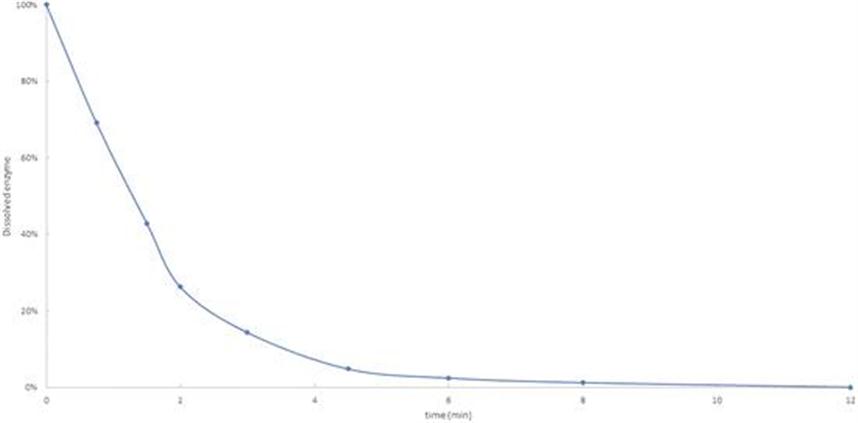
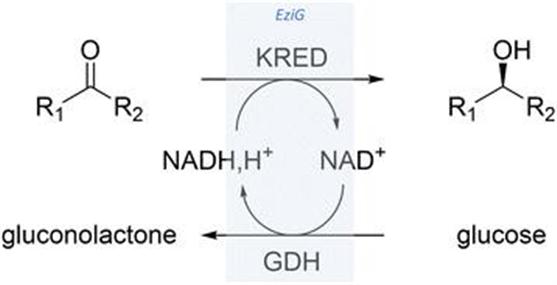
13.2 A General Methodology for Enzyme Reuse
13.2.1 The Potential of Biocatalysis by Far Exceeds Its Current Exploitation
There are a handful of reaction types in which biocatalysis has been established as the method of choice in the formation of high value compounds. These include the production of enantiopure alcohols, amines and esters with lipases (alcohols, esters and amines), transaminases (amines) and ketone reductases (alcohols). A desired enantiomer can be produced by stereoselective synthesis or kinetic resolution, which in most cases gives an obvious economic and environmental benefit.2,4 Though these enzymes and synthesis methods are now relatively well established, there remain many potential cases where they are not used. An estimate by the company Codexis indicates that as much as 30% of the small molecule compounds handled by the pharmaceutical industry today would benefit from using biocatalysis.14 In all likelihood, the actual usage is much lower. The pharmaceutical industry may be leery of rapid changes, due in large part to tight regulations. Still, the economic drive to use enzymes in more processes should have already led to a drastic increase in the application of biocatalysis. What is the reason for the current situation? Arguably, lack of technical knowledge, a widespread unfamiliarity of the molecular features and mode of operation of enzymes, an inadequate technological toolbox, and a habit-driven reliance for the continued use of the vastly more established field of metal catalysis are all significant contributing factors. Nonetheless, and as stated above, we believe that the main barrier to the utilisation of biocatalysts is the high cost contribution of these species in general and their development cost compared to traditional catalysts. Additionally, though economic gain may often be achievable, investment in screening for a suitable enzyme variant is deterred by the uncertainty of the end result as applying high catalyst loadings of non-reusable preparations becomes a necessity.
If a reusable preparation could be assured, there are likely to be a plethora of industrial processes that could be performed under an enzyme catalysis regime in an economically sound manner for non-chiral or lower value products. Enzyme reuse requires some sort of immobilisation of the enzyme, or transformation to a heterogeneous catalyst in some manner. As will be explained below, this has not been a straightforward procedure, and reusable enzymes are therefore scarce. Alcohol resolution with lipase B from Candida antarctica (CalB)15 in the common immobilised form is an example wherein an effective immobilised enzyme is achieved that can be used as a simple heterogeneous catalyst. Immobilised CalB has been extensively studied and is used in various industrial processes.16,17 The wide substrate scope of CalB is surely one reason for its popularity, but the importance of the immobilised form and its simple use should also be highlighted. We believe that if a larger number of immobilised enzymes can be formulated, which are chosen carefully to suit major reaction types, their use will be adopted by the industry in a much more open fashion than for enzymes marketed through the current model of first developing/screening for a specific reaction and then employing non-reusable crude extracts as the frequent preparation of choice.
