10
METALS
INTRODUCTION
Metals are an integral part of world development. From the earliest discovery to the latest and most esoteric materials, knowledge of the properties and applications of metals has increased and created a continuing challenge to find new and improved uses.
Steel has evolved from the earliest cast iron and wrought iron, which still have applications and current usage, to a multitude of grades obtained by alloying and heat-treating. The properties of some grades depend on adding elements such as nickel and chromium to obtain a family of stainless steels; other grades rely on various chemicals to obtain strength characteristics. Desired ductility is obtained by controlling carbon content.
From the tiniest staple used to adhere sheets of paper to the huge columns installed to support skyscrapers, steel is available in many forms, including wire, rod, pipe, tube, sheet, plate, and rolled structural sections.
Hot-rolled steel is used for heavy structural members. Cold-formed steel applications include steel decking, cold-formed metal framing, cold-formed metal joist framing, structural metal stud framing, slotted channel framing, and cold-formed metal trusses. The high strength-to-weight ratio obtained with thin profiles is one of cold-formed steel’s benefits.
Sheet and strip steel can be formed, providing lightweight structural properties for use in building components, door stiffeners, and vehicles. Expanded metal gratings, treads, and screens are made by slitting and stretching steel sheets. Sheets are also used with internal stiffeners to provide hollow metal doors. Thicker plates can be welded together to form shapes larger than those created by rolling. Plates and angles connected by rivets are still in use after many years of service, but now steel plates are welded together to obtain the desired shape.
Stainless steel may be a preferred metal when corrosion resistance is important. Hot-dip galvanizing, and a variety of coatings, are acceptable and proven methods to resist corrosion, when metal is not exposed to excess moisture or other chemicals. Many old steel structures that have been properly maintained are still in service.
Steel specifications are established by ASTM International (ASTM). These standards, as well as standards for the other metals, are developed by consensus committees comprised of producer, user, and general-interest members.
For years, sheet and strip thickness was designated by gauge. This designation does not, however, accurately reflect current industry terminology because gauge sizes may vary by material. Therefore, ASTM, American National Standards Institute (ANSI), American Iron and Steel Institute (AISI), National Association of Architectural Metal Manufacturers (NAAMM), and other standards organizations have discontinued the use of gauge to designate thickness, and instead, use a minimum thickness with tolerance.
Many of the sheet steel standards for hot-rolled, cold-rolled, and zinc-coated steels have been combined into three standards. Thus, the hot-rolled sheet steel standard now covers commercial steels, drawing steels, and high-strength, low-alloy steels. This requires the design professional to identify which designation, type, and grade is desired. The same applies to the cold-rolled steel sheet standard and the zinc-coated standard for galvanized and galvannealed steels. The new designations provide the necessary information, including chemical and mechanical properties, so the user can select the appropriate steel for the end application.
Based on their characteristics, all metals—steel, stainless steel, aluminum, copper alloys (bronze and brass), titanium, and other nonferrous metals—provide the design professional with an palette of materials, which when combined with a consideration of availability, cost, and effectiveness enable creation of either a simple or a complex structure.
The information in this chapter should be supplemented by literature available from many associations, among them: AISI, Aluminum Association, American Institute of Steel Construction, ANSI, ASTM, Copper Development Association, Nickel Development Institute, and NAAMM.
Contributor:
Edward R. Estes, Jr., PE, Techinical Concultant, National Association of Architectural Metal Manufacturers, Norfolk, Virginia.
METAL MATERIALS
PROPERTIES OF METALS
Metals and their alloys are classified in two broad categories: ferrous and nonferrous. Ferrous metals’ main component is iron, and nonferrous metal alloys normally do not contain iron.
FERROUS METALS
Iron, steel, and their alloys are usually the most cost-effective metal choice for structural applications.
Iron that contains no trace of carbon is soft, ductile, and easily worked, but it rusts in a relatively short period of time and is susceptible to corrosion by most acids.
The characteristics of cast iron vary widely among the six basic types: gray, malleable, ductile, white, compacted graphite, and high-alloy iron. All cast irons have high compressive strengths, but tensile and yield strengths vary widely depending on basic type. Cast iron is relatively corrosion-resistant but cannot be hammered or beaten into shapes.
Gray irons are rather brittle because they have a high carbon and silicon content. However, castings of gray iron are excellent for damping purposes (i.e., absorbing vibrations). They are produced in eight ASTM classes or grades, with tensile-strength ratings from 20,000 to 60,000 psi. Applications include decorative shapes, such as fences and posts, gratings, and stair components, as well as utility uses such as manhole covers and fire hydrants.
Malleable iron, which is more expensive than gray iron, has been used for decades in applications that require durability and high ductility. This low-carbon white iron is cast, reheated, and slowly cooled, or annealed, to improve its workability.
Ductile iron is made by adding magnesium to molten iron shortly before the metal is poured into molds. The magnesium alters the surface-tension mechanism of the molten iron and precipitates the carbon out as small spheres, instead of flakes, which make the iron casting more ductile. Ductile iron is less brittle, stiffer, stronger, and more shock-resistant than gray iron. Ductile iron castings are more expensive than gray iron but usually less than malleable iron. Ductile iron is the fastest-growing segment of the metal casting industry.
