
THREE
What a Plant Feels
I will touch a hundred flowers
And not pick one.
—Edna St. Vincent Millay, “Afternoon on a Hill”
Most of us interact with plants every day. At times we experience plants as soft and comforting, like grass in a park during an indulgent midday nap or fresh rose petals spread across silk sheets. Other times they are rough and prickly: we navigate around pesky thorns to get to a blackberry bush on a meander through the woods or trip over a knotted tree trunk that’s worked its way up through the street. But in most cases, plants remain passive objects, inert props that we interact with but ignore while we do so. We pluck petals from daisies. We saw the limbs off unsightly branches. What if plants knew we were touching them?
It’s probably a bit surprising, and maybe even a bit disconcerting, to discover that plants know when they’re being touched. Not only do they know when they’re being touched, but plants can differentiate between hot and cold, and know when their branches are swaying in the wind. Plants feel direct contact: some plants, like vines, immediately start rapid growth upon contact with an object like a fence they can wrap themselves around, and the Venus flytrap purposely snaps its jaws shut when an insect lands on its leaves. And plants seemingly don’t like to be touched too much, as simply touching or shaking a plant can lead to growth arrest.
Of course, plants don’t “feel” in the traditional sense of the term. Plants don’t feel regret; they don’t get a feel for a new job. They do not have an intuitive awareness of a mental or emotional state. But plants perceive tactile sensation, and some of them actually “feel” better than we do. Plants like the burr cucumber (Sicyos angulatus) are up to ten times more sensitive than we are when it comes to touch. Vines from a burr cucumber can feel a string weighing only 0.009 ounce (0.25 gram), which is enough to induce the vine to start winding itself around a nearby object. On the other hand, most of us can only feel the presence of a very light piece of string on our fingers that is about 0.07 ounce (or 2 grams) in weight. Although a plant may be more sensitive to touch than a human being, plants and animals have some surprising similarities when it comes to feeling that touch.

Burr cucumber (Sicyos angulatus)
Our sense of touch transmits vastly different sensations, from a painful burn to the light trace of a breeze. When we come into contact with an object, nerves are activated, sending a signal to the brain that communicates the type of sensation—pressure, pain, temperature, and more. All physical sensations are perceived through our nervous system by specific sensory neurons in our skin, muscles, bones, joints, and internal organs. Through the action of different types of sensory neurons, we experience a vast array of physical sensations: tickling, sharp pain, heat, light touch, or dull ache, to name a few. Just as different types of photoreceptors are specific for different colors of light, different sensory neurons are specific for different tactile experiences. Different receptors are activated by an ant crawling on your arm and by a deep Swedish massage at the spa. Our bodies have receptors for cold and receptors for heat. But each of the different types of sensory neurons acts in essentially the same way. When you touch something with your fingers, the sensory neurons for touch (known as mechanoreceptors) relay their signal to an intermediary neuron that connects up to the central nervous system in the spinal cord. From there, other neurons convey the signal to the brain, which tells us that we’ve felt something.
The principle involved in neural communication is the same for all nerve cells: electricity. The initial stimulus starts a rapid electrochemical reaction known as depolarization, which is propagated along the length of the nerve. This electric wave hits the adjacent neuron, and the wave continues along the new neuron, and so on, until it reaches the brain. A block in the signal at any stage can be catastrophic, as in the case of traumatic spine damage, which cuts this signal, leading to loss of feeling in the limbs that have been affected.
While the mechanisms involved in electrochemical signaling are complex, the basic principles are simple. Just as a battery maintains its charge by housing different electrolytes in different compartments, a cell has a charge owing to different amounts of various salts in and outside the cell. There is more sodium on the outside of cells and more potassium inside. (That’s why salt balances are so important in our diets.) When a mechanoreceptor is activated, let’s say by your thumb touching the space bar on a keyboard, specific channels open up near the point of contact in the cell membrane that allow sodium to pass into the cell. This movement of sodium changes the electric charge, which leads to the opening of additional channels and increased sodium flux. This results in the depolarization that propagates along the length of the neuron like a wave propagating across the ocean.