13.2.2 Unlocking the Potential of Enzymes
Living cells perform a diverse set of enzyme catalysed reactions. Enzyme discovery ranging from bacterium in diverse environments to the astonishing chemistry within our own bodies has given us access to useful catalysts. New catalytic activity can be achieved through engineering of existing enzymes by methods that are now considered robust and effective, and enzymes can be engineered to be more stable in order to withstand the environment within chemical reactors. The time required for developing a biocatalyst for a given reaction is roughly on par with the average time for developing any other catalyst type. In theory, there are plentiful reactions in which enzymes can replace conventional catalysts; however, if the cost contribution of these enzymes in the target process remains too high, the industry will most likely refrain from making the switch. To initiate a switch from already validated catalyst systems, there must be a clearer economic benefit, confidence that the final catalyst will perform as intended, and a genuine belief in the novel technology.
EnginZyme sees a significant benefit in producing biocatalysts that are of an easy-to-use form ready for standard equipment. For the sake of argument, let us assume that there is an enzyme suited for producing a wide range of products from a common pharmaceutical intermediate motif; an enzyme not engineered for one specific reaction, but which can be used for many reactions, and requiring high catalyst loadings in many cases. It is either difficult to bring the cost of producing this enzyme to a level low enough for economic benefit or too much enzyme is needed to produce the product in a reasonable time frame. This theoretical enzyme is employed as a crude preparation that is not reusable, which in turn results in cumbersome product work-up procedures. Such assumptions are not farfetched, and can be exemplified by the case of transaminases and ketone reductases. The disadvantages counteract the otherwise great process improvement. Consequently, the enzyme is only used in a few cases, such as when a low enough catalyst loading is required, or when the product produced is of high value, and work-up procedures are either economically motivated or happen to be simple.
Now, extend the argument for the immobilised CalB mentioned above, whereby an immobilised and thereby reusable preparation is easily attainable and a heterogeneous catalyst with high reusability is achieved, and higher catalyst loadings are now acceptable due to the reusability. Testing and optimisation of reactions can be done with limited effort, and employment in processes is not much different from using standard catalysts. Would this not significantly increase the use of the envisioned enzyme? Goswami and Stewart argue that “…one often does not need to develop the “perfect” enzyme for every case when a pretty good solution will do” and suggest that we “develop enzymes that perform well on whole groups of substrates”.18 As we see it, the strength of this approach would be increased if an effective immobilisation of the enzyme in question could be assured. This is one of the arguments in favour of launching the general immobilisation product EziG.
13.2.3 Immobilised Enzymes for the Pharmaceutical Industry
Biocatalysis is used in the production of food, cosmetics and in several other areas of chemistry; and the potential market for immobilised enzymes is large in all of them. However, EnginZyme has chosen to firstly focus on the pharmaceutical industry, where the need for enantiopure products and the capability to produce complex molecular entities at as low a cost as possible gives an obvious benefit in favour of biocatalysis. Accordingly, pharmaceutical companies have been more keen to employ enzymes in their production processes than, perhaps, in many other branches. Although sales to companies in this industry segment require a significant time investment, this detriment is outweighed by the potential of addressing already developed and verified biocatalytic transformation types. EnginZyme has started by looking at the enzymes that are already present and used; enabling their reuse makes new applications feasible (economically viable), and lowers the costs for current processes.
13.2.4 The Reusable Enzyme Utopia – Enzymes Anchored in Space
The current methods for enzyme reuse can be very effective but, unfortunately, none can be described as being generally applicable. That is, for a given enzyme, there is no method for which a reasonable amount of investment has a sufficiently high degree of certainty to yield an effective reusable preparation. A research project for enzyme reuse, with enzyme immobilisation or other methods, can be a tedious and costly endeavour with an uncertain outcome.
