Consciousness/Arousal (description of patient & timing is most helpful)
• Arousal: spectrum from awake/alert → drowsy → stupor → coma. Terms vague & subjective, so most useful to describe response to increasing stimulation (eg, voice → noxious).
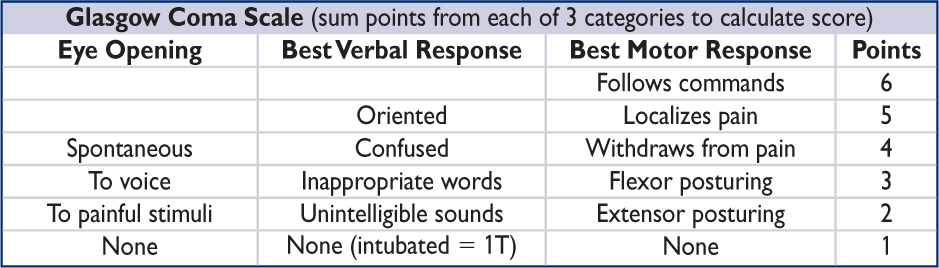
• Coma: lack of response to external stimuli. Degree formalized in Glasgow Coma Scale. Caused by focal lesions in brainstem (reticular activating system), thalamus, or diffuse dysfxn of both cerebral hemispheres. Mimics: locked-in synd., akinetic mutism, catatonia.
• Delirium/acute confusional state: altered attention & awareness, develops over hrs to days, often fluctuating, accompanied by cognitive Δs (eg, disorientation, memory loss, perceptual Δs); sometimes w/ sleep–wake dysregulation, autonomic Δs, emotionality
• Dementia: progressive cognitive impairment developing over mos to yrs; often affects memory, language, visuospatial and executive function; attention often spared
Etiologies of Decreased Responsiveness |
|
1° Neurologic (usually with focal signs) |
Systemic (esp. in elderly or prior CNS injury) |
Vasc: ischemic stroke/TIA, ICH, VST, PRES, vasculitis, pituitary apoplexy Seizure: postictal, status, nonconvulsive Infxn: meningitis, encephalitis, abscess Trauma: TBI, concussion, diffuse axonal injury ↑ intracranial pressure: mass, edema, hydrocephalus, herniation Autoimmune/paraneoplastic enceph. Neurodeg: late-stage (eg, Alzheimer’s) or rapidly progressive (eg, CJD) |
Cardiac: global ischemia, HoTN, HTN enceph Pulmonary: ↓ PaO2, ↑ PaCO2 GI: liver failure, ↑ NH3 Renal: uremia, dialysis, ↓ or ↑ Na, ↓ or ↑ Ca Heme: TTP/HUS, DIC, hyperviscosity Endo: ↓ glc, DKA/HHNS, hypothyr., Addisonian ID: pneumonia, UTI, endocarditis, sepsis Hypothermia & hyperthermia Meds: anticholin., anti-hist., psychotrop., digoxin Toxins/withdrawal: EtOH, sedative, opiate, CO Psychiatric: catatonia, serotonin synd., NMS |
• History (witness & background crucial): tempo, premorbid sx (eg, focal neuro deficits, HA, infxn, pain, falls), medical conditions (eg, dementia, epilepsy, onc, cardiac, psych, infection/immune status), accompanied by head trauma, current meds (eg, sedatives, opioids, anticoag, anticonvulsants, immunosuppressants), drug/alcohol use
• General exam: VS, breathing pattern (eg, Cheyne-Stokes), tongue bite (seizure), nuchal rigidity (meningitis, SAH; do not test if c/f trauma/cervical spine fx), ecchymoses, rash, signs of head trauma (eg, Battle sign, raccoon eyes, hemotympanum, CSF rhinorrhea), asterixis, liver disease stigmata, embolic phenomena/endocarditis, s/s drug use
• Neuro exam (see below): perform off sedatives/paralytics if possible, look for focal deficits suggesting structural cause (eg, stroke, herniation), s/s of ↑ ICP (eg, HA, vomiting, papilledema, abducens nerve palsy, unilateral dilated pupil, ↑ BP/↓ HR, fixed downgaze)
Neuro Exam in Patients with Decreased Responsiveness |
|
Mental status |
Arousal (behavioral response to ↑ intensity of stimulation, GCS) |
Cranial nerves |
Pupils: pinpoint → opiates, pontine lesion; midposition & fixed → midbrain lesion; fixed & dilated → severe anoxic injury, herniation, anti-cholin. Extraocular movements / vestibulo-ocular reflex tests: Oculocephalic maneuver (“doll’s eyes”): nl = eyes move opposite head movement (do not test if possible cervical spine trauma) Vestibular (cold) caloric stimulation: in coma, nl = eyes move slowly to lavaged ear, then quickly away (do not test w tymp memb perf) Corneal reflex, facial grimace to nasal tickle Gag & cough reflexes (with ET tube manipulation if necessary) |
Motor |
Tone, spont movements, flexor/extensor posturing of arms/legs, strength |
Sensory |
Response to painful stimuli: purposeful vs. reflexive/posturing |
Reflexes |
Deep tendon reflexes, Babinski, “triple” flexion (ankle, knee, & hip flexion to noxious stimulation → not suggestive of intact cortical function) |

Initial treatment
• Empiric antibiotics if c/f CNS infection: vancomycin/CTX, consider acyclovir and ampicillin
• Immobilization of C-spine if concern for cervical trauma
• Thiamine 100 mg IV → dextrose 50 g IVP (this order to prevent exacerbation of Wernicke’s)
• If opiates suspected: naloxone 0.01 mg/kg; if BDZ suspected, consider flumazenil 0.2mg IV
• If concern for ↑ ICP ± herniation: ↑ head of bed; osmotherapy w/ mannitol or hypertonic saline; ↑ ventilation; dexamethasone for tumor edema; c/s neurosurgery (? decompress)
Diagnostic studies (Lancet 2014;384:2064)
• All patients: check fingerstick glucose, electrolytes, BUN/Cr, LFTs, CBC, tox screen, U/A
• Based on clinical suspicion:
Labs: NH3, TSH, cort stim, B12, ABG, HIV, ESR, ANA, TPO/anti-TG, BCx, drug levels
Imaging: head CT, then MRI; CTA if c/f stroke/SAH; radiographs to r/o C-spine fracture
Lumbar puncture to r/o meningitis, SAH, or noninfectious inflammation (eg, autoimmune)
EEG to evaluate for nonconvulsive seizures, toxic/metabolic encephalopathy
Further treatment of delirium (NEJM 2017;377:1456)
• Treat underlying acute illness, eliminate precipitating factors, & provide supportive care
• Address sensory & cognitive impairments (frequent reorientation, glasses/hearing aids, etc.)
• Decrease/prevent infection/restraints if possible, remove lines/catheters if unnecessary
• Promote good sleep: reduce noise & nighttime interventions; sedative med if necessary
• Meds: consider antipsychotics (but neither haloperidol nor ziprasidone ↓ delirium duration in ICU Pts; NEJM 2018;379:2506); avoid benzos except in EtOH withdrawal or seizures
ANOXIC BRAIN INJURY (at risk if ≥5 min cerebral hypoxia)
Initial evaluation (Circulation 2010:S768)
• Neuro exam: arousal/verbal, eyes & other cranial nerves, motor response to pain
• Imaging: CT usually not informative w/in first day after arrest, but should be done prior to initiating hypothermia if patient found down or has had head trauma
Targeted temperature management (Circulation 2015;132:2448)
• Indications: comatose (GCS <8) w/in 6h after cardiac arrest (not isolated resp. arrest). Studied only in VT/VF, but consider after asystole or PEA, or 6–12h post-arrest.
