Nofima: Peptide Recovery and Commercialization by Enzymatic Hydrolysis of Marine Biomass
17.5 Functional Properties and Bioactivities of Hydrolyzed Marine Biomass
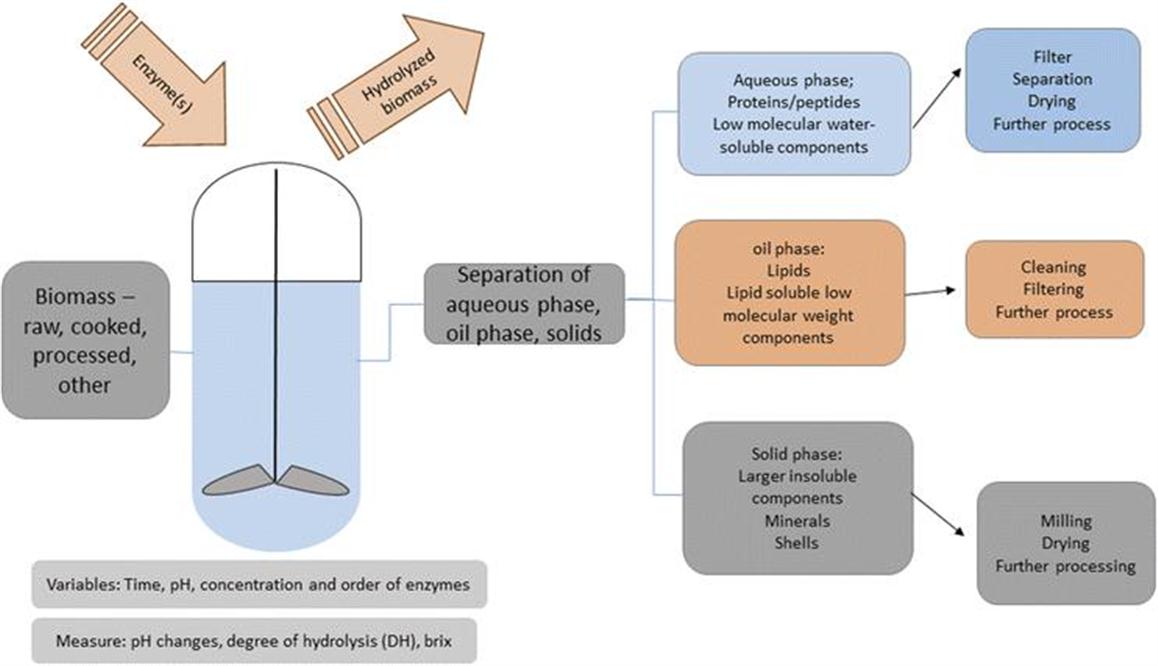
Detailed characterization of protein hydrolysates in terms of their chemical composition, sensory attributes, functional properties and bioactivities is an essential part of product development. A challenge when developing hydrolysis-based products from biomass is the complexity of the biomass, such that there is a multitude of different components in the mixture that affects the hydrolysis reaction. In addition, when using fisheries by-products, the composition of this heterogeneous biomass can vary from day to day, adding a further complexity to the production. This issue is addressed below. Much work has been done on demonstrating the functional properties and bioactivity of peptides derived from marine biomass.6,8,15,24,48,49 As mentioned in the classification of marine biomass, the functional screening will depend on the type of application. Most of the marine biomass that is classified as suitable for human consumption will go through the screening for bioactivity, in some cases category 3 or even category 2 biomass will be tested for bioactivity in those instances where a bioactivity can significantly enhance the biomass value. One example is animal feed where a bioactivity can significantly improve desired traits of the animal.8 In most cases, peptides will be recovered as a mix of peptides in a hydrolysate with a peptide size distribution. The size of the peptides will depend on the degree of hydrolysis of the proteins – the size will vary, but generally an enzymatic digestion that has been allowed to proceed for a longer time will contain smaller peptides.
A challenge of marine proteins is that they often can have an undesirable taste. The taste of the final product will affect its commercial value. Bitter taste is often associated with hydrophobic amino acids that are released, as well as other small molecular components and residual fats. De-bittering techniques have been developed, including solvent extraction, the use of activated carbon on matrices, chromatography techniques, addition of de-bittering components, or hydrolysis with exopeptidases. The research performed indicates that restricting the enzymatic digestion of the biomass to yield larger peptides will reduce undesired taste. However, this can again affect the bioavailability of the peptides.21,28,29,50 Many of the de-bittering techniques are not easily implemented in large scale production; however, hydrolysate de-bittering with exopeptidases has shown promising results and is amenable to an industrial scale hydrolysis process.11,20 The physicochemical properties such as water binding, emulsifying and foaming abilities are also important in order to obtain valuable products from the marine hydrolysates,8,17 and are part of the deciding factors when evaluating the commercial value of the hydrolysate.