At the end of a neuron, at the junction where it meets an adjacent one, the action potential leads to a rapid change in the concentration of an additional ion, calcium. This calcium spike is necessary for the release of neurotransmitters from the active neuron, which are received by the next neuron. Neurotransmitters binding to the second neuron initiate new waves of action potentials. These spikes in electrical activity exemplify the ways in which nerves communicate, whether from a receptor to the brain or from the brain to a muscle to cause a movement. The ubiquitous cardiac monitors in hospitals depict this kind of electrical activity as it relates to heart function—a spike of activity followed by a recovery, which is repeated again and again. Mechanosensory neurons send similar spikes of activity to the brain, and the frequency of the spikes communicates the strength of the sensation.
But touch and pain are biologically not the same phenomena. Pain does not simply result from an increase in the signals emanating from touch receptors. Our skin features distinct receptor neurons for different types of touch, but it also has unique receptor neurons for different types of pain. Pain receptors (called nociceptors) require a much stronger stimulus before they send action potentials to the brain. Advil, Tylenol, and other pain relievers work because they specifically mute the signal coming from the nociceptors but not the mechanoreceptors.
So human touch is a combination of actions in two distinct parts of the body—cells that sense the pressure and turn this pressure into an electrochemical signal, and the brain that processes this electrochemical signal into specific types of feelings and initiates a response. So what happens in plants? Do they have mechanoreceptors?
Venus’s Trap
The Venus flytrap* (otherwise known as Dionaea muscipula) is the quintessential example of a plant that responds to touch. It grows in the bogs of the Carolinas, where the soil lacks nitrogen and phosphorous. To survive in such a nutritionally poor environment, Dionaea has evolved the amazing ability to garner nutrition not only from light but from insects—and small animals as well. These plants carry out photosynthesis, like all green plants, but they moonlight as carnivores, supplementing their diet with animal protein.
(* The “Venus” part of the plant’s name has little to do with science and much to do with the rather lewd imaginations of nineteenth-century English botanists. See www.sarracenia.com/faq/faq2880.html.)
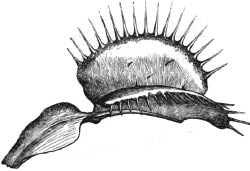
Venus flytrap (Dionaea muscipula)
The leaves of the Venus flytrap are unmistakable: they end in two main lobes connected by a central midrib, and the edges of the two lobes are bordered by long protrusions, called cilia, that resemble the teeth of a comb. These two lobes, connected on one side by a hinge, are normally spread at an angle, forming a V-like structure. The internal sides of the lobe have pink and purple hues and excrete nectar that is irresistible to many creatures. When an unassuming fly, a curious beetle, or even a small, meandering frog crawls across the surface of the leaves, the two leaves spring together with surprising force, sandwiching the unsuspecting prey and blocking its escape with its jail bars of interlocking cilia.* The trap closes at an astounding speed: unlike our futile attempts to swat at a pesky fly, the Venus flytrap springs shut in less than one-tenth of a second. Once activated, the trap excretes digestive juices that dissolve and absorb the poor prey.
(* See www.youtube.com/watch?v=ymnLpQNyI6g for a great example of the Venus flytrap in action.)
The amazing characteristics of the Venus flytrap led Charles Darwin, who was among the first scientists to publish an in-depth study of the plant and other carnivorous flora, to describe it as “one of the most wonderful [plants] in the world.” Darwin’s interest in carnivorous plants illustrates how naive curiosity can lead a trained scientist to groundbreaking discoveries. Darwin begins his 1875 treatise Insectivorous Plants in this way: “During the summer of 1860, I was surprised by finding how large a number of insects were caught by the leaves of the common sun-dew [plant] (Drosera rotundifolia) on a heath in Sussex. I had heard that insects were thus caught, but knew nothing further on the subject.” From knowing virtually nothing about the matter, Darwin became the foremost expert on carnivorous plants, including the Venus flytrap, in the nineteenth century, and indeed his work is still referenced today.