Ductile irons are produced in strength ratings from 55,000 to 130,000 psi. Ductile castings using a special austempering heat-treating process offer much higher tensile strengths, ranging from 125,000 to 230,000 psi. Called ADI castings, they rival or surpass certain alloy steel castings in tensile and yield strengths.
White iron castings, which are extremely hard and brittle, are used primarily in industrial machinery parts that experience high wear and require abrasion resistance.
The characteristics of compacted graphite iron fall between those of gray and ductile iron. The properties of this metal are so difficult to control during production that very few metal casters manufacture it.
High-alloy irons are gray, ductile, or white irons with an alloy content of 3 percent to more than 30 percent. Their properties are significantly different from those of unalloyed irons.
Wrought iron or steel is relatively soft, corrosion- and fatigueresistant, and machinable. It is easily worked, making it ideal for railings, grilles, fences, screens, and various types of decorative metal. It is commercially available in bars, rods, tubing, sheets, and plates.
Carbon steel is iron that contains low to medium amounts of carbon. Higher carbon content increases metal strength and hardness but reduces its ductility and weldability. The corrosion resistance of carbon steels is improved by galvanizing, which is a hot-zinc dipping process, or applying an organic coating. Some architectural uses include structural shapes such as welded fabrications or castings, metal framing and joists, fasteners, wall grilles, and ceiling suspension grids.
High-strength low-alloy (HSLA) steels have better corrosion resistance than carbon steels, and they are chosen when weight is a consideration and higher strength is specified. Low-alloy steels are seldom used in exterior architectural applications that involve water runoff because adjacent materials could become stained with rust.
Typical elements used to modify steel include the following:
• Aluminum, for surface hardening.
• Chromium, for corrosion resistance.
• Copper, for atmospheric corrosion resistance.
• Manganese in small amounts, for additional hardening; in larger amounts, for better wear resistance.
• Molybdenum, combined with other metals such as chromium and nickel, to increase corrosion resistance and raise tensile strength without reducing ductility.
• Nickel, to increase tensile strength without reducing ductility; in high concentrations, nickel improves corrosion resistance.
• Silicon, to strengthen low-alloy steels and improve oxidation resistance; larger amounts produce hard, brittle castings that are resistant to corrosive chemicals.
• Sulfur, for free machining.
• Titanium, to prevent intergranular corrosion of stainless steels.
• Tungsten, vanadium, and cobalt for hardness and corrosion resistance.
Stainless steels are at least 11.5 percent chromium. Nickel is added to boost atmospheric corrosion resistance; molybdenum is added when maximum corrosion resistance is needed, such as when iron will come into contact with saltwater. Stainless steel is used in construction for flashing, coping, fasciae, wall panels, floor plates, gratings, handrails, hardware, fasteners, and anchors. Decorative shapes and statuary can be cast in stainless steel.
NONFERROUS METALS
Nonferrous metals and their alloys can be categorized into seven major groups for architectural applications: those based on aluminum, copper (pure copper, brasses, and bronzes), lead, zinc, tin, nickel, and magnesium. Another approach is to divide nonferrous alloys into two groups: heavy metals (copper-, zinc-, lead-, and nickel-based) and light metals (aluminum- and magnesium-based).
ALUMINUM
The nonferrous metal workhorse for architectural applications is aluminum. It has good forming and casting characteristics and offers good corrosion resistance. When exposed to air, aluminum does not oxidize progressively because a hard, thin oxide coating forms on the surface and seals the metal from its environment.
Aluminum and its alloys, numbering in the hundreds, are widely available in common commercial forms. Aluminum alloy sheets can be formed, drawn, stamped, or spun. Many wrought or cast aluminum alloys can be welded, brazed, or soldered, and aluminum surfaces readily accept a wide variety of finishes, both mechanical and chemical.
Although it is light in weight, commercially pure aluminum has a tensile strength of about 13,000 psi. Most aluminum alloys lose strength at elevated temperatures. At subzero temperatures, on the other hand, aluminum is stronger than at room temperature but no less ductile. Cold-forming the metal may nearly double its tensile strength. Aluminum can be further strengthened by alloying it with other elements such as manganese, silicon, copper, magnesium, zinc, or lithium. The manganese-based aluminum alloy 3003 is used for roofing, sheet metal, siding, and electrical conduit.
BRASS, COPPER, AND BRONZE
Good thermal and electrical conductivity, corrosion resistance, and easy forming and joining make copper and its alloys useful in construction. However, copper and many of its alloys have relatively low strength-to-weight ratios, and their strength is even further reduced at elevated temperatures. These metals are offered in rod, plate, strip, sheet, and tube shapes; forgings; castings; and electrical wire.
These metals can be grouped according to composition in several general categories: copper, high-copper alloys, and many types of brass and bronze. Monel metal is a copper-nickel alloy that offers excellent corrosion resistance, and is often used for corrosion-resistant fasteners.
Bronze was originally a copper-tin alloy, but today aluminum bronzes, silicon bronzes, and leaded phosphor bronzes are more common. Phosphor bronze is a copper-tin-phosphorus alloy, and leaded phosphor bronze is composed of copper, lead, tin, and phosphorus.