The bioactivity will of course affect the commercial potential of any given product. To determine bioactivity in the hydrolysate, screening is performed upon fractionation of the hydrolysate followed by in vitro bioassays to determine effects. Marine hydrolysates have been demonstrated to have several effects in humans (i.e. effects as functional foods and in the pharmaceutical area), including antioxidant and anticoagulant effects, and cardioprotective, antimicrobial, anti-diabetic and appetite suppressive effects, as well as improving nutritional status in people with nutritional needs. Antitumor, antiviral and neuroprotective effects have also been demonstrated. Upon initial screening, the hydrolysate is further fractionated and bioactivity effects can be further studied in several in vitro and in vivo assays, which have been extensively reviewed elsewhere.19,20,27,51,52 These processes are laborious and can usually only be applied in the developmental phase. A different and interesting approach is the use of multivariate analysis to relate the chemical fingerprint of a given hydrolysate with the corresponding sensory, functional and pharmacological properties. For example, van der Ven et al. developed Fourier-transform infrared spectroscopy (FTIR)-based multivariate regression models for the prediction of functional parameters (i.e. solubility, emulsification and foaming) and sensory properties (i.e. bitterness) of whey and casein hydrolysates.53 Such an analytical and statistical approach is a beneficial alternative to measuring hydrolysate properties using the traditional biochemical assays especially in the scale up phase of the process. Overall, both the complexity of food processing by-products and a multistage action of catalytic enzymes poses significant challenges in creating the relevant biochemical understanding prior to product (i.e. protein hydrolysate) development. Knowledge of such biochemical parameters is mandatory, particularly in product development for higher-paying markets (i.e. for human consumption). In the processes described here, the biomass is utilized in the process such that protein, minerals and fats are not wasted. In other pharmaceutical processes, a bioactive peptide is identified from marine biomass; however, the compound is then synthesized chemically and used in drugs. In these pharmaceutical processes, the biomass is not utilized in the same way, such that proteins, minerals and fats in by-products are still wasted. Scale-up of laboratory developed processes and recent advances in the bioprocessing of marine biomass has allowed for more products that consume or use up the biomass to be developed; however, there are still challenges faced in the commercialization phase, and so the issue of scale up will be addressed next.
A lot of research is being carried out on the exploitation and valorization of low value marine biomass, especially remaining raw materials. Many of the high value products have been produced at lab scale, both within pharma, food and feed, but the number of project making the translation from lab scale to market is not very high. A few products have reached the market, but there is a need for research enabling the conversion of low value biomass into commercial products and at the same time enabling scale-up, marketing and consumer preferences. The demonstration phase is a critical phase of the development of sustainable products from under-utilized biomass. As mentioned above, by-products are complex and heterogeneous biomasses with a substantial, uncontrollable variation in quality due to differences in composition (e.g. protein structure and amino acid composition), oxidation state of oil and protein components, the amounts and activity of spoilage enzymes, and the microorganisms present in the material. It is therefore crucial to develop processing methods that can handle these variations, in order to obtain a stable end-product quality. The scale-up of a laboratory developed process can be challenging. Many factors can hamper the scale up. One challenge can be that the hydrolysis process on a large scale (2000 L plus) does not proceed as it does at laboratory scale. Another challenge can be that infrastructure that is readily available at laboratory scale can be either hard to access or not applicable to larger scales, or alternatively be very costly in larger scale. The demonstration phase is often challenging in terms of investments as the access to capital in this phase can be scare while the costs of scale-up of innovation and trial in the market are typically high.54,55 Let us first focus on the scale-up of the process itself. Due to differences in heating, mixing, sheer flow and the formation of inhibitory products that were not significant at smaller scale, the speed and the result of the hydrolysis can differ from laboratory to larger scale. By scaling the process in a step-wise manner, going from smaller scale and increasing the volume 10× for each step, the issues can be more easily resolved, as the cause of the changes can be more easily identified. Another way of improving the scale-up process is to ensure proper monitoring of the process. Robust process design was introduced by the Japanese quality guru Taguchi around 1950, and has found use in biotechnology.56 The method aims at finding process settings that minimize the effect of the so-called nuisance variables (e.g. raw material properties), and thereby obtaining a consistent product regardless of the raw material variation. Tailor-made hydrolysates do not only need to have the desired target functionality, but also satisfy a multitude of other requirements such as sensory and physiochemical properties. At the same time, it is important to maximize yield and maintain good quality of the oil and mineral fractions. The so-called desirability functions is a well-established method for multi-response optimization,57 making it possible to set different weights on the responses.