• Exclusions: pregnancy, CV instability despite pressors/assist devices, other cause of coma, persistent ↓ O2. Relative contraindications: major head trauma, coagulopathy/bleeding, major surgery <14d, systemic infection/sepsis.
• Target temp: 32–36°C × ≥24h. Initial studies showing benefit targeted 32–34°C, but subsequent study showed ≈ outcomes for 36°C vs. 33°C (NEJM 2013;369:2197). Some still target 32–34°C and reserve 36°C for Pts w/ contraindic to more aggressive cooling.
• Method: ice packs to head/neck/torso; cooling blankets; cooling vest or endovascular catheter. Goal to achieve target temp <6h (but no benefit to prehosp cooling; JAMA 2014;311:45). Pts should be sedated/paralyzed while cooled. MAP goal >70. Start rewarming 24h after cooling is initiated (rewarm ≤0.5°C per h).
• In Pts not cooled or after rewarming Pts who were cooled: prevent fever (goal <36°C) for ≥48h post arrest
• Complications
Dysrhythmias (brady most common): if significant or hemodynamic instability → rewarm
Coagulopathy (can receive lytics, GP IIb/IIIa inhibitors, etc.); monitor PT & PTT
Infection: monitor surveillance blood cultures during cooling
Hyperglycemia during cooling, hypoglycemia w/ rewarming; stop insulin if glc <200 mg/dL
Hypokalemia during cooling, hyperkalemia w/ rewarming; keep K 4–5 mEq/L
Ongoing evaluation
• Neuro exam: daily focus on coma exam. No exam finding is reliable <24 h or on sedation. Should be off sedation for adequate time (depends on dose, duration, Pt’s metabolism).
• Labs: daily CBC, PT/PTT, electrolytes. Serum neuron-specific enolase (NSE) on days 1–3.
• Imaging: noncontrast CT 24 h after arrest; if unrevealing, consider MRI around days 3–5
• EEG: consider in all to exclude seizures; greatest risk during rewarming
• Somatosensory evoked potentials (SSEP): helpful for prediction of poor outcome if cortical responses are absent bilaterally; perform 48 h after arrest (72 h if cooled)
Prognosis (Nat Rev Neuro 2014;10:190)
• Prior to cooling era, poor prognosis at 72 h if absent pupillary & corneal reflexes and no motor response to pain; or absent SSEPs at 48 h. With cooling, unclear if prior measures as reliable. Overall ~12% survive to hosp. d/c; VT/VF 25-40%, PEA ~10%, asystole ~2%.
• Prognosis requires multifactorial assessment based on age, exam, comorbidities, ancillary data. Poor signs: absent brainstem reflexes, Rx-resistant myoclonus, EEG w/ absent background/reactivity, NSE >33, diffuse hypoxic injury on MRI. If doubt, err on more time.
Definitions & clinical manifestations (Epilepsia 2017;58:522)
• Seizure: transient neurologic symptoms due to excessive synchronous neuronal activity; may be provoked by a reversible factor lowering the seizure threshold, or unprovoked
• Epilepsy: ≥2 unprovoked seizures occurring >24 h apart or 1 unprovoked seizure w/ ≥60% probability of further seizures over the next 10 y (see below for prognostication)
• Generalized seizures (involves brain diffusely)
Tonic-clonic (grand mal):
Aura (sec to mins): premonition with paresthesias, focal motor contractions, abnormal smells/tastes, fear, depersonalization, déjà vu, autonomic changes, automatisms
Ictal period (sec to mins): lateral gaze and head deviation, tonic contraction of muscles → intermittent relaxing and tensing of muscles, tongue biting, urinary incontinence, pooling of secretions, incontinence
Postictal period (mins to h): slowly resolving period of confusion, disorientation, and lethargy. May be accompanied by focal neurologic deficits (Todd’s paralysis).
Absence (petit mal): transient lapse of consciousness w/o loss of postural tone, usu pedi
Myoclonic (infantile spasms & juvenile myoclonic epilepsy): sudden, brief contraction
• Focal seizures (involves discrete brain area, often associated with a structural lesion)
w/o impaired awareness: focal motor/autonomic sx (formerly “simple partial seizure”) or focal sensory/psychic symptoms (eg, aura)
w/ impaired awareness: dyscognitive features (formerly “complex partial seizure”)
evolving to bilateral, convulsive seizure (formerly “secondarily generalized seizure”)
• Status epilepticus: continuous convulsive seizure ≥5 min or >2 seizures w/o resolution of postictal encephalopathy; life threatening
• Nonconvulsive status epilepticus: alteration of awareness (ranging from confusion to coma) w/o motor manifestations of seizure; dx with EEG
Differential diagnosis
• Syncope (Lancet Neurol 2006;5:171)
Feature |
Seizure |
Syncope |
Aura |
Unusual behavior/automatisms |
Diaphoresis, nausea, tunnel vision |
Convulsions |
Variable duration |
Usually <10 sec |
Postictal state |
Yes; can be ≥30 min |
None or short |
Other clues |
Tongue biting, incontinence |
Skin pallor, clamminess |
• Nonepileptic seizure (aka “psychogenic”): may see side-to-side head turning, asymmetric large-amplitude limb movements, hip thrusting, diffuse shaking w/o LOC, crying/talking during event; diagnosis requires spell capture on EEG with no EEG correlate
• Other: metabolic disorders (eg, alcoholic blackouts, hypoglycemia), migraine, TIA, transient global amnesia, narcolepsy (cataplexy), nonepileptic myoclonus, tics, asterixis
Etiologies of seizures (vary strongly by age)
• Without focal lesion: genetic predisposition to seizures or epilepsy syndrome; alcohol withdrawal, illicit drugs; meds (eg, β-lactams, bupropion, fluoroquinolones, tramadol, MNZ, meperidine, CsA); electrolyte (hyponatremia) & other metabolic (eg, uremia, liver failure, hypoglycemia); autoimmune encephalitis, idiopathic (~60%)
• With focal lesion: tumor, trauma, stroke, subdural hematomas, posterior reversible encephalopathy syndrome, mesial temporal sclerosis, abscess, focal cortical dysplasia
Clinical evaluation (JAMA 2016;316:2657)
• History key in differentiating seizure from other causes of transient loss of consciousness. Must talk to witnesses. Ask about prodrome, unusual behavior before spell, type & pattern of abnl movements incl. head turning & eye deviation (gaze preference usually away from seizure focus), loss of responsiveness.
• Recent events: illnesses/fevers, head trauma, sleep deprivation, stressors
• PMH: prior seizures or ⊕ FHx; prior CNS infection, stroke or head trauma; dementia
• Medications (new or noncompliance), alcohol and illicit drug use
• General physical exam should include the skin, looking for neuroectodermal disorders (eg, neurofibromatosis, tuberous sclerosis) that are a/w seizures
• Neurologic exam should look for focal abnormalities → underlying structural abnormality
Diagnostic studies (Neurology 2007;69:1996)
• Lab: full lytes, BUN, Cr, glc, LFTs, tox screen, AED levels (valproic acid and phenytoin have therapeutic range; levetiracetam level rarely useful unless ? noncompliance), illicit drug screen
• Routine EEG (~30 min): may help determine risk of seizure recurrence after 1st-time unprovoked seizure. Caveat: interictal EEG nl in 50% of Pts w/ epilepsy, and interictal epileptiform activity (spikes or sharp waves) seen in up to 2% of nl population; EEG w/in 24h, sleep deprivation and repeated studies ↑ dx yield of EEG.