We now know that the Venus flytrap feels its prey and senses if the organism crawling around inside its trap is the right size to consume. There are several large black hairs on the pink surface of the inside of each lobe, and the hairs act as triggers that spring the trap closed. But one hair being touched is not enough to spring the trap; studies have revealed that at least two have to be touched within about twenty seconds of each other. This ensures that the prey is the ideal size and won’t be able to wiggle out of the trap once it closes. The hairs are extremely sensitive, but they are also very selective. As Darwin noted in his book Insectivorous Plants:
Drops of water, or a thin broken stream, falling from a height on the filaments [hairs], did not cause the blades to close . . . No doubt, the plant is indifferent to the heaviest shower of rain . . . I blew many times through a fine pointed tube with my utmost force against the filaments without any effect; such blowing being received with as much indifference as no doubt is a heavy gale of wind. We thus see that the sensitiveness of the filaments is of a specialized nature.
Even though Darwin described in great detail the series of events leading to trap closure and the nutritional advantage of the animal protein to the plants, he couldn’t come up with the mechanism of the signal that differentiated between rain and fly and enabled the rapid imprisonment of the latter. Convinced that the leaf was absorbing some meaty flavor from the prey on the lobes, Darwin tested all types of proteins and substances on the leaf. But these studies were for naught, as he could not induce trap closure with any of his treatments.
His contemporary John Burdon-Sanderson made the crucial discovery that explained the triggering mechanism once and for all. Burdon-Sanderson, a professor of practical physiology at University College in London and a physician by training, studied the electrical impulses found in all animals, from frogs to mammals, but from his correspondence with Darwin became particularly fascinated by the Venus flytrap. Burdon-Sanderson carefully placed an electrode in the Venus flytrap leaf, and he discovered that pushing on two hairs released an action potential very similar to those he observed when animal muscles contract. He found that it took several seconds for the electrical current to return to its resting state after it had been initiated. He realized that when an insect brushes up against the hairs inside the trap, it induces a depolarization that is detected in both lobes.
Burdon-Sanderson’s discovery that pressure on two hairs leads to an electrical signal that is followed by the trap closing was one of the most important of his career and was the first demonstration that electrical activity regulates plant development. But he could only hypothesize that the electric signal was the direct cause of trap closure. More than one hundred years later, Alexander Volkov and his colleagues at Oakwood University in Alabama proved that the electric stimulation itself is the causative signal for the trap’s closing. They applied a form of electric shock therapy to the open lobes of the plant, and it caused the trap to close without any direct touch to the trigger hairs. Volkov’s work and earlier research in other labs also made it clear that the trap remembers if only a single trigger hair has been touched, and then it waits until a second hair is triggered before closing. Only very recently did this research shed light on the mechanism that allows the Venus flytrap to remember how many of its hairs have been triggered, which I’ll explore in chapter six. Before we get to the ways in which plants remember, we need to take some time with the connection between the electric signal and the movement of the leaves.
Water Power
Burdon-Sanderson observed that the electrical pulse he detected in the closing Venus flytrap was very similar to the action of a nerve and a contracting muscle. While the action potentials in the absence of nerves were clear to him, the mechanism of movement in the absence of muscles was obscure. To Burdon-Sanderson’s knowledge, the action potential in the plant had no clear muscle-like target to act on to induce the trap’s closing.
Studies of Mimosa pudica provided a wonderful experimental system to understand the world of leaf movements, which could then be generalized to other plants. The Mimosa pudica is native to South and Central America but is now grown worldwide as an ornamental because of its fascinating moving leaves. Its leaves are hypersensitive to touch, and if you run your finger down one of them, all the leaflets rapidly fold inward and droop. They reopen several minutes later, only to rapidly close once more if you touch them again. The name pudica reflects this drooping movement. It means “shy” in Latin. The plant is also known throughout many regions as “the sensitive plant.” Its unusual behavior is referred to as “false death” in the West Indies, and it is referred to as the “don’t touch me” plant in Hebrew and the “shy virgin” in Bengali.
The Mimosa’s characteristic drooping and opening action is very similar to that of the Venus flytrap even at the level of electrophysiology. This was noticed by Sir Jagadish Chandra Bose, a noted physicist turned plant physiologist from Calcutta, India. While carrying out research in the Davy Faraday Research Laboratory of the Royal Institution of Great Britain, Bose reported to the Royal Society in a lecture in 1901 that touch initiated an electric action potential that radiated the length of the leaf, resulting in the rapid closing of the Mimosa leaflets. (Unfortunately, Burdon-Sanderson was highly critical of Bose’s work and recommended that his Mimosa paper be rejected from the Proceedings of the Royal Society of London, though subsequent studies in many labs have since shown that Bose was indeed correct.)