Hydrolysis of biomass is commonly performed in large batch-based reactor tanks; however, an industrial improvement on production of peptide-based products from marine biomass is the introduction of continuous hydrolysis in large-scale production. In this setup, the hydrolysis is performed continually through smaller pipe-like constructions while being pushed from one end, the starting point, to the end-point of the hydrolysis. The process is monitored by recording viscosity in the biomass hydrolysate throughout the process, and the addition of monitoring-techniques as described below can improve the process additionally by slowing real-time adjustments of the processing settings and thus better targeting the desired end product quality. Parameters like temperature and pH can be modulated along the process, and the addition of different enzymes to sequentially break down components in the biomass can also lead to an improved product.
Earlier and on-going studies at Nofima show that characterization of both the hydrolysis process and the resulting hydrolysates using the classical wet chemistry approaches is a challenging task with a considerable risk of inaccurate or otherwise misleading results. Recent developments in analytical technologies open up several new possibilities in process monitoring as well as product and raw material characterization. In process monitoring, the degree of hydrolysis is an important parameter that can be measured using a variety of different methods including TNBS and OPA,31,32 with the alternative approach based on titration of released protons (pH Stat) in the course of the hydrolysis.32,58 Another valuable parameter used for characterization of protein hydrolysates is the molecular weight distributions (MWD).59 Unlike DH%, MWD is a direct measure of the composition of the hydrolysate and relates to important quality parameters such as intestinal absorption, viscosity and bitterness. A major limitation of both MWD and DH% measurements is the lengthy analysis time, thus restricting their use as on-line monitoring tools in industrial setups. In this regard, the fast and non-invasive spectroscopic techniques are interesting process monitoring tools with a promising potential for industrial applications.60 FTIR is also one of the techniques that potentially can be used to monitor the breakdown of proteins in hydrolysis reactions.61,62 Thus, by using on-line or near real-time monitoring techniques one can significantly improve the scale-up of the hydrolysis process. As the biomass that is being processed is complex and can vary, the optimal parameters and the process can vary for each production. By allowing for continuous monitoring, the product produced can be more stable as it allows for small adjustments in process parameters in the large-scale production.
In addition to the process challenges and recent development in large scale production of peptide-based products from marine biomass, access to both infrastructure and capital can be a challenge. The demonstration phase has been described as the valley of death in product commercialization.55 The access to demonstration plants can help alleviate the hurdle of the demonstration phase. Demonstration plants are flexible small scale production plants where companies or academic entities can test a process that has been developed at laboratory scale. A demonstration plant allows the company or academia to test out a process without having to make large investments in costly scale-up and production infrastructure. A product prototype can be made in this process and this product prototype can be tested in the market. The prototype assessment of the product in the market is an important part of product development as it will help evaluate whether the product can be sold in one or several markets as predicted in initial analyses, and that there is a customer segment willing to pay for the product. In addition to allowing for testing in the market, performing a prototype production will provide estimates of production costs and capital expenditures that will be associated with the product. As the demonstration plant will be built to be flexible and contain infrastructure that can be adapted to a number of processes, the cost figures will only be estimates. If the product is to be produced in a production plant designed specifically for the developed process, the production costs will likely be lower, and the yield will be higher. Figure 17.2 is a schematic illustration of the infrastructure available at Biotep, a demonstration plant that is owned and operated by Nofima. The different infrastructure is connected by pipes that can direct the biomass to the desired infrastructure. Different infrastructure is noted on the figure, and can also be viewed on the full 3D movie of the plant that is available (https://vimeo.com/174329346) for further explanation. Located outside of the area shown in Figure 17.2 are cooling and storage facilities for the different products. The plant is approved for food grade production, and according to EU regulations44 all food and feed grade products have to be separated. The receiving room and the room with the reactor tanks (top left, Figure 17.2) are in what is called the unclean zone. All biomass is inactivated and pasteurized before being moved to the clean zone, which starts in the top-right room with the holding tanks and the filtration systems and continues further to the drying room and the storage and exit for the products.