• Long-term EEG monitoring (hrs to days): if suspicion for non-convulsive status or non-epileptic seizures; video monitoring may help w/ nonepileptic seizures
• MRI to r/o structural abnormalities; ↑ Se w/ fine coronal imaging of frontal & temporal lobes
• LP (if no space-occupying lesion on imaging): if suspect meningoencephalitis (eg, fever, ↑ WBC, nuchal rigidity) or autoimmune encephalitis and in all HIV ⊕ Pts
Treatment (Neurology 2015;84:1705; Lancet 2015;385:884)
• Treat any underlying precipitants, including CNS infections, intoxication, withdrawal, etc.
• Antiepileptic drug (AED) Rx usually reserved for Pts w/ ≥2 unprovoked seizures, single seizure w/ high risk of recurrence (see below), or underlying structural abnormality. Provoked seizures generally treated by addressing underlying cause; consider AED if status epilepticus on presentation, focal neuro exam, postictal Todd’s paralysis.
• After 1st unprovoked sz, weigh risks of recurrence vs AED. ↑ risk of recurrence if abnl EEG, MRI, or nocturnal sz. If EEG & MRI nl → 65% sz-free at 5 y (Lancet Neurol 2006;5:317).
• Immediate treatment w/ AED after 1st unprovoked seizure ↓ risk of recurrence over 2 y, but does not Δ long-term prognosis
• If AED Rx indicated, choice dependent on type of seizure, side effects, cost, mechanism of elimination (if hepatic or renal insufficiency), teratogenesis, and drug interactions
• Introduce gradually, monitor carefully
• May consider withdrawal of meds if seizure free (typically for at least 1 y) and normal EEG
• Individual state laws mandate seizure-free duration before being allowed to drive
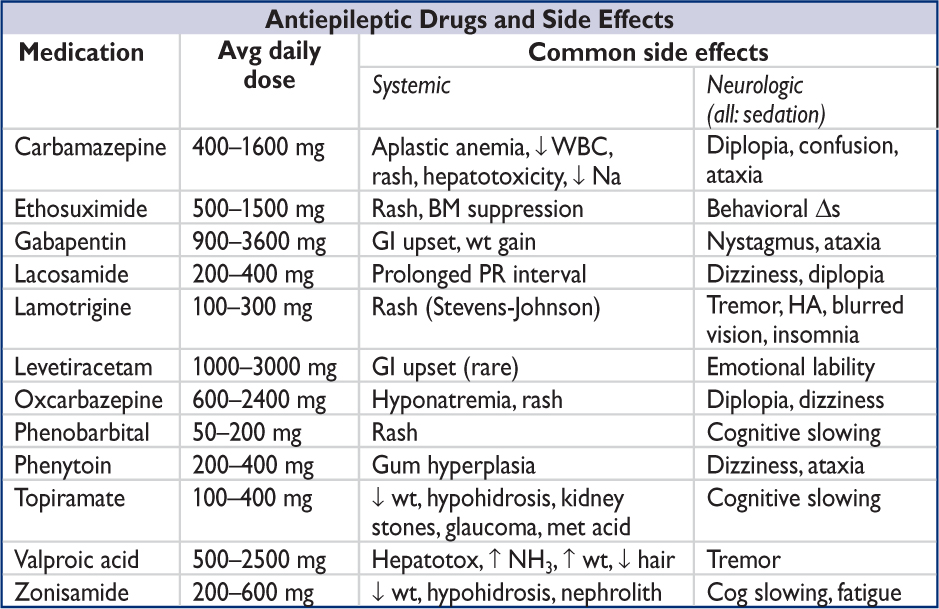
(NEJM 2008;359:166; Lancet Neurol 2011;10:446)
Status epilepticus (Epilepsy Curr 2016;16:48)
• ABCs: vital signs, oral airway or endotracheal intubation. Place Pt in semiprone position to ↓ risk of aspiration. Obtain IV access. Give thiamine, dextrose, IV normal saline.
• STAT glc, metabolic panel, CBC, tox screen, lactate, AED levels, consider head CT, LP
• Start standing AED after loading dose.

PE, phenytoin equivalents. *Consider PR diazepam if no IV access and IM midazolam is contraindicated.
Clinical manifestations
• Minor withdrawal sx (6–48 h after last drink): mild anxiety, tremulousness, HA
• Withdrawal seizures: typically w/in 48 h after last drink; if unRx’d, 1/3 → delirium tremens
• Alcoholic hallucinosis: isolated hallucinations (typically visual) 12–48 h after last drink
• Delirium tremens (DT): disorientation, agitation, hallucinations, ↑ HR & BP, fever, diaphoresis; begins 48–96 h after last drink, lasts 5–7 d
• Consider other dx: CNS infxn or bleed, sz, drug O/D, coingestions, acute liver failure, GIB
• Ten-item scale (CIWA-Ar) used to assess and manage alcohol withdrawal (see Appendix)
Treatment (NEJM 2003;348:1786)
• Benzodiazepines (BDZ)
Drug: diazepam (long-acting w/ active metab; ↓ risk of recurrent withdrawal), lorazepam (short half-life), chlordiazepoxide, oxazepam (no active metab; good if cirrhosis)
Dosing: typically start w/ diazepam 10–15 mg IV q10–15min (or lorazepam 2–4 mg IV q15–20min) until appropriate sedation achieved, then titrate to CIWA-Ar scale, evaluating q1h until score <8 × 8 h, then q2h × 8 h, and if stable, then q4h (JAMA 1994;272:519)
• If refractory to BDZ prn → BDZ gtt, phenobarb, dexmedetomidine, or propofol (& intubation)
• Avoid βB (mask sx)
• Mechanical restraints as needed until chemical sedation achieved
• Volume resuscitation as needed; thiamine then glc to prevent Wernicke’s encephalopathy (ataxia, ophthalmoplegia, short-term memory loss); replete K, Mg, PO4
• Prophylaxis: if min sx or asx (ie, CIWA score <8) but prolonged heavy EtOH consumption or h/o withdrawal seizures or DTs → chlordiazepoxide 25–100 mg (based on severity of EtOH use) q6h × 24 h, then 25–50 mg q6h × 2 d
Differential diagnosis
• Includes a variety of sx. Disequilibrium: sense of imbalance, gait disturbance; vertigo: perception of spinning; near syncope: lightheadedness due to cerebral hypoperfusion.