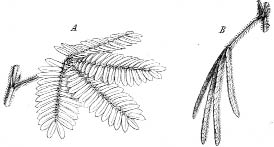
Mimosa pudica
Studies revealed that when the electric signal acts on a group of cells called the pulvinus, which are the motor cells that move the leaves, it leads to the drooping behavior of the Mimosa’s leaves. To understand how the pulvinus moves the leaves in the absence of muscles, we have to understand a little bit of basic plant cell biology. The plant cell contains two main parts. The protoplast, similar to cells in animals, resembles a water balloon: a thin membrane surrounds a liquid interior. This interior contains several microscopic parts, including the nucleus, mitochondria, proteins, and DNA. What’s unique about plant cells is that the protoplast is enclosed within the second part, a boxlike structure called the cell wall. The cell wall gives a plant its strength in the absence of a supporting skeleton. In wood, cotton, and nutshells, for example, the cell walls are thick and sturdy, while in leaves and petals the walls are thin and pliable. (We are incredibly dependent on cell walls, in fact, as they are used to create paper, furniture, clothing, ropes, and even fuel.)
Normally, the protoplast contains so much water that it presses strongly on the surrounding cell wall, which allows plant cells to be very tight and erect and to support weight. But when a plant lacks water, there’s little pressure on the cell walls, and the plant wilts. By pumping water in and out of cells, the plant can control how much pressure is applied to the cell wall. The pulvinus cells are found at the base of each Mimosa leaflet and act as mini hydraulic pumps that move the leaves. When the pulvinus cells are filled with water, they push the leaflets open; when they lose water, the pressure drops, and the leaves fold into themselves.
Where do the electric action potentials come into play? They are the critical signal that tells the cell whether to pump water in or out. Under normal conditions, when the Mimosa’s leaves are open, the pulvinus cells are full of potassium ions. The high concentration of potassium inside the cell relative to the outside causes water to enter the cell in a futile attempt to dilute the potassium, which results in great pressure on the cell wall—and in erect leaves. Potassium channels are opened when the electric signal reaches the pulvinus, and as the potassium leaves the cell, so does the water. This causes the cells to become flaccid. Once the signal has passed, the pulvinus pumps potassium into the cells again, and the resulting influx of water opens up the leaf again. Calcium, the same ion critical for neural communication in humans, regulates the opening of the potassium channels, and as we’ll see, it is essential for a plant’s response to touch.
A Negative Touch
In the early 1960s, Frank Salisbury was studying the chemicals that induce flowering in cocklebur (Xanthium strumarium), a weed found throughout North America and most notorious for its little football-shaped burrs, which are commonly found clinging to hikers’ clothing. To understand how the plant grew, Salisbury and his team of technicians at Colorado State University decided to measure the daily increase in leaf length by going out into the field and physically measuring the leaves with a ruler. To his bewilderment, Salisbury noticed that the leaves being measured never reached their normal length. Not only that, as the experiment continued, they eventually turned yellow and died. But the leaves on the same plant that were not handled and measured thrived. As Salisbury explained, “We were confronted with the remarkable discovery that one can kill a cocklebur leaf simply by touching it for a few seconds each day!”

Cocklebur (Xanthium strumarium)
As Salisbury’s interests lay elsewhere, a decade passed before his observation was put into broader context. Mark Jaffe, a plant physiologist who was based at Ohio University in the early 1970s, recognized that this touch-induced growth inhibition is a general phenomenon in plant biology. He coined the cumbersome term “thigmomorphogenesis” from the Greek roots thigmo- (touch) and morphogenesis (creation of shape) to describe the general effect of mechanical stimulation on plant growth.