In short, existing techniques can be grouped into three categories, of which numerous examples can be found in academic publications: (1) encapsulation/entrapment of enzymes in a permeable material, (2) cross-linking of the intact enzyme, and (3) binding to a suitable (inert) carrier material. In the case of industrial use, methods in the first category are being marketed by companies such as LentiKat’s,19 which encapsulates the enzyme in lentil-shaped particles, and Zymtronix,20 which uses a self-assembling magnetic porous material to entrap the enzyme. As reflected in the acronym, the Dutch company CLEA (Cross-Linked Enzyme Aggregates) Technologies B.V. are experts in the second category.21 The most commonly utilised method, however, is binding the enzyme to a suitable carrier, such as a porous plastic bead or a silica based material. Resindion22 and Purolite23 offer a variety of different types of porous carrier materials that can be tested with the target enzyme, and others, such as ChiralVision,24 offer services to perform and optimise the immobilisation. Despite these available options, enzyme immobilisation is not a standard technology in the industry because the available methods are effective only for a few verified enzymes, lack general applicability and/or require screening and development work to function in a process for a given enzyme. Moreover, the established methods often bring significant diffusion limitations and enzyme deactivation, which hampers their effectiveness. Furthermore, these methods all require additional costs for preparing the reusable enzyme. Arguably, if 90% activity is lost, more than ten-time reusability is required to ensure that a company sees a return on investment (ROI) for any method. Such high activity loss is not uncommon, yet ten-time reuse is a considerable challenge.
Enzymes can generally be assumed to be most catalytically effective in the form in which they were evolved, i.e. dissolved in aqueous medium, free to have molecular and structural mobility of their different parts, and with their active site easily reachable from the surrounding space. Based on this notion, the ideal immobilisation method would magically anchor enzyme moieties in three dimensions, evenly distributed in a reaction vessel. This would maintain the enzyme in its natural form while also enabling reuse, as the reaction mixture can be removed and a new one added. To date there is obviously no known technology that fulfils these criteria, but the principle can be used as a model to design immobilisation methods, which was the case for EziG.
13.2.5 The EziG Technology
EziG is a material based on a rational design approach, specifically intended for use in biocatalysis and conceived with the utopic immobilisation described above as a model from which as few changes as possible should be made. For practical reasons, encapsulation and cross-linking could not be implemented, since these processes inhibit the natural form and alter the direct environment of the enzyme. Rather, it was necessary to identify a carrier material that could function as closely as possible to a magic anchor. The choice was a specific controlled-pore glass (CPG), a material that has interconnecting pores, which enables more efficient mass transfer to the interior than other porous materials. The compromise required from a ‘magic’ anchor in space is a porous silica skeleton that allows a solvent to effectively reach any given surface point. An important factor is the ability for reactants to reach the enzyme, i.e. efficient mass transfer into the porous material. Poor mass transfer is a common cause for low catalytic activity in general; a problem that is specifically pronounced for immobilised enzyme preparations.
The CPG concept has been used for enzyme immobilisation for decades, but is generally not regarded as a viable option since its performance does not outweigh its high cost. The main issue has been the nature of its silica-like surface, which is not stable to hydrolysis and may also cause denaturation of the enzymes themselves. Although the mass transfer is favourable, activity loss is observed and the number of cycles of reuse are limited. Therefore, we have covered the porous surface of the CPG with an organic polymer layer, thus forming hybrid CPG, which is stable and not harmful to enzymes. Now, a suitable micro-environment for the enzyme is achieved in an accessible pore, and utilisation of a favourable mass transfer is thereby made possible while maintaining an active enzyme. The organic polymer completely covers the interior and exterior surfaces of the particle, and it is stable towards aqueous environments and most organic solvents. The glass skeleton does not swell and is dense enough to allow sedimentation. Microscopic swelling of the organic polymer may occur in organic solvents, but this does not significantly increase the bulk volume of the porous material. At this point, the carrier is suited for adsorption of the enzyme, or for binding with a variety of known methods used for common resins. None of these are generally applicable for a given enzyme, however, so many techniques would have to be tested on a case-by-case basis, the amount of active bound enzyme would often be low, and loss of activity would occur as a consequence of binding at different points on the enzyme surface through distortion of the enzyme’s structure.