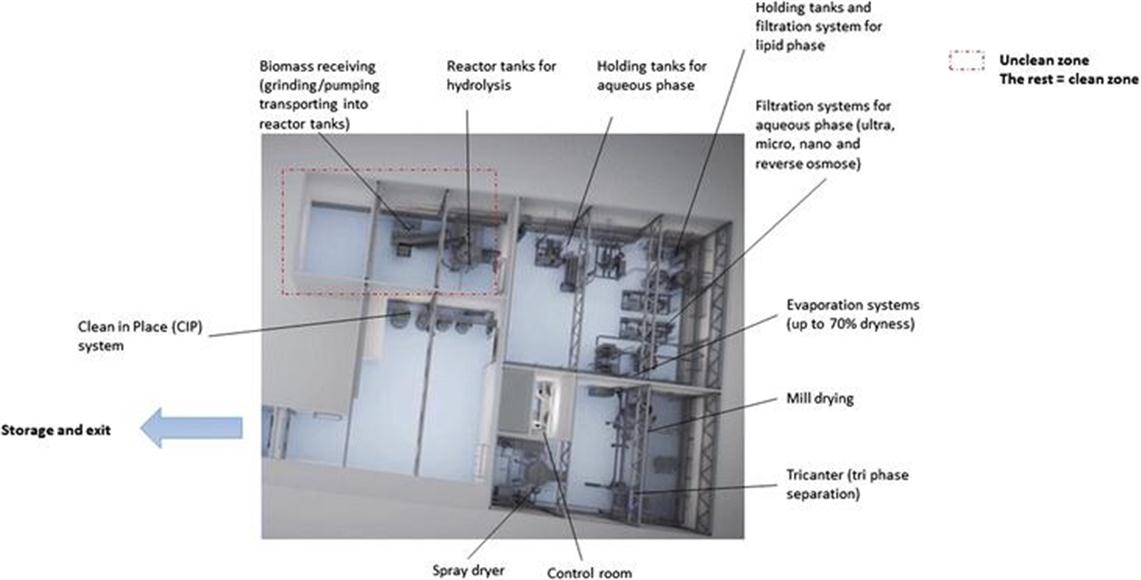
Figure 17.2 Illustration of Biotep demonstration scale up plant. The different parts of the infrastructure are marked on the picture and an explanation is available on-line (https://vimeo.com/174329346) and at Biotep.no.
References
1. Nofima, Nofima Annual Report 2015, 2015.
2. M. Pedersen, A. Mortensen, B. Dreyer, P. Honkanen, H. Nilsen, K. Ø. Midling, O. Andreassen, B. I. Bendiksen and P. Olsen, Forskningsvirksomheten 2015-Årsrapport til Nærings og Fiskeridepartementet, Nofima.no, 2016.
3. E. Dauksas, E. Falch, R. Slizyte and T. Rustad, Process Biochem., 2005, 40, 2659.
4. J. Raa, A. Gildberg and J. N. Olley, Crit. Rev. Food Sci. Nutr., 1982, 16, 383.
5. N. Yan and X. Chen, Nature, 2015, 524, 155.
6. A. Baiano, Molecules, 2014, 19, 14821.
7. S. Damodaran, K. L. Parkin and O. R. Fennema, Fennema's Food Chemistry, Fourth Edition, CRC Press, Taylor & Francis Group, Boca Raton, 2007.
8. A. Gildberg, J. Aquat. Food Prod. Technol., 2004, 13, 3.
9. V. Venugopal, F. Shahidi and T. C. Lee, Crit. Rev. Food Sci. Nutr., 1995, 35, 431.
10. L. F. de Arruda, R. Borghesi, A. Brum, M. R. D'Arce and M. Oetterer, Food Sci. Technol. (Campinas), 2006, 26, 749.
11. A. E. Ghaly, V. V. Ramakrishnan, M. S. Brooks, S. M. Budge and D. Dave, J. Microb. Biochem. Technol., 2013, 5, 107.
12. H. A. M. Hermansson, E. Sivik and E. Skjoldebrand, Lebensm.-Wiss. Technol., 1971, 4, 201.
13. L. F. d. Arruda, R. Borghesi and M. Oetterer, Braz. Arch. Biol. Technol., 2007, 50, 879.
14. H. O. Hultin and S. D. Kelleher, US Patent 6,451,975, 2000.
15. I. Undeland, S. D. Kelleher and H. O. Hultin, J. Agric. Food Chem., 2002, 50, 7371.
16. H. G. Kristinsson and H. O. Hultin, J. Agric. Food Chem., 2003, 51, 5103.
17. H. G. Kristinsson and B. A. Rasco, Crit. Rev. Food Sci. Nutr., 2000, 40, 43.
18. H. G. Kristinsson, A. E. Theodore and B. Ingadottir, in Maximising the Value of Marine By-products, ed. F. Shahidi, Woodhead Publishing, Cambridge, 2007, pp. 144–168.