Of course, plants are exposed to multiple tactile stresses such as wind, rain, and snow, and animals regularly come into contact with many of them. So in retrospect, it isn’t so surprising that a plant would retard its growth in response to touch. A plant feels what type of environment it lives in. Trees growing high on a mountain ridge are often exposed to strong winds, and they adapt to this environmental stress by limiting their branch development and growing short, thick trunks. The same species of tree grown in a protected valley, on the other hand, will be tall, thin, and full of branches. Growth retardation in response to touch is an evolutionary adaptation that increases the chances that a plant can survive multiple, and often violent, perturbations. Indeed, from an ecological point of view, a plant faces many of the same choices that we would if we were building a home. What types of resources should go into the foundation? How about the frame? If you live in an area with low wind levels, or low risk for earthquakes, then your resources may go into the outward appearance of your home. But in an area of high wind levels, or high risk for earthquakes, your resources have to go into a substantial foundation and frame.
What holds true for trees also holds true for our little mustard plant Arabidopsis thaliana that we met in the first chapter. An arabidopsis plant that’s touched a few times a day in the lab will be much squatter, and flower much later, than one that’s left to its own accord. Simply stroking its leaves three times a day completely changes its physical development. While this change in overall growth takes many days for us to witness, the initial cellular response is actually quite rapid. In fact, Janet Braam and her colleagues at Rice University demonstrated that simply touching an arabidopsis leaf results in a rapid change in the genetic makeup of the plant.
That Braam discovered this phenomenon at all was quite serendipitous. Initially, as a young research fellow at Stanford University, she was interested not in the effect of touch on plants but rather in the genetic programs activated by plant hormones. In one of her experiments designed to elucidate the effect of the hormone gibberellin on plant biology, she sprayed arabidopsis leaves with this hormone and then checked which genes were activated by the treatment. She discovered several genes that were rapidly turned on following her spray treatment, and she assumed that they were responding to the gibberellin. But it turned out that their activity increased after they were sprayed with any number of substances—even water.
Not to be defeated, Braam pressed on, trying to figure out why these genes were activated even by water. She experienced a true eureka moment when she realized that the common factor in the treatments was the physical sensation of being sprayed with the solutions. Braam hypothesized that the genes she discovered were responding to the physical treatment of the leaves. To test this, she continued her experiment, but rather than spraying the plants with water, she simply touched them. To her satisfaction, the same genes that had been induced by spraying with the hormone, or with water, were also activated by touching the plants. Braam understood that her newfound genes were clearly activated by touch, and she aptly named them the “TCH genes” since they were induced by touching the plants.
Further understanding the importance of this discovery necessitates a quick exploration of how genes work in general. The DNA found in the nucleus of each cell that makes up an arabidopsis plant contains about twenty-five thousand genes. At the simplest level, each gene encodes one protein. While the DNA is the same in each cell, different cells contain different proteins. For example, a cell in a leaf contains proteins different from those in a root cell. The leaf cell contains proteins that absorb light for photosynthesis, while the root cell contains proteins that help it absorb minerals from the soil. Various cell types contain different proteins because different genes are active—or more exactly, different genes are transcribed—in each type of cell. While some genes are transcribed in all cells (like the genes needed for making membranes, for example), most genes are transcribed in only specific subsets of cell types. So while each cell has the potential to turn on any of the twenty-five thousand genes, in practice only several thousand genes are active in a particular cell type. To further complicate matters, many genes are also controlled by the external environment. Some genes are transcribed in leaves only after the leaves see blue light. Some are transcribed in the middle of the night, some after a heat spell, some after a bacterial attack, and some after touch.
What are these touch-activated genes? The first of the TCH genes Braam identified encode proteins involved in calcium signaling in the cell. As we’ve seen earlier, calcium is one of the important salt ions that regulates both the cell’s electrical charge and communication between cells. In plant cells, calcium helps maintain cell turgor (as in the pulvinus cells in the case of the Mimosa plant) and is also part of the plant cell wall. Calcium is essential for humans and other animals to propagate electric signals from neuron to neuron, and it is also necessary for muscle contraction. Although we do not yet know all of the ways in which calcium regulates such diverse phenomena at the same time, it is a field of intense study.
Scientists do know that following a mechanical stimulation like the shaking of a branch or a root hitting a rock, the concentration of calcium ions in a plant cell peaks rapidly and then drops. This spike affects the charge across the cell membrane, but it also directly affects multiple cellular functions as a “second messenger,” a mediator molecule that relays information from specific receptors to specific outputs. This free soluble calcium is not very efficient on its own in causing some response, because most proteins can’t bind calcium directly; hence calcium, in both plants and animals, usually works in conjunction with a small number of calcium-binding proteins.