Therefore, a well defined and general binding method was sought. Selective binding of target proteins with affinity tags is a standard procedure in protein purification applications.25 The simplest form is use of a poly-histidine tag (His-tag), whereby usually a chain of six residues of the amino acid histidine are added to a chosen end of a protein (N- or C-terminal), which enables binding to metal ions. For purification purposes, a specific binding and the ability to easily remove the protein from a resin after washing are beneficial. Commonly, immobilised metal affinity chromatography (IMAC) involves the use of nickel or cobalt ions chelated to a resin, thereby giving a specific binding to the His-tag, which is simple to break by addition of dissolved imidazole. IMAC with His-tagged proteins is regarded as a general method for purification and is common practice at laboratory scale. EziG uses this technique for binding enzymes, but in this case nickel or cobalt is replaced with iron in ionic form. Although iron is slightly less specific for His-tags, it is non-toxic and the binding is more stable in the sense that, most commonly, little or no enzyme leaching occurs. Thereby, EziG immobilisation is claimed to be general for any enzyme produced with an accessible His-tag. Rather than binding directly on the native enzyme surface in different ways depending on the enzyme in question, by binding to the tag that is usually connected to the enzyme via a linker, the point of binding is now defined. The distortion of the enzyme is minimal, though reversible interactions of the carrier’s surface with the enzyme still occur. Details of the EziG enzyme carrier material are presented in Figure 13.1.
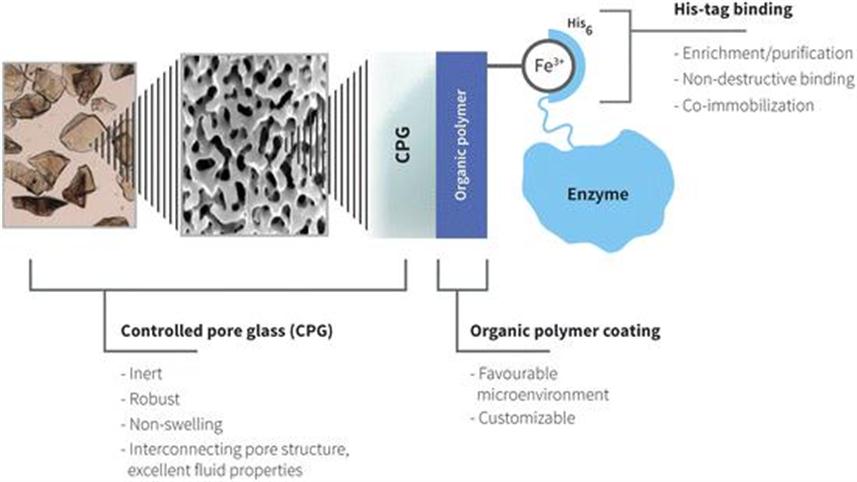
Figure 13.1 Schematic picture of the EziG enzyme carrier, an engineered material for biocatalytic applications. The external and internal porous surface of CPG is covered with an organic polymer layer, which is derivatised to chelate Fe(III), to which His-tagged enzyme can be bound.
By utilising the specificity of the affinity tag, immobilisation on EziG can be performed without prior purification of the enzyme, in contrast to other techniques in which purification may be required to reach a workable enzyme loading on a carrier. The frequently used crude preparations, i.e. cell extracts, can be directly applied to EziG to achieve a reusable preparation. Additionally, different enzymes can easily be co-immobilised on the same carrier for cascade reactions.