19. R. C. F. Cheung, T. B. Ng and J. H. Wong, Mar. Drugs, 2015, 13, 4006.
20. M. Hayes and K. B. Tiwari, Int. J. Mol. Sci., 2015, 16(9), 22485.
21. P. A. Sujith and T. V. Hymavathi, As. J. Food Ag-Ind., 2011, 4, 365.
22. T. Rustad, I. Storrø and R. Slizyte, Int. J. Food Sci. Technol., 2011, 46, 2001.
23. M. Chalamaiah, B. Dinesh kumar, R. Hemalatha and T. Jyothirmayi, Food Chem., 2012, 135, 3020.
24. J. T. Ryan, R. P. Ross, D. Bolton, G. F. Fitzgerald and C. Stanton, Nutrients, 2011, 3(9), 765.
25. C. C. Udenigwe and R. E. Aluko, J. Food Sci., 2012, 77(1), R11.
26. Marealis AS, http://www.marealis.com/, accessed February 2017.
27. A. Gildberg, J. A. Arnesen, B.-S. Sæther, J. Rauø and E. Stenberg, Process Biochem., 2011, 46, 2205.
28. T. Aspevik, PhD, University of Bergen, 2016.
29. T. Aspevik, C. Totland, P. Lea and Å. Oterhals, Process Biochem., 2016, 51, 1006.
30. J. Adler-Nissen, S. Eriksen and H. S. Olsen, Plant Foods Hum. Nutr., 1983, 32, 411.
31. D. Spellman, E. McEvoy, G. O’Cuinn and R. J. FitzGerald, Int. Dairy J., 2003, 13, 447.
32. S. M. Rutherfurd, J. AOAC Int., 2010, 93, 1515.
33. S. Chakraborty, N. Kaushik, P. S. Rao and H. N. Mishra, Compr. Rev. Food Sci. Food Saf., 2014, 13, 578.
34. B. M. Nestl, B. A. Nebel and B. Hauer, Curr. Opin. Chem. Biol., 2011, 15, 187.
35. H.-D. Belitz, W. Grosch and P. Schieberle, Food Chemistry, Fourth Revised and Extended Edition, Springer-Verlag, Berlin, 2009.
36. M. B. Rao, A. M. Tanksale, M. S. Ghatge and V. V. Deshpande, Microbiol. Mol. Biol. Rev., 1998, 62, 597.
37. A. Demirci, G. Izmirlioglu and D. Ercan, in Food Processing, Principles and Applications, Second Edition, ed. S. Clark, S. Jung and B. Lamsal, John Wiley & Sons, Inc., Hoboken, 2014, pp. 107–136.
38. J. Adler-Nissen, in Enzymes in Food Processing, Third Edition, ed. G. Reed, Academic Press, London, 1993, pp. 159–203.
39. R. Hatti-Kaul, U. Törnvall, L. Gustafsson and P. Börjesson, Trends Biotechnol., 2007, 25, 119–124.
40. D. R. Headon and G. Walsh, Biotechnol. Adv., 1994, 12(4), 635.
41. Enzyme Company Guide, 2015, Edition Sustainable Chemistry Solutions, Inc., 2015, p. 517. Available at http://www.bio-catalyst.com/2015-enzyme-company-guide-now-available/, last accessed August 2017.
42. D. Agyei and M. K. Danquah, Biotechnol. Adv., 2011, 29, 272.
43. J. Wasswa, J. Tang and X. Gu, Food Rev. Int., 2007, 23, 159.
44. European Commission, Regulation (EC) No 1069/2009 of the European Parliament and of the Council of 21 October 2009 laying down health rules as regards animal by-products and derived products not intended for human consumption and repealing Regulation (EC) No 1774/2002 (Animal by-products Regulation), http://eur-lex.europa.eu/eli/reg/2009/1069/2014-01-01.