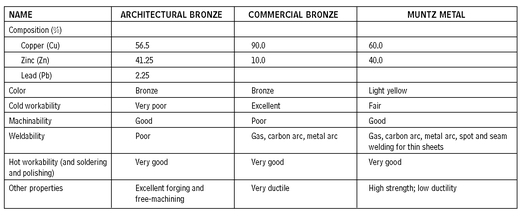
Brass is copper with zinc as its principal alloying element. It is important to know that some brass alloys may be called bronzes even though they have little or no tin in them. Some common nonbronze brass alloys are commercial bronze (90 percent copper, 10 percent zinc), naval brass (60 percent copper, 29 percent zinc, and 1 percent tin), Muntz metal (60 percent copper, 40 percent zinc), and manganese bronze (58 percent copper, 39 percent zinc, and 1 percent tin and iron). When a metal is identified as bronze, the alloy may not contain zinc or nickel; if it does, it is probably brass. Architectural brasses and bronzes are actually all brasses; they are used for doors, windows, door and window frames, railings, trim and grilles, and finish hardware. Muntz metal, also called malleable brass, is a bronze alloy resembling extruded architectural bronze in color. It is available in sheet and strip and is used in flat surfaces in architectural compositions in connection with extruded architectural bronze.
Copper-based alloys characteristically form adherent films that are relatively impervious to corrosion and protect the base metal from further attack. In exterior applications certain alloys darken rather rapidly, from brown to black. Under most outdoor weather conditions, however, copper surfaces, such as roofs or statuary, develop a blue-green patina. Lacquer coatings can help retain the original alloy color.
TYPES AND PROPERTIES OF BRASS
10.1
Contributors:
Edward R. Estes, Jr., PE, Technical Consultant, National Association of Architectural Metal Manufacturers, Norfolk, Virginia; Robert C. Rodgers, PE, Richmond Heights, Ohio.
LEAD
An extremely dense metal, lead is corrosion-resistant and easily worked. Alloys are added to it to improve properties such as hardness and strength. Typical applications of lead include roof and wall accessories, sound and vibration control, and radiation protection. It can be combined with tin alloy to plate iron or steel, which is commonly called terneplate.
It is important to note that lead vapors and dust are toxic if ingested, so care must be taken regarding how and where this metal is used.
ZINC
Although it is corrosion-resistant in water and air, zinc is brittle and low in strength. Its major use is in galvanizing (dipping hot iron or steel in molten zinc), although zinc is also used to create sand-cast or die-cast components. Major building industry uses are roofing, flashing, nails, plumbing hardware, galvanizing structural components, and decorative shapes.
TIN
Key properties of tin are its low melting point (450°F), relative softness, good formability, and readiness to form alloys. Principal uses for tin are as a constituent of solder, a coating for steel (tinplate, terneplate), and an alloy with other metals that can be cast, rolled, extruded, or atomized. Tin is most popular as an alloy for copper, antimony, lead, bismuth, silver, and zinc. Pewter alloys contain 1 to 8 percent antimony and 0.5 to 3 percent copper. Alloy metal in tin solders ranges from 40 percent lead to no lead and 3.5 percent silver.
NICKEL
Whitish in color, nickel is used for plating other metals or as a base for chromium plating. Nickel polishes well and does not tarnish. It is also widely applied as an additive in iron and steel alloys, as well as other metal alloys. Nickel-iron castings are more ductile and more resistant to corrosion than conventional cast iron. Adding nickel makes steel more resistant to impact.
CHROMIUM
A hard, steel-gray metal, chromium is commonly used to plate other metals, including iron, steel, brass, and bronze. Plated cast shapes can be brightly polished and do not tarnish. Several steel alloys, such as stainless plate, contain as much as 18 percent chromium. Chromium does not rust, which makes chromium alloys excellent for exterior uses.
MAGNESIUM
Lightest of all metals used in construction, pure magnesium is not strong enough for general structural functions. For comparison, if a block of steel weighs 1000 lbs, equal volumes of aluminum and magnesium weigh 230 lbs and 186 lbs, respectively. Combining other metals such as aluminum with magnesium results in lightweight alloy materials used in ladders, furniture, hospital equipment, and automobile wheels.
METAL CORROSION
Corrosion, which is caused by galvanic action, occurs between dissimilar metals or between metals and other materials when sufficient moisture is present to carry an electrical current. The galvanic series shown in Table 10.3 is a useful indicator of corrosion susceptibility caused by galvanic action. The metals listed are arranged in order from the least noble (most reactive to corrosion) to the most noble (least reactive to corrosion). The farther apart two metals are on the list, the greater the deterioration of the less noble metal will be if the two come in contact under adverse conditions.
Metal deterioration also occurs when metal comes in contact with chemically active materials, particularly when moisture is present. For example, aluminum corrodes when in direct contact with concrete or mortar, and steel corrodes when in contact with certain treated woods.