Among these, the most studied is calmodulin (calcium-modulated protein). Calmodulin is a relatively small but very important protein, and when it binds with calcium, it interacts with, and modulates the activity of, a number of proteins involved in processes in human beings—such as memory, inflammation, muscle function, and nerve growth. Getting back to plants, Braam showed that the first TCH gene encoded calmodulin. In other words, when you touch a plant, be it arabidopsis or papaya, one of the first things it does is make more calmodulin. Most likely, a plant makes more calmodulin to work with the calcium that it releases during the action potentials.
Thanks to the continuing work of Braam and other scientists, we now know that over 2 percent of arabidopsis genes (including, but not limited to, genes encoding calmodulin and other calcium-related proteins) are activated after an insect lands on its leaf, an animal brushes up against it, or the wind moves its branches. This is a surprisingly large number of genes, which indicates just how far-reaching a plant’s response is when it comes to mechanical stimulation and survival.
Plant and Human Feeling
We can feel a varied and complex mixture of physical sensations due to the presence of specialized mechanosensory receptor nerves and due to a brain that translates these signals into sensations with emotional connotations. These receptors enable us to respond to a vast array of tactile stimulations. A specific mechanosensory receptor called Merkel’s disks detects sustained touch and pressure on our skin and muscles. Nociceptors in our mouths are activated by capsaicin, the ultrahot chemical found in chili peppers, and nociceptors signal that our appendix is inflamed before an appendectomy. Pain receptors exist to enable us to withdraw from a dangerous situation or to let us know of a potentially dangerous physical problem inside our bodies.
While plants feel touch, they don’t feel pain. Their responses are also not subjective. Our perception of touch and pain is subjective, varying from person to person. A light touch can be pleasurable to one person or an annoying tickle to another. The basis for this subjectivity ranges from genetic differences affecting the threshold pressure needed to open an ion channel to psychological differences that connect tactile sensations with associations such as fear, panic, and sadness, which can exacerbate our physiological reactions.
A plant is free from these subjective constraints because it lacks a brain. But plants feel mechanical stimulation, and they can respond to different types of stimulation in unique ways. These responses do not help the plant avoid pain but modulate development to best suit the ambient environment. An amazing example of this was provided by Dianna Bowles and her team of researchers at the University of Leeds. Earlier research had shown that wounding a single tomato leaf leads to responses in the unwounded leaves on the same plant (similar to the types of research outlined in chapter two). These responses include the transcription of a class of genes called proteinase inhibitors in the intact leaves.

Tomato (Solanum lycopersicum)
Bowles was curious to know more about the nature of the signal from a wounded leaf to an unharmed leaf. The accepted paradigm was that a secreted chemical signal was transported in the veins of a wounded leaf to the rest of the plant. But Bowles hypothesized that the signal was electric. To test her hypothesis, she burned one tomato leaf with a hot steel block and found that an electric signal could be detected in the stem of the same plant at a distance from the wounded leaf. The plant could still detect the signal even if she iced the stem-like structure that connects the leaf to the stem (called a petiole). She found that icing the petiole blocked chemical flow from the leaf to the stem—but not electrical flow. Moreover, when she iced the petiole of the burned leaf, the untreated leaves still transcribed the proteinase inhibitor genes. The leaf did not feel pain. The tomato responded to the hot metal not by moving away from it but by warning its other leaves of a potentially dangerous environment.
As sessile, rooted organisms, plants may not be able to retreat or escape, but they can change their metabolism to adapt to different environments. Despite the differences between the ways plants and animals react to touch and other physical stimulations at the organismal level, at the cellular level the signals initiated are hauntingly similar. Mechanical stimulation of a plant cell, like mechanical stimulation of a nerve, initiates a cellular change in ionic conditions that results in an electric signal. And just like in animals, this signal can propagate from cell to cell, and it involves the coordinated function of ion channels including potassium, calcium, calmodulin, and other plant components.
A specialized form of mechanoreceptor is also found in our ears. So if plants can sense touch as a result of the mechanoreceptors similar to the ones in our skin, can they also hear by sensing sound through mechanoreceptors similar to the ones in our ears?