• Vertigo Ddx:
Peripheral
BPPV: dislodged canaliths in semicircular canal; episodic rotatory vertigo (<1 min episodes), triggered by changes in position; Rx: Epley/BBQ roll maneuver
Meniere’s disease: ↑ endolymphatic pressure in inner ear; episodic rotatory vertigo (min-hrs), N/V, aural fullness, hearing loss, tinnitus; Rx: diuretics, ↓ salt
Vestibular neuritis: sudden-onset w/ gait ataxia; if w/ hearing loss = labyrinthitis
Central
Posterior circulation stroke/TIA: “5 Ds” of dizziness, diplopia, dysarthria, dysphagia, dystaxia; sudden onset (resolves after mins in TIA, persists in stroke)
Other: migraine, Chiari, epilepsy, MS, tumors, drugs/meds, concussion
Initial evaluation
• Hx: ask open-ended questions (description by Pt may be unreliable), pace of illness, episodic vs. chronic, meds, other sx of posterior circ including diplopia, dysarthria, ataxia
Exam |
Peripheral Causes |
Central Causes |
Orthostatics |
⊕ in orthostatic syncope |
Typically absent |
Eye movements |
Nystagmus unidirectional if present, never vertical, suppressed w/ fixation |
Nystagmus bidirectional, often vertical, not suppressed w/ fixation |
Hearing |
May be impaired in some peripheral causes of vertigo |
Normal (rarely unilat. hearing loss in AICA-territory stroke) |
Coord./gait |
Normal |
May reveal limb, trunk, gait ataxia |
• HINTS testing (Stroke 2009;40:3504)
Head impulse test: Pt fixates on examiner’s nose during rapid passive head turn; presence of “catch-up saccade” supports peripheral dysfunction to side of turn
Nystagmus (see table above)
Test of skew: vertical refixation saccade on alternating eye cover supports central cause
• Dix-Hallpike test: Pt sitting → lying back w/ 45º head tilt; elicits rotatory nystagmus after delay of secs; fatigues if repeated; ⊕ suggests BPPV w/ affected ear down
• Supine Roll test: nystagmus elicited by head turn while patient supine; when ⊕ suggests BPPV w/ affected ear down (lateral canal, 8% of cases)
• Studies: ECG, basic labs, if concerning s/s on HINTS → MRI brain
• Treatment: reposition maneuv. for BPPV, vestib. PT; anti-hist., sedatives or anti-emetics
ISCHEMIC STROKE
Etiologies
• Embolic: artery → artery, cardioembolic (~30% due to AF; NEJM 2014;370:2478), paradoxical
• Thrombotic: large vessel (atherosclerosis) vs. small vessel (“lacunar,” lipohyalinosis of small arteries, often related to smoking, HTN, hyperlipidemia, & DM)
• Other: dissection, vasculitis, vasospasm, hypercoag, hypoperfusion, endocarditis, venous
Clinical manifestations
• Timing: embolic → sudden onset; thrombotic → may have stuttering course
Stroke Syndromes by Vascular Territory |
|
Artery |
Deficits |
ICA → Ophth |
Amaurosis fugax (transient monocular blindness) |
ACA |
Hemiplegia (leg > arm), abulia, urinary incontinence, primitive reflexes |
MCA |
Hemiplegia (face & arm > leg); hemianesthesia; homonymous hemianopia Aphasia if dom. hemisphere: sup. div. → expressive; inf. div → receptive Apraxia & neglect if nondom. hemisphere. |
PCA |
Macular-sparing homonymous hemianopia; alexia w/o agraphia Thalamic syndromes with contralateral hemisensory disturbance |
Vertebral, PICA |
Wallenberg syndrome = numbness of ipsilateral face and contralateral limbs, diplopia, dysarthria, dysphagia, ipsilateral Horner’s, hiccups |
Basilar |
Pupillary Δs (midbrain=dilated, pons=pinpoint), long tract signs (quadriplegia, sensory loss), CN abnl, cerebellar dysfxn. Top of basilar → “locked in” synd. |
Cerebellar |
Vertigo, N/V, diplopia, dysarthria, nystagmus, ipsilateral limb ataxia |
Lacunar (arterioles) |
5 major syndromes: pure hemiplegia, pure hemianesthesia, ataxic hemiparesis, dysarthria + clumsy hand, mixed sensorimotor |
Transient ischemic attack (TIA)
• Sudden deficit due to cerebral ischemia; no stroke on imaging; most resolve in <1 h
• Ddx: seizure, migraine, hypoglycemia, amyloid spells, TGA, anxiety
• Risk of subsequent stroke ~2% by 1 wk (NEJM 2016;374:1533). Can stratify based on ABCD2: Age ≥60 y (+1); BP ≥140/90 (+1); Clin features: unilat. weak. (+2), speech impair. w/o weakness (+1); Duration ≥60 (+2) or 10–59 min (+1); DM (+1)
Physical exam
• General: murmurs, carotid & subclavian bruits, peripheral emboli, endocarditis stigmata
• Neurologic exam, NIH stroke scale (http://www.ninds.nih.gov/doctors/NIH_Stroke_Scale.pdf)
Acute workup
• Electrolytes, Cr (relevant for contrast); glc, CBC, coags (see exclusion criteria for lysis)
• Cardiac biomarkers, 12-lead ECG, tox screen
• STAT CT to r/o ICH prior to lysis. (Se ICH ≈ MRI, CT faster). Early signs of stroke: hyperdense artery, loss of gray-white differentiation, edema, insular ribbon. CT can be nl initially, and not Se for small & brainstem. CTA if considering endovascular intervention.
Acute treatment of ischemic stroke (Lancet 2017;389:641; Stroke 2018;49:e46)
• Thrombolysis (IV): tPA 0.9 mg/kg (max 90 mg), w/ 10% as bolus over 1 min, rest over 1 h
consider if onset w/in 4.5 h, ∅ ICH, ∅ contraindic. (incl. current/prior ICH; head trauma or stroke w/in 3 mo; intracranial neoplasm, AVM or aneurysm; recent intracranial/intraspinal surgery; active internal bleeding; noncompressible arterial puncture; ↑ BP; multilobar infarct; plt <100k, INR >1.7, on Xa inhib, PTT >40, glc <50)
0–3 h: 12% absolute ↑ in good neuro outcome (min/no disability), 5.8% absolute ↑ in ICH, trend toward 4% absolute ↓ mortality
3–4.5 h: 7.4% absolute ↑ in good neuro outcome, 1.8% absolute ↑ in ICH, ∅ mortality benefit (nb, trial excluded patients with previous strokes + DM)
Data for TNK, Rx up to 9 h, and for MRI imaging to guide Rx (NEJM 2018;378:1573 & 379:611; 2019;380:1795)
• BP: lower to <185/110 to consider lysis; if lyse keep <180/105 × 24 h (consider labetalol or nicardipine), o/w permissive HTN unless >220/120 or sx; if sx HoTN consider vasopressors
• Initiate ASA w/in 24–48 h; avoid anticoagulation w/in 24 h of lysis; see below for long-term Rx
• Cerebral edema → herniation: 1–5 d post large MCA or cerebellar strokes, ↑ risk in young. Elevate HOB >30°; mannitol ± 23% NaCl. Hemicraniectomy ↓ mortality (NEJM 2014;370:1091). Neurosurgery consult in select MCA and all large cerebellar strokes.
• Endovascular thrombectomy indicated if w/in 6 h of sx onset, pre mRS 0-1, occlusion in ICA or MCA, NIHSS ≥6 (clinical severity), ASPECTS ≥6 (CT based likelihood of recovery) (NEJM 2015;372:11, 1009, 1019, 2285 & 2296; Lancet 2016;387:1723). May extend to 6–24 h if mismatch between infarct size and clinical deficits or stroke penumbra (NEJM 2018;378:11 & 708).