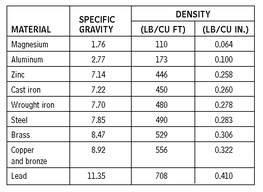
WEIGHTS OF METALS FOR BUILDINGS
10.2
THE GALVANIC SERIES
10.3
| Anode (least noble) + | Magnesium, magnesium alloys |
| Zinc |
| Aluminum 1100 |
| Cadmium |
| Aluminum 2024-T4 |
| Steel or iron, cast iron |
| Chromium iron (active) |
| Ni-Resist |
| Type 304, 316 stainless (active) |
| Hastelloy “C” |
| Lead, tin |
| Electric current flows from positive (+) to negative (-) | Nickel (active) |
| Hastelloy “B” |
| Cathode (most noble)— | Brasses, copper, bronzes, copper-nickel alloys, monel |
| Silver solder |
| Nickel (passive) |
| Chromium iron (passive) |
| Type 304, 316 stainless (passive) |
| Silver |
| Titanium |
| Graphite, gold, platinum |
Pitting and concentration cell corrosion are other types of metal deterioration. Pitting takes place when particles or bubbles of gas are deposited on a metal surface. Oxygen deficiency under these deposits sets up anodic areas, which cause pitting. Concentration cell corrosion is similar to galvanic corrosion; the difference is in the electrolytes. Concentration cell corrosion can be produced by differences in ion concentration, oxygen concentration, or foreign matter adhering to the surface.
SHAPING AND FABRICATION OF METALS
Many different manufacturing processes are applied to metal to produce structural forms and shapes required in the construction and ornamentation of buildings.
• Rolling hot or cold metal between pressurized rollers produces most of the readily available, standard construction material shapes. Baked enamel-coated aluminum is cold rolled to make siding and gutters.
• In the extruding process, heated metal ingots or bars are pushed through a die orifice to produce a wide variety of simple and complex shapes. Sizes are limited only by the size or capacity of the die.
• Casting is a process in which molten metal is poured into molds or forced into dies and allowed to solidify in the shape of the mold or die. The casting process is used with virtually all metals; however, surface quality and physical characteristics are greatly affected by the metal alloy and casting process selected. Almost all metals can be cast in sand molds. Only aluminum, zinc, and magnesium are ordinarily cast in metal dies in what is called either a die-casting or permanent-mold process. Round, hollow building products such as cast-iron pipe for plumbing and sewer applications are made by centrifugal casting machines.
• In the drawing process, either hot or cold metal is pulled through dies that alter or reduce its cross-sectional shape to produce architectural product configurations. Common drawn products are sheets, tubes, pipes, rods, bars, and wires. Drawing can be used with all metals except iron.
• Forging is the process of hammering hot metal or pressing cold metal to a desired shape in dies of a harder metal. The process usually improves the strength and surface characteristics of the metal. Aluminum, copper, and steel can be forged.
• Machining is used to finish areas of castings or forgings that require highly precise fits or contours. Shapes can also be machined from heavy plate or solid blocks of metal.
• Bending produces curved shapes in tubing, pipe, and extrusions.
• Brake forming of metal plate or sheet metal is a process of making successive pressings to achieve shapes with straight-line angles.
• In the spinning process, ductile types of sheet metal (usually copper or aluminum) are shaped with tools while being spun on an axis.
• Embossing and coining stamps metal with textured or raised patterns.
• Blanking shears, saws, or cuts metal sheets with a punch press to achieve a desired configuration.
• Perforating punches or drills holes through flat plate or sheet metal.
• Piercing punches holes through metal without removing any of the metal.
• Fusion welding is used to join metal pieces by melting filler metal (welding rod) and the adjacent edges briefly with a torch and then allowing the molten metal to solidify. Two common types of fusion welding are electric-arc and gas. Electric arc or metallic arc welding normally uses metal welding rods as electrodes in the welding tool.
• Gas welding is also known as oxyacetylene welding because it uses a mixture of oxygen and acetylene to fuel the flames produced by the blowtorch. Oxyacetylene blowtorches are widely used in construction work to cut through metal structural beams and metal plates.
MELTING TEMPERATURES OF METALS
10.4
| MELTING TEMPERATURES |
|---|
| BASE METAL | DEGREES CELSIUS | DEGREES FAHRENHEIT |
|---|
| Aluminum | 660 | 1220 |
| Antimony | 631 | 1168 |
| Cadmium | 321 | 610 |
| Chromium | 1857 | 3375 |
| Cobalt | 1495 | 2723 |
| Copper | 1083 | 1981 |
| Gold | 1064 | 1947 |
| Iron | 1535 | 2795 |
| Lead | 328 | 622 |
| Magnesium | 649 | 1200 |
| Manganese | 1244 | 2271 |
| Nickel | 1453 | 2647 |
| Silver | 962 | 1764 |
| Tin | 232 | 450 |
| Zinc | 420 | 788 |
| Zirconium | 1852 | 3366 |
Contributors:
Robert C. Rodgers, PE, Richmond Heights, Ohio; Edward R. Estes, Jr., PE, National Association of Architectural Metal Manufacturers, Norfolk, Virginia.
• Soldering is a metal joining process that uses either hard or soft solder. The metal pieces being joined together do not melt as they do in the welding process because solders melt at much lower temperatures. Soft solders consist of tin with a high percentage of lead, and melt at temperatures of 360° to 370°F. Hard solders are composed of tin and a low content of antimony or silver, and melt at temperatures ranging from 430° to 460°F.