Commonly, porous materials used for enzyme immobilisation can be loaded to carry up to around 10% w/w enzyme. Since a large fraction of this is often deactivated, and other proteins are also bound (unless the immobilisation is performed from a purified preparation), the loading of active enzyme is usually limited to single digit percentages. With EziG, however, examples of up to 30% w/w active enzyme are produced. Depending on the reaction rate, the virtual activity of enzymes thus bound varies. In fast reactions, diffusion limitations do decrease the rate somewhat despite the efficient mass transfer. Fast reactions are commonly chosen for simple assays in laboratory testing, in which case the expected activity for the ensuing synthesis reaction is often underestimated, since it is usually not on a par with the assay reaction in terms of reaction rate. For slower reactions, the EziG-immobilised enzyme can reach the same activity as the dissolved enzyme, or extend even higher when stabilising effects come into play.
13.2.6 Standardised Procedure for Immobilisation
By using the affinity tag technique for binding to a tailored material suitable for biocatalysis, immobilisation can be performed by a standardised procedure and active reusable enzyme can generally be expected. Thereby, a great deal of uncertainty is removed and motivation to plan the development of a biocatalytic process with immobilised enzyme is enhanced. Notably, some enzymes require slightly modified procedures to compensate for enzyme specific features such as surface hydrophobicity, multimeric structures, and addition of stability enhancing agents. Accordingly, EziG is currently produced in three varieties with varying surface hydrophobicity. The binding of enzyme in active form is very rarely an issue, in contrast to common resins, and the focus can now shift to optimising the target reaction to accommodate for the introduction of the porous material. EnginZyme shares a vision whereby the idea of being able to reuse essentially any commercially interesting biocatalyst will be included in the decision making and cost estimates made by industrial chemical process designers when assessing the various options at hand.
13.2.7 Lower Cost Materials versus High Performance
The result of immobilising an enzyme on the designed material is generally a high mass loading of reusable enzyme with high retained activity (see examples below); however, it is not possible to produce this material without a significantly higher cost compared to other commonly used materials for enzyme immobilisation. With its sophisticated material properties,26 EziG is significantly costlier than porous plastic, for example. To evaluate economic feasibility, one must calculate the total cost of producing a reusable enzyme per unit of activity, and then conduct a comparison with the non-reusable alternative. A comparison of cost for producing the immobilised enzyme alone is redundant. When compared with low cost materials for immobilising enzymes, and including the cost of wasted enzyme because of activity loss, EziG is almost certainly the most economical choice in the vast majority of cases. The general immobilisation procedure is straightforward and can be performed from crude extracts, enzyme activity is maintained and high loadings of active enzyme are achieved; the higher cost of EziG is outweighed by these cost savings, as exemplified by the general case below.
To simplify, imagine a common case of a porous plastic material on which an enzyme is immobilised, thereby losing 90% of the activity, with a mass loading of 5%. The cost of enzyme for producing a given amount of activity has thereby roughly increased by a factor of ten, and the amount of carrier material needed to immobilise the enzyme must be 190 times that of the enzyme mass needed in the non-immobilised case (ten times more enzyme and 5% w/w enzyme loading gives a total mass of 200 kg for a preparation of equal activity to 1 kg of non-immobilised enzyme). Without including the cost for the carrier material, along with other factors such as costs for immobilisation procedures, increased volume in the reaction vessel, etc., the enzyme alone must still be reused more than ten times for the immobilisation to give any benefits.
The same case with EziG may show a mass loading of 20% and a maintained activity level of 90% compared to the dissolved enzyme. The increased cost for the enzyme is not significant, and the mass amount of carrier needed is not more than four times that of the enzyme. To reach the activity equal to 1 kg of non-immobilised enzyme, ∼1.1 kg enzyme and ∼4.4 kg of EziG is needed, giving a total mass of around 5.5 kg for the immobilised preparation. For the majority of enzymes for immobilisation on the available support resins available today, the difference in enzyme and carrier mass material needed more than compensates for the higher cost of EziG. Since the immobilisation can be done directly from a crude extract, few or no additional steps need be added to the process. The number of recycling loops necessary for reaching a saving varies with enzyme cost, but for the average enzyme this number is usually in the single-digit range. Additionally, since the binding with the His-tag is reversible, the same carrier material can often be reloaded with fresh enzyme after exhausting the first batch of bound material and then recycled within the given process, thus avoiding any risk of cross-contamination. When this scenario is possible, the cost contribution from the carrier becomes insignificant.