs sunlight and therefore gets warmer. Its warmth melts more snow, and with positive feedback melting accelerates until all the snow is gone. When the net trend is towards cooling, the same process operates in reverse. Just now the floating ice of the polar basin is rapidly melting and is an example of the Budyko effect in operation.
2) As the oceans warm, so the area covered by nutrient-poor water increases, making the ocean less friendly for algae. This reduces the rate of pump down of carbon dioxide and the generation of white reflecting marine stratus clouds.
3) On the land, increasing temperature tends to destabilize tropical forests and lessen the area they cover. The land that replaces the forest lacks cooling mechanisms and is hotter, and so, like the snow, the forest melts away.
4) Richard Betts, in a 1999 Nature paper, first observed that the Boreal forests in Siberia and Canada are dark and heat absorbing. As the world grows warmer they extend their range and so absorb more heat.
5) As forest and algal ecosystems die their decomposition releases carbon dioxide and methane into the air. In a warming world this also acts as a positive feedback.
6) Large deposits of methane are held in ice crystals within molecular–sized voids, called clathrates. These are stable only in the cold or under high pressure. As the Earth warms there is an increasing risk of these clathrates melting, with the escape of large volumes of methane, which is twenty-four times as potent a greenhouse gas as carbon dioxide.
There are almost certainly other systems, both geophysical and geophysiological, that affect climate that we have not so far discovered, but the rate of global warming suggests that there is no large negative feedback that would countervail temperature rise. The only system we do know of that acts in negative feedback† is the long-term weathering sink for carbon dioxide, called ‘rock weathering’.† This is the bio-chemical process by which carbon dioxide dissolved in rain water reacts with calcium-silicate rocks. Vegetation on the rocks greatly enhances the removal of carbon dioxide, and the greater warmth leads to faster vegetation growth, making a stronger sink for carbon dioxide. But too much heat on the land masses could turn this also to positive feedback. There is also a negative feedback caused by fierce tropical storms, which stir the water sufficiently to draw up nutrients from below the surface layer and so allow algal blooms. We do not yet know how large an effect this has on climate.
Past and present atmosphericpollution with carbon dioxide and methane is similar to the natural release of these gases fifty-five million years ago, when comparable quantities of carbon entered the atmosphere. Then the temperature rose about 8°C in the temperate northern regions and 5°C in the tropics; the consequences of this heating lasted 200,000 years.
Until recently we accepted that the evolution of organisms takes place according to Darwin’s vision, and the evolution of the material world of rocks, air and ocean according to textbook geology. But Gaia Theory sees these two previously separated evolutions as part of a single Earth history, where life and its physical environment evolve as a single entity. I find it helpful to think that what evolves are the niches, and organisms negotiate for their occupancy.
The ideas I have just presented are part of the basis of Gaia Theory, but a full explanation would require an account of how self-regulation works. In some ways this is not just difficult, it is impossible: emergent phenomena like life, consciousness and Gaia resist explanation in the traditional cause-and-effect sequential language of science. Emergence has similarities with the quantum phenomena of ‘entanglement’, and we may never be fully able to explain them. What we can do is express them in the language of mathematics and use them in the cornucopia of our inventions. Engineers are well able to design complex self-regulating systems, such as automatic pilots for ships, aircraft and spacecraft; communications engineers and cryptologists are already making devices that exploit quantum entanglement. But I doubt if any of them have a conscious mental image of their inventions; they develop and understand them intuitively.
To recapitulate, the part of Gaia thinking that most confuses is the question: what is self-regulation? What first amazed me about the Earth system was its capacity to stay close to the right temperature and the right chemical composition for life and to have done so for over three billion years, a quarter of the time the universe is thought to have existed. But for many years after the intuition of Gaia, I had no idea how it worked.
When I was about ten years old I was taken by my mother and father on winter Sundays from our home in Brixton to South Kensington. Their destination was the Victoria and Albert Museum, filled with art treasures, and mine was the Science Museum. Like most boys of that time, 1928 to 1932, I was fascinated by mechanical things and wanted to know how they worked. One of the exhibits was a working model of the steam engine, complete with James Watt’s famous governor. This device regulates the engine’s speed, and it consists of a vertical shaft driven by the engine on which is mounted two arms that carry iron balls at their ends. The arms are hinged to the shaft so that, as the shaft rotates, the balls swing out. The faster the engine runs, the higher the balls are lifted; a second pair of arms connected to those carrying the rotating balls simply lifts a lever controlling the flow of steam from the boiler of the engine. The faster the engine runs the more the steam valve is closed. It was obvious to me as a child that the engine would settle down to run at a constant speed, and that simply by changing the setting of the connection to the steam valve the speed could be set as high or as low as one wished. This was an early example of a control system using a negative feedback to govern the otherwise uncontrollable engine. Without it, the machine would race and perhaps shake itself to pieces when the steam pressure was high, or stop or run too slowly when the pressure was low. But was it really this simple?
James Clerk Maxwell was arguably the greatest physicist of the nineteenth century; in his mind the forces of magnetism and electricity were brought together in a comprehensive electromagnetic theory, a theory that laid the foundations of much of modern physics. Maxwell is reported to have said, a few days after seeing Watt’s spinning ball governor, ‘It is a fine invention, but try as I may, its analysis defies me.’ Maxwell’s puzzlement was not so surprising. Simple working regulators, the physiological systems in our bodies that regulate our temperature, blood pressure and chemical composition, and simple models like Daisyworld, are all outside the sharply defined boundary of Cartesian cause-and-effect thinking. Whenever an engineer like Watt ‘closes the loop’ linking the parts of his regulator and sets the engine running, there is no linear way to explain its working. The logic becomes circular; more importantly, the whole thing has become more than the sum of its parts. From the collection of elements now in operation a new property, self-regulation, emerges – a property shared by all living things, mechanisms like thermostats, automaticpilots, and the Earth itself.
The philosopher Mary Midgley in her pellucid writing reminds us that the twentieth century was the time when Cartesian science triumphed. It was a period of excessive hubris and called itself the century of certainty; at its start there were eminent physicists saying, ‘there are only three things left to discover’, and at the end they were seeking the ‘theory of everything’. Now in the twenty-first century we are beginning to take seriously the remark of that truly great physicist, Richard Feynman, about quantum theory: ‘anyone who thinks they understand it probably does not.’ The universe is a much more intricate place than we can imagine. I often think our conscious minds will never encompass more than a tiny fraction of it all and that our comprehension of the Earth is no better than an eel’s comprehension of the ocean in which it swims. Life, the universe, consciousness, and even simpler things like riding a bicycle, are inexplicable in words. We are only just beginning to tackle these emergent phenomena, and in Gaia they are as difficult as the near magic of the quantum physics of entanglement. But this does not deny their existence.