• Brazing, which is sometimes called hard soldering, also joins two pieces of metal together by torch melting a filler rod material between them. The filler has a high content of copper and melts between 800° and 900°F.
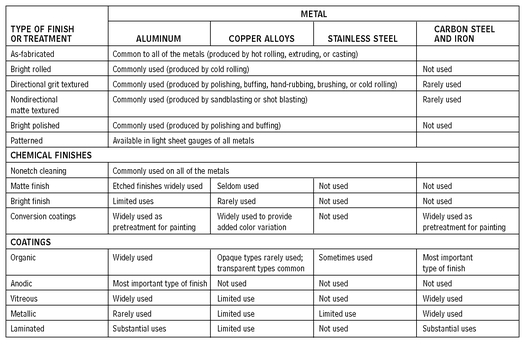
FINISHES ON METALS
GENERAL
The finishes commonly used on architectural metals fall into three categories:
• Mechanical finishes are the result of physically changing the surface of the metal through mechanical means. The forming process itself or a subsequent procedure is performed either before or after the metal is fabricated into an end-use product.
• Chemical finishes are achieved by means of chemicals, which may or may not have a physical effect on the surface of the metal.
• Coatings are applied as finishes, either to the metal stock or to the fabricated product. These coatings either change the metal itself, through a process of chemical or electrochemical conversion, or they are simply applied to the metal surface.
Application environments, service requirements, and aesthetics together determine which metal finish or coating is best to specify. Finishes are usually selected for both appearance and function; chromium plating on metal bathroom water faucets and handles, or baked enamel on sheet metal lighting fixtures, for example, must be attractive as well as functionally protective.
For structural and exterior metal building products, such as structural metal framing, steel siding, and exterior lighting, function and operating environments are more important criteria. From a design standpoint, it is important to recognize how finishes and coatings resist wear, and corrosion. To choose the right coating or finish, design professionals must understand which material or process is best suited for a specific application.
MECHANICAL FINISHES
Mechanical finishes fall into the following five categories:
• As-fabricated finishes are the texture and surface appearance given to a metal by the fabrication process.
• Buffed finishes are produced by successive polishing and buffing operations using fine abrasives, lubricants, and soft fabric wheels. Polishing and buffing improve edge and surface finishes and render many types of cast parts more durable, efficient, and safe.
• Patterned finishes are available in various textures and designs. They are produced by passing an as-fabricated sheet between two matched-design rollers, embossing patterns on both sides of the sheet, or between a smooth roller and a design roller, embossing or coining on one side of the sheet only.
• Directional textured finishes are produced by making tiny parallel scratches on the metal surface using a belt or wheel and fine abrasive, or by hand-rubbing with steel wool. Metal treated this way has a smooth, satin sheen.
• Peened finishes are achieved by firing a stream of small steel shot at a metal surface at high velocity. The primary aim of shotpeening is to increase the fatigue strength of the component; the decorative finish is a by-product. Other nondirectional textured finishes are produced by blasting metal, under controlled conditions, with silica sand, glass beads, and aluminum oxide.
CHEMICAL FINISHES
Chemical finishes are produced in four ways.
• Chemical cleaning cleanses the metal surface without affecting it in any other way. This finish is achieved with chlorinated and hydrocarbon solvents and inhibited chemical cleaners or solvents (for aluminum and copper) and pickling, chlorinated, and alkaline solutions (for iron and steel).
• Etched finishes produce a matte, frosted surface with varying degrees of roughness by treating the metal with an acid (sulfuric and nitric acid) or alkali solution.
• The bright finish process, not used widely, involves chemical or electrolytic brightening of a metal surface, typically aluminum.
• Conversion coating is usually categorized as a chemical finish, but since a layer or coating is produced by a chemical reaction, it could be considered a coating. Conversion coatings typically prepare the surface of a metal for painting or for receiving another type of finish, but they are also used to produce a patina or statuary finish. A component is treated with a diluted solution of phosphoric acid or sulfuric acid and other chemicals that convert the surface of the metal to an integral, mildly protective layer of insoluble crystalline phosphate or sulphate. Such coatings can be applied by either spray or immersion and provide temporary resistance in a mildly corrosive environment. They can be used for gray, ductile, and malleable iron castings as well as steel castings, forgings, or weldments, such as railings and outdoor furniture.
COATINGS
Organic coatings on metal can provide protection and may also be decorative. When protection is the sole purpose, primers or undercoats, pigmented topcoats in hidden areas, and clear finishes are used. Organic coatings used for decorative and protective applications include pigmented coatings, clear finishes used for gloss, and transparent or translucent clear finishes with dyes added.
Organic coatings usually fall under the general categories of paints, varnishes, enamels, lacquers, plastisols, organisols, and powders. Literally hundreds of different organic coating formulations offer an almost unlimited range of properties.
Many organic coatings are applied with brushes and rollers, but dipping and spraying of coatings account for most industrial and commercial building projects. Dipping is useful for coating complex metal parts, but spraying is used for most architectural applications. Spraying is fast and inexpensive, and new computer-controlled applicators can follow even complex curvatures. Conventional spraying, however, has two disadvantages. First, there is no easy, inexpensive way to collect and reuse the coating material. And, second, when solvent-based coatings are used, environmental restrictions need to be taken into consideration.