Workup to assess for etiology/modifiable risk factors
• Cardiac: prolonged Holter for AF (eg, 10 d at presentation, 3 & 6 mo detects in 14%); TTE to r/o thrombus/veg, w/ bubble study to r/o PFO/atrial septal aneurysm if suspect embolic
• Vessel imaging: CTA or MRA head/neck; carotid U/S w/ Doppler if contraindic to CTA/MRA
• Labs: lipids, HbA1c, TSH, homocysteine, Lp(a), hypercoag w/u (if <65 y or cryptogenic stroke; ideally drawn before starting anticoag), ESR/CRP, blood cx if s/s systemic infection
• MRI helpful if dx of stroke unclear (esp. post circ) or to define stroke subtype, age, exact size
DWI bright/ADC dark = earliest finding in acute ischemia (~w/in mins, up to days)
T2-FLAIR: hyperintense w/in hrs, persists for wks; PWI differentiates irreversibly infarcted core vs. viable penumbra; T1 fat-sat (neck vessels) if suspicious for dissection
Secondary stroke prevention (NEJM 2012;366:1914)
• Antiplatelet therapy: different agents likely have similar efficacy
ASA ↓ death & repeat stroke; equal to warfarin in nonembolic stroke (NEJM 2001;345:1444)
clopidogrel: marginally superior to ASA, slightly ↑ ICH (Lancet 1996;348:1329)
clopidogrel + ASA (vs. ASA alone): Rx for 21 d in minor strokes/TIA → ↓ risk of stroke, ? ↑ ICH; longer Rx not better & ↑ ICH (NEJM 2013;369:11 & 2018;379:215; BMJ 2018;363:k5108) Rx for 90 d if stroke due to intracranial athero (NEJM 2011;365:993)
• Anticoagulation (AC): consider for AF (qv), cardiac/paradoxical emboli (except bacterial endocard); large extra-dural dissections; hypercoag; bridge to CEA in sx carotid stenosis
Hold off on AC in large strokes for ~2–4 wk given risk of hemorrhagic conversion
• Long-term SBP target 120–139 mmHg (JAMA 2011;306:2137)
• ↓ LDL-C (<< 70 mg/dL): ↓ recurrence w/ statin PCSk9i added to statin (NEJM 2017;376:1713)
• Fluoxetine: improved motor recovery after 3 mo (Lancet Neurol 2011;10:123)
• Carotid revascularization (NEJM 2013;369:1143)
CEA (if surgical morbidity & mortality ≤6%) indicated for:
sx stenosis 70–99% (benefit ↑ for males, >75 y, ≤2 wk from stroke) → 65% ↓ RR of repeat stroke, slight benefit for 50–69% stenosis (NEJM 1991;325:445; Lancet 2004;363:915)
asx stenosis 70–90%, <79 y: 50% ↓ RR of repeat stroke (Lancet 2010;376:1074)
Stenting: c/w CEA, periprocedural stroke ↑ (esp. in elderly) & MI ↓ (but many asx), subseq. stroke rate ≈ (NEJM 2016;374:1011 & 1021; Lancet 2016;387:1305; Lancet Neuro 2019;18:348)
Patent foramen ovale (PFO; in ~27% of population) (NEJM 2005;353:2361)
• ↑ stroke risk: ≥4 mm separation, R→L shunting at rest, ↑ septal mobility, atrial septal aneurysm
• If PFO & stroke/TIA: no benefit of warfarin over ASA (Circ 2002;105:2625), but consider if at high risk for or has DVT/PE. Closure ↓ recurrence by ≥50% if ↑ risk features (see above) and absence of factors suggesting alternate etiology (NEJM 2017;377:1011, 1022, 1033). RoPE score: age (+1 for each decade <70); cortical stroke on imaging (+1); HTN, DM, h/o stroke/TIA, smoker (+1 for each absent risk factor). Consider closure if >7 (JAHA 2018;7:1).
INTRACRANIAL HEMORRHAGE (ICH)
Classification by location
• Hemorrhagic strokes: intraparenchymal hemorrhage (IPH) & subarachnoid hemorrhage (SAH)
• Other ICH: epidural hematoma (EDH) & subdural hematoma (SDH)
Etiologies
• AVM, aneurysm, cerebral venous sinus thrombosis → IPH or SAH
• HTN (basal ganglia, cerebellum, brainstem), cerebral amyloid (lobar), tumor (esp. w/ melanoma, renal cell CA, chorio-CA, thyroid CA) → IPH
• Trauma → all locations (nb, IPH or SAH caused by trauma technically not a stroke)
Clinical manifestations (Lancet 2017;389:655 & NEJM 2017;377:257)
• ↓ consciousness, N/V, HA, progressive focal neurologic deficits
• SAH: thunderclap HA, onset w/ exertion; nuchal pain/rigidity; LOC. EDH: initial lucid interval.
Workup (Acad Emerg Med 2016;23:963)
• STAT CT brain, angio (CT-A or conventional) if suspicious for vascular source
• ? LP for xanthochromia if no evid of ICH on CT (although ⊖ LR 0.01) & suspicious for SAH
• Coags (PT, PTT, INR)
Management (Crit Care Med 2016;44:2251; JAMA 2019;321:1295)
• Reverse coagulopathy, INR <1.4. Plt >100k, no need for plt tx if on antiplt Rx (? if ↑ ICH), DDAVP if uremic. 2-3 mo after recovers, can restart antiplt mono Rx (Lancet 2019;393:2013).
• BP control w/ art line, nicardipine or labetalol gtt. SBP goal <140 for 1st 24 h, then <160 (NEJM 2013;368:2355 & 2016;375:1033), though BP goals controversial (NEJM 2016;375:1033)
• SAH: endovasc coiling vs. surg clipping (depends on location, comorbid.; Lancet 2015;385:691) of aneurysm/AVM; nimodipine to ↓ risk of vasospasm (monitor w/ TCDs), seizure Ppx
• Surg evac: EDH; SDH if >1 cm or rapid ↑; IPH: no obvious benefit (Lancet 2013;382:397)
• Venous sinus thrombosis: start anticoagulation, manage ↑ ICP and seizures as needed

PERIPHERAL NEUROPATHIES
Etiologies based on presentation
• Mononeuropathy (1 nerve): acute → trauma; chronic → entrapment, compression, DM, Lyme. Common: median n. (carpal tunnel); ulnar n. (elbow or wrist); radial n. (spiral groove); com. peroneal n. (fibular head w/ leg crossing); lat. femoral cutan. n. (inguinal lig)
• Mononeuropathy multiplex (axonal loss of multiple, noncontig. nerves): vasculitic synd. (eg, PAN, Churg–Strauss, Wegener’s, SLE, RA, Sjögren’s, cryo, HCV), DM, Lyme, HIV, leprosy, hereditary neurop. w/ pressure palsies, infiltrative (sarcoid, lymphoma, leukemia)
• Polyneuropathy (multiple symmetric nerves, generally length dependent): 30% idiopathic;
W/ autonomic features: DM, EtOH, paraneoplastic, B12 def, amyloid, chemo, 1° dysauto
Painful (small fiber nerves): DM, EtOH, amyloid, chemo, sarcoid, heavy metals, porphyria
Demyelinating. Acute: AIDP (Guillain-Barré), diphtheria. Subacute: meds (taxanes), paraneoplastic. Chronic: idiopathic, DM, CIDP, anti-MAG, HIV, hypothyroidism, toxins, paraproteinemia, hereditary (eg, CMT).
Axonal. Acute: acute motor axonal neuropathy, porphyria, vasculitis, uremia, critical illness. Subacute: EtOH, sepsis, paraneoplastic, meds (cisplatin, paclitaxel, vincristine, INH, ddI, amio). Chronic: DM, uremia, lead, arsenic, HIV, paraproteinemia, B12 defic.