45. R. Moita and P. C. Lemos, J. Biotechnol., 2012, 157, 578.
46. T. Keshavarz and I. Roy, Curr. Opin. Microbiol., 2010, 13, 321.
47. M. Chandrasekaran and A. H. Bahkali, Saudi J. Biol. Sci., 2013, 20, 105.
48. P. A. Harnedy and R. J. FitzGerald, J. Funct. Foods, 2012, 4, 6.
49. F. Shahidi, in Maximising the Value of Marine By-products, Woodhead Publishing, 2007, DOI: 10.1016/B978-1-84569-013-7.50028-4, pp. xxi-xxv.
50. H.-O. Kim and E. C. Y. Li-Chan, J. Agric. Food Chem., 2006, 54, 10102.
51. S.-K. Kim and I. Wijesekara, J. Funct. Foods, 2010, 2(1), 1.
52. D. Zdzieblik, S. Oesser, M. W. Baumstark, A. Gollhofer and D. König, Br. J. Nutr., 2015, 114, 1237.
53. C. van der Ven, S. Muresan, H. Gruppen, D. B. A. de Bont, K. B. Merck and A. G. J. Voragen, J. Agric. Food Chem., 2002, 50(24), 6943.
54. C. Hendry, P. Harborne and J. Brown, Energy Policy, 2010, 38, 4507.
55. H. Hellsmark, J. Frishammar, P. Söderholm and H. Ylinenpää, Res. Policy, 2016, 45, 1743.
56. K.-L. Tsui, IIE Trans., 1992, 24, 44.
57. S. Das, A. Bhattacharya, S. Haldar, A. Ganguly, S. Gu, Y. P. Ting and P. K. Chatterjee, Sustainable Mater. Technol., 2015, 3, 17.
58. S. M. Rutherfurd, J. AOAC Int., 2010, 93(5), 1515.
59. P. G. Tello, F. Camacho, E. Jurado, M. P. Paez and E. M. Guadix, Biotechnol. Bioeng., 1994, 529–532.
60. J. P. Wold, M. O'Farrell, M. Høy and J. Tschudi, Meat Sci., 2011, 89, 317.
61. C. Ruckebusch, N. Nedjar-Arroume, S. Magazzeni, J.-P. Huvenne and P. Legrand, J. Mol. Struct., 1999, 478, 185.
62. N. A. Poulsen, C. E. Eskildsen, M. Akkerman, L. B. Johansen, M. S. Hansen, P. W. Hansen, T. Skov and L. B. Larsen, Int. Dairy J., 2016, 61, 44.
63. N. Urala and L. Lähteenmäki, Food Qual. Prefer., 2007, 18, 1.
64. D. N. Cox and G. Evans, Food Qual. Prefer., 2008, 19, 704.
65. J. J. McCluskey, N. Kalaitzandonakes and J. Swinnen, Annu. Rev. Resour. Econ., 2016, 8, 467.
66. Q. Chen, S. Anders and H. An, Food Qual. Prefer., 2013, 28, 419.
67. M. Siegrist, Trends Food Sci. Technol., 2008, 19, 603.
68. M. Siegrist, N. Stampfli and H. Kastenholz, Appetite, 2008, 51, 526.
69. A. H. Matin, E. Goddard, F. Vandermoere, S. Blanchemanche, A. Bieberstein, S. Marette and J. Roosen, Int. J. Consum. Stud., 2012, 36, 149.
70. B. Magnuson, I. Munro, P. Abbot, N. Baldwin, R. Lopez-Garcia, K. Ly, L. McGirr, A. Roberts and S. Socolovsky, Food Addit. Contam., Part A: Chem., Anal., Control, Exposure Risk Assess., 2013, 30, 1147.
71. I. W. Y. Cheung and E. C. Y. Li-Chan, Food Chem., 2010, 122, 1003.
72. J. Cheng, W. Zhang, X. Zhang, F. Han, X. Li, X. He, Q. Li and J. Chen, JAMA Intern. Med., 2014, 174, 773.
73. Antihypertensive Effect and Safety of Peptides Derived From Coldwater Shrimp (MARE), https://clinicaltrials.gov/ct2/show/NCT01583582, accessed March 2017.
74. Effect and Safety of Marealis RPC (Refined Peptide Concentrate) in Mild or Moderate Hypertensive Subjects, https://clinicaltrials.gov/ct2/show/NCT01974570, accessed March 2017.
75. A. G. Tacon and M. Metian, Ambio, 2009, 38, 294.
76. S. I. Siikavuopio, P. James, E. Stenberg, T. Evensen and B.-S. Sæther, Fish. Res., 2017, 188, 121.