Electrodeposition, an increasingly popular alternative to spraying, is similar to electroplating, except that organic resins are deposited instead of metal. Electrodeposition is based on the principles of electrophoresis—the movement of charged particles in a liquid under the influence of an applied electrical charge. Electrodeposition offers several advantages: The coating builds up to a uniform thickness without runs or sags, very little paint is wasted, low levels of volatile organic compounds (VOCs) are emitted, and coatings can be deposited even into deeply recessed areas of a complex shape. Electrodeposition also has disadvantages: Coating thickness is limited, and because only one coat can be applied this way, subsequent coats must be sprayed.
Powder coating is perhaps the best known environmentally acceptable painting process. It offers three major advantages. One, because the paints are solventless, they are safer and sustainable; two, the paints cost less; and, three, they are more durable.
Powdered paints are formulated in much the same way as solvent-based paints, with the same pigments, fillers, and extenders, but are dry at room temperatures. Heat-reactive or “heat-latent” hardeners, catalysts, or cross-linking components are used as curing agents.
Powder coatings are either thermoplastic or thermosetting. Thermoplastic coatings (e.g., vinyl, polyethylene, and certain polyesters), as the term implies, are melted by heat during application. Before such coatings are applied, the surface must be primed to ensure adhesion. Thermosetting paints undergo a chemical change; they cannot be remelted by heat. Thermosets do not require a primer. Coating powders include epoxies, polyurethanes, acrylics, and polyesters.
The two most common methods of applying powdered finishes to metal are spraying and dipping, the same as those used for solvent-based paint. Electrostatic spraying is used to apply powder films 1 to 5 mil in thickness. A mixture of air and powder moves from a hopper to a spray applicator. The mixture is charged electrostatically as it passes through the applicator, causing it to stick to any grounded metal object. Powder that falls to the floor is recycled.
For coatings thicker than 5 mil, fluidized-bed dipping is used. The powder is placed in a special tank into which air is blown, turning the powder into a fluidlike mass. Objects are dipped in the “fluid” and then baked to cure the finish.
COMPARATIVE APPLICABILITY OF VARIOUS FINISHES FOR ARCHITECTURAL APPLICATIONS
10.5
NOTE
10.5 For more information, see the Metal Finishes Manual for Architectural and Metal Products, published by the Architectural Metal Products Division of the National Association of Architectural Metal Manufacturers.
Contributor:
Robert C. Rodgers, PE, Richmond Heights, Ohio.
STRUCTURAL METAL FRAMING
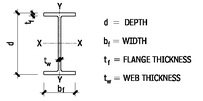
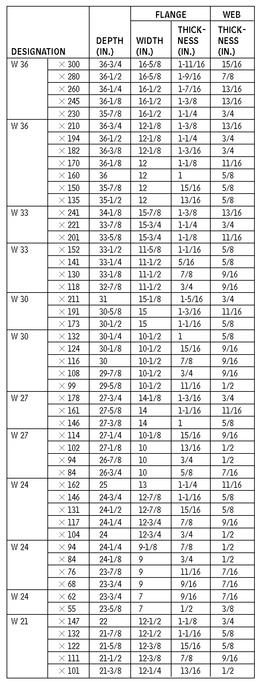
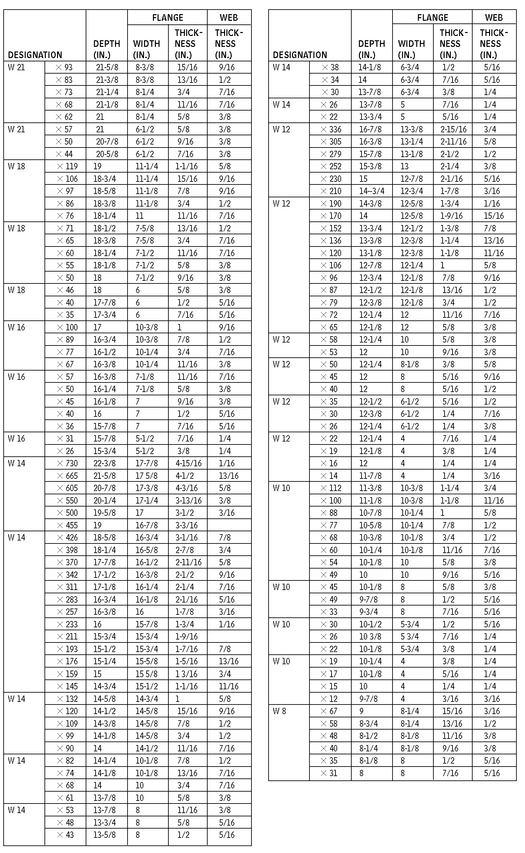
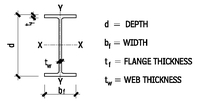
W AND M STEEL SHAPES
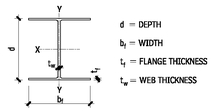
W SHAPES—DIMENSIONS FOR DETAILING
10.6
Contributor:
American Institute of Steel Construction, Chicago, Illinois.