Clinical manifestations
• Weakness, fasciculations, cramps, numbness, dysesthesias (burning/tingling), allodynia
• ± Autonomic dysfxn (orthostasis, constipation, urinary retention, impotence, abnl sweating)
• Depressed or absent DTRs (may be normal in small fiber neuropathy)
Diagnostic studies
• Distal symmetric polyneuropathy: CBC, lytes, BUN/Cr, HbA1C, B12, TSH, ESR, SPEP + IF
• EMG/NCS (often no change in 1st 10–14 d or in small-fiber neuropathy)
• Based on H&P: LFTs, ANA, anti-Ro/La, HIV, Cu, Lyme, RPR, UA, UPEP+IF, ACE, ANCA, heavy metals, LP (AIDP/CIDP), cryo, paraneoplastic Abs, genetic testing. Autonomic testing/skin bx (small fiber), nerve bx (mononeuropathy multiplex), fat pad bx (amyloid).
• MRI if possible radiculopathy or plexopathy (after EMG)
Pharmacologic treatment of neuropathic pain (Lancet Neurol 2015;14:162)
• Gabapentin, pregabalin, TCAs (nortriptyline, amitriptyline), SNRIs (duloxetine, venlafaxine)
• 2nd line: tramadol, topicals (lido, capsaicin); 3rd line: nerve block, botulinum toxin A
GUILLAIN-BARRÉ SYNDROME (GBS)
Definition & epidemiology (Nat Rev Neurol 2014;10:469)
• AIDP (60–80%); acute motor axonal neuropathy (AMAN; 7–30%; a/w anti-GM1, anti-GD1a Abs; worse prognosis); Miller Fisher synd. (ophthalmoplegia & ataxia; a/w anti-GQ1b Ab)
• Incidence 1–2 per 100,000; most common acute/subacute paralysis
• Precipitants in 60%: viral illness (influenza, CMV, EBV, HIV, Zika), URI (Mycoplasma), gastroenteritis (Campylobacter), Lyme, immunizations (no proven risk w/ current), surgery
Clinical manifestations (Lancet 2016;388:717)
• Pain (55–90%), distal sensory dysesthesias & numbness often 1st sx, back pain common
• Progressive symmetric paralysis in legs and arms over hrs to days; plateau in 1–4 wk
• Hypoactive then absent reflexes. <10% w/ reflexes on presentation, but all develop hypo/areflexia during course. Minority of AMAN w/ preserved reflexes throughout.
• Resp failure requiring mech vent occurs in 25%; autonomic instability & arrhythmias in 60%
Diagnostic studies (results may be normal in first several days)
• LP: albuminocytologic dissociation = ↑ protein w/o pleocytosis (<10 WBCs) seen in up to 64% of Pts. ↑ protein in ½ in 1st wk, ¾ by 3rd wk of sx. Unlikely to be GBS if WBC >50
• EMG/NCS: ↓ conduction velocity, conduction block, abnl F-waves; can be nl in 1st 2 wk
• FVC & NIF: to assess for risk of resp. failure (cannot rely on PaO2 or SaO2 alone)
• Plasma exchange or IVIg of equal efficacy (Neuro 2012;78:1009); steroids not beneficial
• Supportive care with monitoring in ICU setting if rapid progression or resp. failure
• Watch for autonomic dysfunction: labile BP, dysrhythmias (telemetry)
• Erasmus GBS outcome score can help w/ prognostication (Lancet Neurol 2007;6:589). Most recover near baseline in 1 y; 3–5% mortality. Residual deficits: pain, fatigue.
MYASTHENIA GRAVIS (MG)
Definition & epidemiology (Lancet Neurol 2015;14:1023; NEJM 2016;375:2570)
• Autoimmune disorder with Ab against acetylcholine receptor (AChR, 80%), muscle-specific kinase (MusK, 4%), lipoprotein-related protein 4 (LRP4, 2%), or other NMJ proteins
• Prevalence: 1 in 7500; affects all ages, peak incidence 20s–30s (women), 60s–70s (men)
• 15% of AchR MG a/w thymoma; 30% of pts w/ thymoma develop AchR MG
Clinical manifestations
• Fluctuating weakness w/ fatigability (worse w/ repetitive use, relieved by rest)
• Cranial muscles involved early → 60% present initially w/ ocular sx (ptosis, diplopia); 20% will only have ocular sx; 15% w/ bulbar (difficulty chewing, dysarthria, dysphagia)
• Limb weakness proximal > distal; DTRs preserved; minimal/no atrophy
• MusK MG (F >> M): mostly cranial/bulbar, neck, and resp weakness
• Exacerb. triggered by stressors: URI, surgery, preg/postpartum, meds (eg, Mg, AG, macro-lides, FQ, procainamide, phenytoin, D-penicillamine). Prednisone can worsen sx acutely.
• Myasthenic crisis = sx exacerbation, risk of respiratory compromise
• Cholinergic crisis = weakness due to overtreatment with anticholinesterase meds; may have excessive salivation, abdominal cramping and diarrhea; rare at normal doses
Diagnostic studies
• Bedside: ptosis at baseline or after >45 sec of sustained upgaze; improved ptosis with ice pack over eyes for 2–5 min (Se 77%, Sp 98%)
• Neostigmine test: temporary ↑ strength; false ⊕ & ⊖ occur; premedicate w/ atropine
• EMG: ↓ response with repetitive nerve stimulation (vs. ↑ response in Lambert-Eaton)
• Anti-AChR Ab (Se 80%, 50% if ocular disease only, Sp >90%); muscle specific receptor tyrosine kinase (MuSK) Ab; AchR modulating Ab
• CT or MRI of thorax to evaluate thymus (65% hyperplasia, 10% thymoma)
Treatment
• Thymectomy if thymoma and in Ab ⊕ Pts w/o thymoma (NEJM 2016;375:511)
• Cholinesterase inhibitor (eg, pyridostigmine) is most rapid acting (benefit in 30–60 min). Less effective for MusK MG. Side effects: cholinergic stim (brady, diarrhea, drooling).
• Immunosuppression: prednisone (benefit in wks; don’t start during crisis) + AZA (benefit in 6–15 mo). If no response: mycophenolate, rituximab, MTZ, CsA. Goal to taper off steroids
• Myasthenic crisis: treat precipitant; consider d/c cholinesterase inhibitor if suspect cholinergic crisis. IVIg or plasmapheresis; if no response, high-dose glucocorticoids (in monitored setting b/c risk for initial worsening). ICU if rapid or severe (follow FVC, NIF).
MYOPATHIES
Etiologies
• Hereditary: Duchenne, Becker, limb-girdle, myotonic, metabolic, mitochondrial
• Endocrine: hypothyroidism, hyperparathyroidism, Cushing syndrome
• Toxic: statins, fibrates, glucocorticoids, zidovudine, alcohol, cocaine, antimalarials, colchicine, penicillamine
• Infectious: HIV, HTLV-1, trichinosis, toxoplasmosis
• Inflammatory: polymyositis, dermatomyositis, inclusion body myositis, anti-HMGCR
Clinical manifestations
• Progressive or episodic weakness (not fatigue)
• Weakness most often symmetric, proximal > distal (stairs, rising from sitting, etc.)
• ± Myalgias (though not prominent or frequent), cramps, myotonia (impaired relaxation)
• May develop either pseudohypertrophy (dystrophies) or mild muscle atrophy
• Assoc. organ dysfxn: cardiac (arrhythmia, CHF), pulmonary (ILD), dysmorphic features
Diagnostic studies
• CK, aldolase, LDH, electrolytes, ALT/AST, PTH, TSH, ESR, HIV
• Autoantibodies: ANA, RF, anti-Jo1, antisynthetase, anti-Mi-2, anti-SRP, anti-HMGCR (if statin use), 5TN1CA (in inclusion body myositis)
• EMG/NCS: low-amplitude, polyphasic units w/ early recruitment, ± fibrillation potentials
• Muscle biopsy, molecular genetic testing (where indicated)
• Age-appropriate cancer screening if polymyositis or dermatomyositis suspected
Primary headache syndromes (International Headache Society Classification)
• Tension-type: bilateral, pressure-like pain of mild–mod intensity, not throbbing or aggra-vated by physical activity. A/w photophobia or phonophobia, not N/V. Freq a/w myofascial sensitivity in neck/head. Triggers: stress, sleep deprivation, dehydration, hunger. Episodic HA Rx: NSAIDs, acetaminophen (risk of med overuse HA); chronic HA Rx: TCAs.
• Cluster HA and other trigeminal autonomic cephalalgias (TACs) (Continuum 2018;24:1137)
Characterized by unilateral headache a/w ipsilateral autonomic sx (rhinorrhea, red/tearing eye, miosis, ptosis, lid edema, sweating), subtypes differentiated by timing.
Cluster: ♂ > ♀, unilateral pain w/ autonomic sx & restlessness; attacks 15 min–3 h, up to 8/d (circadian). Ppx: CCB (verapamil). Rx: high-flow O2 (12–15 L/min), sumatriptan.
Paroxysmal hemicrania: similar to cluster, but ♀ > ♂, attacks 2–30 min. Rx: indomethacin.
Hemicrania continua: ♀ > ♂, ice pick–like pain lasting >3 mo. Rx: indomethacin.
Short-lasting unilateral neuralgiform HA (SUNA/SUNCT): ♂ > ♀, excruciating, stabbing, electrical pain, 5 sec–4 min, up to 200×/d. Rx: lamotrigine, gabapentin, topiramate.
• Migraine: see below
Secondary causes of headaches
• Traumatic: post-concussion, SAH, SDH, postcraniotomy
• ↑ ICP: mass (tumor, abscess, vascular malformations, ICH), hydrocephalus, idiopathic intracranial hypertension (pseudotumor cerebri), altitude-associated cerebral edema
• ↓ ICP: post-LP headache, CSF leak/dural tear, overshunting
• Vascular: stroke (esp. posterior circ), dissection, vasculitis (incl. temporal arteritis), reversible cerebral vasoconstriction syndrome (RCVS), ICH, venous sinus thrombosis
• Meningeal irritation: meningitis, SAH
• Extracranial: sinusitis, TMJ syndrome, glaucoma
• Systemic: hypoxia (OSA), hypercapnia, dialysis, HTN, cardiac cephalalgia, hypoglycemia, ↓ TSH, pheo, medication overuse (analgesics), withdrawal (caffeine, opioids, estrogen)
Clinical evaluation (JAMA 2006;296:1274 & 2013;310:1248)
• History: onset (sudden vs. gradual), quality, severity, location, duration, triggers, alleviating factors, positional component, hormonal triggers (menstruation), preceding trauma, associated sx (visual Δs, “floaters,” N/V, photophobia, focal neurologic sx), medications (analgesics), substance abuse (opioids, caffeine), personal/family hx of HA
• General and neurologic exam (including funduscopic exam, visual fields)
• Warning signs (should prompt neuroimaging)
Explosive onset (vasc); “worst HA of my life” (SAH, RCVS); meningismus (SAH, infxn)
Positional: lying > standing (↑ ICP); N/V (↑ ICP; migraines)
Visual sx: diplopia, blurring, ↓ acuity (GCA, glaucoma, ↑ ICP); eye pain (glaucoma, trigeminal autonomic cephalalgia, optic neuritis)
Abnl neuro exam (struct. lesion, poss. in migraine); ↓ consciousness (± fever): infxn, ICH
Age >50 y; immunosuppression (CNS infections, PRES)
• Imaging: CT or MRI; consider CTA (beading in vasculitis/RCVS/vasospasm), CTV/MRV
• LP if ? SAH (✔ for xanthochromia), idiopathic intracranial HTN (✔opening press); image first!
MIGRAINE (NEJM 2017;377:553)
Definition & clinical manifestations (Lancet 2018;391:1315)
• Epidemiology: affects 15% of women and 6% of men; onset usually by 30 y
• Migraine w/o aura (most common): ≥5 attacks lasting 4–72 h with both (a) N/V or photophobia & phonophobia, and (b) ≥2 of following: unilateral, pulsating, mod–severe intensity, or aggravated by routine activity
• Migraine w/ aura: ≥2 attacks w/: (a) aura defined as ≥1 fully reversible sx: visual Δs (flickering spots, visual loss), sensory sx (paresthesias, numbness), speech disturbance; and (b) unilateral progression of sx(s) over ≥5 but ≤60 min; and (c) HA w/in 60 min of aura
• Aura may occur w/o HA (“acephalgic migraine”), must r/o TIA/stroke (typically rapid onset)
• If motor weakness, consider sporadic or familial hemiplegic migraine: aura of reversible motor weakness (up to 24 h), a/w CACNA1A, ATP1A2, or SCN1A mutations
• Precipitants: stress, foods (cheese, chocolate, MSG), fatigue, EtOH, menses, exercise
Treatment
• Abortive Rx: 5-HT1 agonists (triptans) effective if given early in migraine attack; contraindicated if motor aura, CAD, prior stroke. Also consider acetaminophen, caffeine, NSAIDs (ketorolac), steroids, Mg, metoclopramide, prochlorperazine, valproate, dihydroergotamine (caution if CAD, recent triptan use). Avoid butalbital, opioids.
• Prophylaxis: valproic acid, topiramate, β-blockers (propranolol first-line), TCAs, Mg, B2, botox, anti-CGRP & receptor mAbs (femanezumab, erenumab; NEJM 2017;377:2113 & 2123)
Differential diagnosis of back pain
• Musculoskeletal: involving spine (vertebra, facet joints), paraspinal muscles and ligaments, sacroiliac joint, or hip joint. Spondylolisthesis, vertebral fx, OA, inflam. spondyloarthritis (RA, ankylosing spondylitis, reactive, psoriatic), musculoligamentous “strain,” myofascial pain syndrome, trochanteric bursitis.
• Spinal cord (myelopathy)/nerve root (radiculopathy):
Degenerative/traumatic: disc herniation, foraminal or lumbar stenosis, spondylolisthesis
Neoplastic: lung, breast, prostate, RCC, thyroid, colon, multiple myeloma, lymphoma
Infectious: osteomyelitis/discitis, epidural abscess, zoster, Lyme, CMV, HIV, spinal TB
• Referred pain from visceral disease:
GI: PUD, cholelithiasis, pancreatitis, pancreatic cancer
GU: pyelonephritis, nephrolithiasis, uterine or ovarian cancer, salpingitis
Vascular: aortic dissection, leaking aortic aneurysm
Initial evaluation (Lancet 2017;389:736)
• History: location, radiation, trauma, wt loss, cancer hx, fever, immunocompromised, IV drug use, neurologic sx, saddle anesthesia, bowel/bladder sx (retention, incont.)