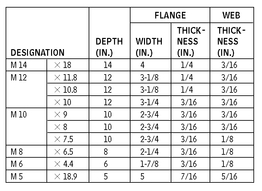
M SHAPES—DIMENSIONS FOR DETAILING
10.7
S, HP, C, MC, AND L STEEL SHAPES
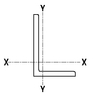
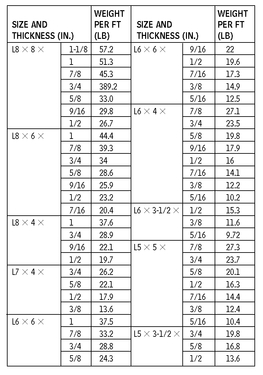
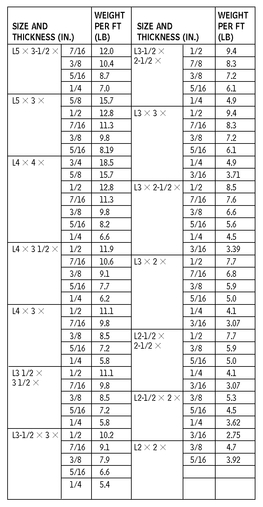
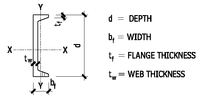
ANGLES—DIMENSIONS FOR DETAILING
10.8
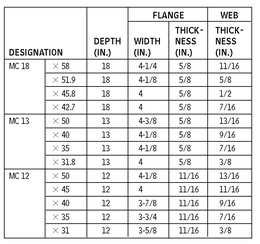
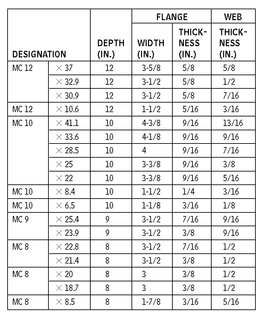
MISCELLANEOUS CHANNELS—DIMENSIONS FOR DETAILING
10.9
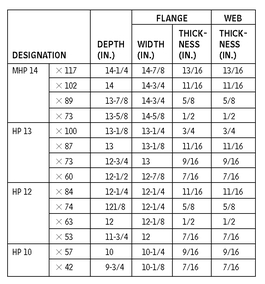
HP SHAPES—DIMENSIONS FOR DETAILING
10.10
Contributor:
American Institute of Steel Construction, Chicago, Illinois.
METAL TUBING AND PIPES
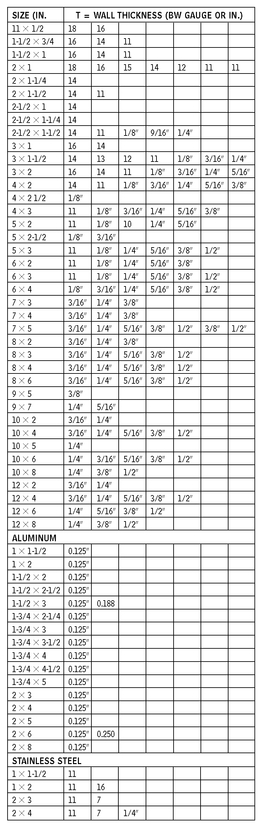
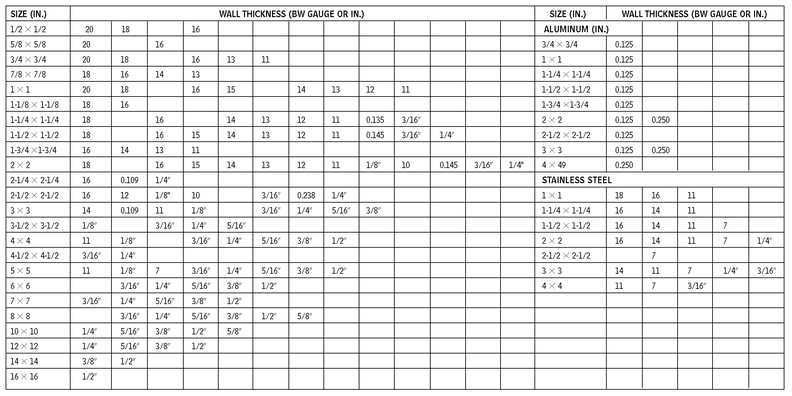
RECTANGULAR TUBING—STEEL
10.11
RECTANGULAR AND SQUARE TUBING
10.12
RECTANGULAR ALUMINUM TUBING (IN.)
10.13
| SIZE × t | SIZE × t | SIZE × t |
|---|
| 1-1/2 × 1-1/2 × 1/8 | 1-3/4 × 3-1/2 × 1/8 | 2 × 4 × 1/8 |
| 1 × 2 × 1/8 | 1-3/4 × 4 × 1/8 | 2 × 5 × 1/8 |
| 1-1/2 × 2-1/2 × 1/8 | 1-3/4 × 4-1/2 × 1/8 | 3 × 5 × 1/8 |
| 1-3/4 × 2-1/4 × 1/8 | 1-3/4 × 5 × 1/8 | |
| 1-3/4 × 3 × 1/8 | 2 × 3 × 1/8 | |
SQUARE ALUMINUM TUBING (IN.)