• General physical exam: local tenderness, ROM, signs of infection or malignancy; paraspinal tenderness or spasm in musculoskeletal strain
• Signs of radiculopathy (sharp/lancinating pain radiating into limb):
Spurling sign (cervical radiculopathy): radicular pain w/ downward force to extended & ipsilaterally rotated head; 30% Se, 93% Sp
Straight leg raise (sciatica or lumbosacral radiculopathy): radicular pain at 30–70°; ipsilateral: 92% Se, 28% Sp; crossed (contralateral leg raised): 28% Se, 90% Sp
Patrick/FABER test (sacroiliac joint syndrome): severe pain on hip external rotation; 70% Se, 100% Sp
Neurogenic claudication in lumbar stenosis (see table on next page)
• Neurologic exam: full motor (including sphincter tone), sensory (including perineal region; note dermatomal patterns), and reflexes including bulbocavernous, anal wink (S4), and cremasteric (L2)
• Red flags: upper motor neuron signs (hyperreflexia, upgoing toes), cauda equina or conus medullaris syndromes (saddle anesthesia, bowel or bladder dysfunction, reduced rectal tone, loss of sacral reflexes), pain at rest or at night
• Laboratory (depending on suspicion): CBC w/ diff, ESR/CRP, Ca, PO4, CSF, BCx
• Neuroimaging: low yield if nonradiating pain, high false ⊕ rate (incidental spondylosis); depending on suspicion: X-rays, CT or CT myelography, MRI, bone scan
• EMG/NCS: may be useful to distinguish root/plexopathies from peripheral neuropathies
SPINAL CORD COMPRESSION
Clinical features
• Etiologies: tumor (vertebral mets, intradural meningioma/neurofibroma), epidural abscess or hematoma, vascular malformation (dural AV fistula), degenerative dis. (spondylosis)
• Acute: flaccid paraparesis and absent reflexes (“spinal shock”)
• Subacute–chronic: spastic paraparesis and hyperreflexia (upgoing toes ± ankle clonus)
• Posterior column dysfunction in legs (loss of vibratory and/or proprioceptive sense)
• Sensory loss below level of lesion (truncal level ± bilateral leg sx is clue for cord process)
Evaluation & treatment
• Empiric spine immobilization (collar, board) for all trauma patients
• STAT MRI (at and above clinical spinal level, with gadolinium) or CT myelogram
• Emergent neurosurgical and/or neurology consultation. Urgent radiation therapy ± surgery for compression if due to metastatic disease (Lancet Oncol 2017;18:e720).
• Empiric broad-spectrum antibiotics ± surgery if c/f epidural abscess
• High-dose steroids depending on cause:
Tumor: dexamethasone 16 mg/d IV (usually 4 mg q6h) with slow taper over wks
Trauma: methylprednisolone 30 mg/kg IV over 15 min then 5.4 mg/kg/h × 24 h (if started w/in 3 h of injury) or × 48 h (if started 3–8 h after injury) (Cochrane 2012:CD001046)
NERVE ROOT COMPRESSION
Clinical features (NEJM 2015;372:1240)
• Radicular pain aggravated by activity (esp. bending, straining, coughing), relieved by lying
• Sciatica = radicular pain radiating from buttocks down lateral aspect of leg, often to knee or lateral calf ± numbness and paresthesias radiating to lateral foot. Caused by compression of nerve roots, plexus, or sciatic nerve.
• <65 y: 90% from disc herniation. ≥65 y also w/ more degenerative contributors: ligamentous hypertrophy, osteophyte formation, facet arthropathy, neural foraminal narrowing
• Spinal stenosis: central canal narrowing → root compression via direct impingement, CSF flow obstruction, vascular compromise
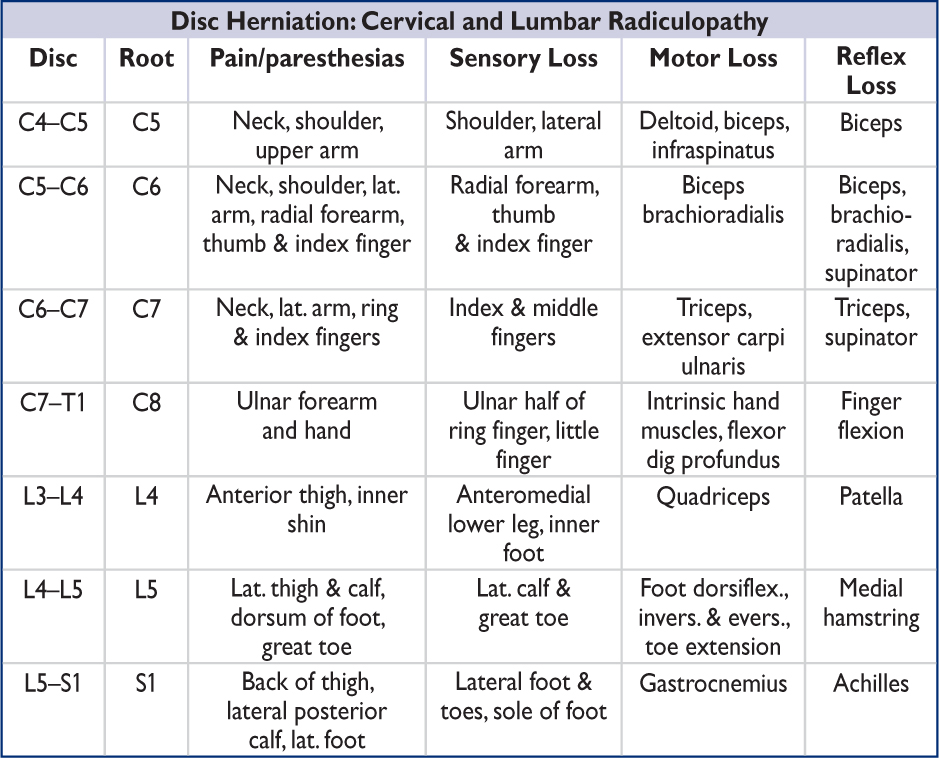
Nb, lumbar disc protrusion tends to compress the nerve root that exits 1 vertebral level below the protrusion.
Neurogenic vs. Vascular Claudication |
||
Features |
Neurogenic Claudication |
Vascular Claudication |
Cause |
Lumbar spinal stenosis (with nerve root compression) |
Peripheral artery disease (with limb ischemia) |
Pain |
Radicular back/buttock pain Radiating down legs |
Cramping leg pain Mostly in calves; radiating up legs |
Worse with |
Walking & standing Hyperextension/lying prone |
Walking Biking |
Better with |
Bending forward, sitting |
Rest (standing or sitting) |
Other sx |
Numbness/paresthesias |
Pale, cool extremity |
Exam |
± Focal weakness, ↓ reflexes ↓ Lumbar extension Preserved pulses |
Diminished/absent pulses (dorsalis pedis/posterior tibialis) Pallor |
Diagnostic studies |
MRI lumbar spine CT myelogram (if no MRI) EMG/NCS |
Arterial Doppler studies Ankle-brachial index (ABI) Arteriography |
Treatment |
PT (flexion exercise), NSAIDs, epidural steroid injections (ESI) Surgery (if other Rx fails) |
Modify vascular risk factors, exercise rehab, antiplatelet Rx, revascularization |
Nb, diagnosis complicated by overlap between presentations & possibility of both diagnoses in the same patient. (NEJM 2007;356:1241 & 2008;358:818)
Evaluation & treatment of nerve root compression (NEJM 2016;374:1763)
• MRI if sx not improved after 6 wk of conservative tx; if non-diagnostic, consider EMG/NCS
• Conservative: avoid bending/lifting; soft collar (cervical radiculopathy); NSAIDs; muscle relaxants; lidocaine patch/ointment; Rx neuropathic pain (see “Peripheral Neuropathies”); physical therapy. Insufficient evidence to recommend oral steroids.
• Avoid opiates when possible; risks outweigh benefits in noncancerous back pain
• Spinal epidural steroid injections (ESI): limited short-term relief of refractory radicular pain
• Surgery: cord compression or cauda equina syndrome; progressive motor dysfunction; bowel/bladder dysfunction; failure to respond to conservative Rx after 3 mo