10.14
| SIZE × t | SIZE × t | SIZE × t |
|---|
| 1/2 × 1/2 × 1/8 | 1-1/2 × 1-1/2 × 1/8 | 3 × 3 × 1/5 |
| 3/4 × 3/4 × 1/8 | 1-3/4 × 1-3/4 × 1/8 | 4 × 4 × 1/8 |
| 1 × 1 × 1/16 | 2 × 2 × 1/8 | 4 × 4 × 1/4 |
| 1-1/4 × 1-1/4 × 1/8 | 2 × 2 × 1/4 | |
| 1-1/2 × 1-1/2 × 5/64 | 3 × 3 × 1/8 | |
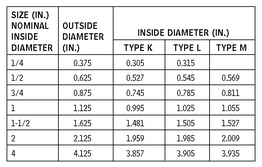
ROUND TUBING—COPPER
10.15
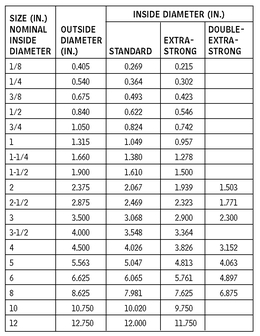
ROUND PIPE—STEEL
10.16
ROUND TUBING AND PIPE
10.17
NOTES
10.14 Rectangular and square tubing with sharp corners is usually used for metal specialties.
10.15 Round tubing, usually manufactured for mechanical purposes, is used for metal specialties. Round tubing is measured by the outside diameter and the wall thickness by gauge, fractions, or decimals of an inch. Round tubing is used where a high-grade finish is required and exact diameters are necessary.
Round tubing is available in steel, aluminum, copper, stainless steel, and other metals. Consult manufacturers for availability of materials and sizes.
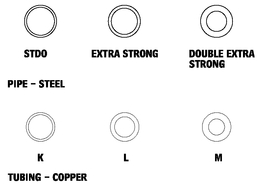
10.16 Round pipe is made primarily in three weights: standard, extra-strong (or extra-heavy), and double-extra-strong (or double-extra-heavy). Outside diameters of the three weights of pipe in each size are always the same: extra wall thickness is always on the inside, and therefore reduces the inside diameter of the heavier pipe. All sizes are specified by what is known as the “nominal inside diameter.” Round pipe is also available in aluminum and stainless steel. Consult manufacturer for sizes.
Contributor:
HMC Architects, Incorporated, Ontario, California.
SQUARE TUBING—STEEL
10.18
GRATINGS
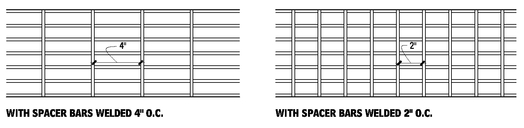
RECTANGULAR BAR GRATING (WELDED OR PRESSURE-LOCKED)
10.19
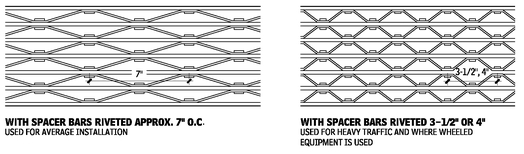
RETICULATED GRATING (RIVETED)
10.20
NOTE
Rectangular bar grating is fabricated from rectangular bearing bars of steel, stainless steel, or aluminum or aluminum I-bars, with cross bars at right angles. Cross bars may be square, rectangular, or of another shape, and be pressure-locked, swaged, or welded to the bearing bars. They may have open ends or ends banded with flats, approximately the same size as the bearing bars. Standard bar spacing include 7/16, 15/16, and 1-3/16 in. The 7/16-in. close mesh spacing which provides 1/4-in. clear opening may be more suitable for pedestrian traffic areas, eliminating openings too big for crutches, wheelchairs, and the heels on women’s shoes. For sizes, tolerances, details, and load tables, refer to ANSI/NAAMM MBG 531, Metal Bar Grating Manual, published by the National Association of Architectural Metal Manufacturers.
NOTE
Reticulated grating is fabricated from rectangular bearing bars of steel, stainless steel, or aluminum, and continuous crimped connecting (reticuline) bars riveted to the bearing bars. They may have open ends or ends banded with flats, approximately the same size as bearing bars, welded across the ends. Normal bar spacing is 3/4, 1-1/8, or 2-5/16 in. For sizes, tolerances, details, and load tables, refer to ANSI/NAAMM MBG 532, Heavy-Duty Metal Bar Grating Manual, published by the National Association of Architectural Metal Manufacturers.
SEE ALSO
Cold-Formed Metal Framing
Cold-Formed Metal Joist Framing
Cold-Formed Metal Trusses
Metal Fastenings
Metal Gratings
Metal Specialties
Metals
Radiation Protection
Roof and Wall Accessories
Steel Decking
Slotted Channel Framing
Sound and Vibration Control
Steel Siding
Structural Metal Framing
Contributors:
HMC Architects, Inc., Ontario, California; Charles F. D. Egbert, AIA, Washington, DC; Vincente Cordero, AIA, Arlington, Virginia; Edward R. Estes, Jr., Norfolk, Virginia.