TEACHER’S NOTES
Section 1. Organization of Matter
1.4. Identifying and Naming Isotopes: “EggCeptional” Isotopes
Prior to making the egg isotopes, decide what items you will use to represent protons, neutrons, and electrons. Suggestions include craft red buttons for protons, blue buttons for neutrons, and yellow beads for electrons. You can give all students the same isotope, or you can vary it. For instance, for sodium-23, you would put in eleven red buttons, eleven yellow beans, and twelve blue buttons. If you use different isotopes for different students, they could later switch eggs and practice figuring out isotope numbers.
1.5. Chemical and Physical Changes: Examining Paper for Change
Place the following items in envelopes for each lab group. Prior to the changes, each piece of paper should be of equal size.
Burned paper
Paper that has a circle cut out of the middle
Paper folded over three times into a square
Paper that has been soaked in water and dried
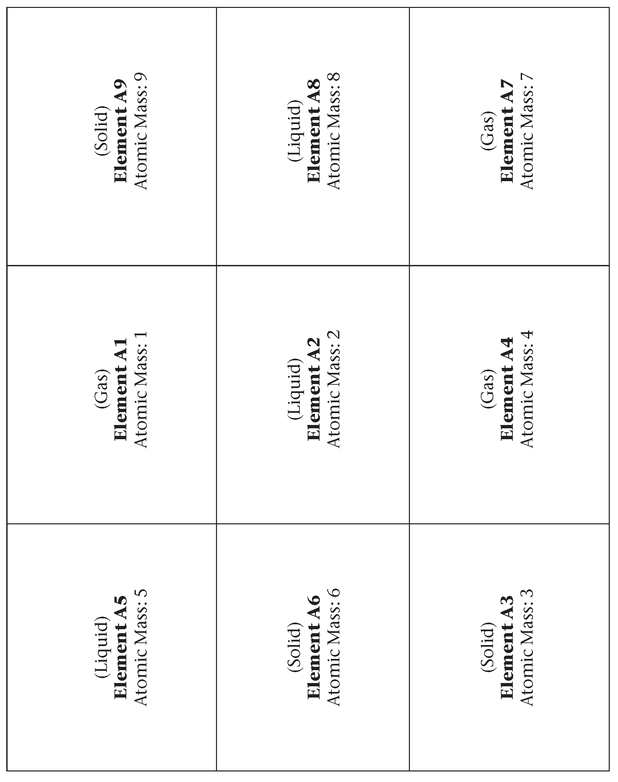
1.11. Mendeleev’s Periodic Table: It Was All in the Cards
Make a photocopy of the following page for each student. Students will cut out the cards themselves.
Section 2. Interactions of Matter
2.1. Acids and Bases: Cabbage Juice Indicators
Before the activity, prepare cabbage juice by chopping a red cabbage into large chunks and boiling for about 15 minutes. Strain the juice from the cabbage and store in the refrigerator until needed. For each lab group, prepare a well plate or 5 shallow dishes of solutions. Label 5 wells (or 5 dishes) as A1, A2, A3, A4, and A5. Half-fill as follows:
A1: vinegar
A2: ammonia solution
A3: lemon juice
A4: baking soda solution
A5: Alka-Seltzer solution
To prepare the baking soda solution, stir about 1 teaspoon of baking soda in a cup of water. For the Alka-Seltzer solution, mix one Alka-Seltzer tablet in a cup of water.
2.4. Exothermic and Endothermic Reactions: Hot Packs and Cold Packs
Prior to the activity, prepare a bag of calcium chloride for each student by placing 3 tablespoons of calcium chloride powder in a small plastic bag and sealing it. Label the bag as calcium chloride. Also prepare a bag of ammonium nitrate for each student by placing 3 tablespoons of ammonium nitrate in a small plastic bag, sealing it, and labeling each of these bags as ammonium nitrate.
2.5. Chemical Reactions: Alka-Seltzer and Water Temperature
To save on the amount of Alka-Seltzer you need for the activity, break each tablet in half and give students half-size pieces rather than whole pieces.
2.9. Single Replacement Reactions: Turning Iron into Copper
Place 2 teaspoons of copper sulfate in a resealable plastic bag for each student. Put 2 tablespoons of water into a small cup for each student.
Section 3. Energy of Motion
3.12. The Three Classes of Levers: Lots of Levers and Lots of Class
Prior to the activity, photocopy ten or more pictures of levers in action for your students. Cut these pictures apart and place them in envelopes so that each student has ten pictures representing different types of levers. Suggestions include a wheelbarrow, a screwdriver prying off the lid of a paint can, a batter hitting a baseball, a person sweeping, a hinged nutcracker, a claw hammer pulling out a nail, scissors, tweezers, a hinged door, and a teeter-totter or seesaw. Be sure to include a picture of a wheelbarrow with a mound of dirt or sand in it, because you will use this image in the activity.
Section 4. Heat, Light, and Sound Waves
4.12. Energy Conductors and Insulators: The Cook’s Choice
Prepare a large container of hot water for each student prior to class. The container should be large enough to fit a plastic, metal, and wooden cooking spoon in it. Water should be hot, but not scalding, so that students do not get burned.
Section 5. Magnetism and Electricity
5.2. Closed Circuits: A Battery, a Bulb, and a Paper Clip
You may want to give the students the bulb with a wooden clothespin around it, so they can hold onto the clothespin rather than the bulb when attempting to light it.
5.3. Electrochemical Cell: Nine-Volt Battery Electrolysis
Prior to the activity, sharpen some pencils enough to provide graphite. When preparing the water dish, dissolve some sodium sulfate to provide the electrolytes that will enable the water to conduct electricity. You can place alligator clips on the ends of the copper wire if you wish, so that students do not have to wind the bare copper wire around the ends of the pencils.
5.6. Schematic Circuit Diagrams: Seeing the Circuit
Prepare the envelopes ahead of time for each student by placing a one-meter length of string, three resistor symbols, a battery symbol, and four arrows in each envelope.
Section 6. The Cell
6.6. Cell Transport: When It Come to Cells, Small Is Good
Before class, prepare 2 potato cubes for each student or lab group. Cubes do not have to be exactly 1 cm3 and 3 cm3.
6.11. Photosynthesis and Respiration: Formula Scramble
Type or write the following words and symbols on individual pieces of paper, and place a complete set in each student’s envelope:
oxygen
carbon dioxide
glucose
water
+
+
sunlight
chlorophyll
energy
oxygen
carbon dioxide
glucose
water
+
+
+
If you do not mind the activity taking more than five minutes, you can ask students to write these words on slips of paper and cut them out so that you need not prepare the envelopes beforehand.
Section 7. Genetics
7.10. Protein Synthesis: Modeling Transcription
Prepare the envelope of red bases by writing the following letters on individual pieces of red construction paper and cutting them out. Label this envelope “DNA bases.”
Prepare the envelope of blue bases by writing the following letters on individual pieces of blue construction paper and cutting them out. Label this envelope “RNA bases.”
If you prefer, you can have students write the letters on different colored construction paper and then cut them out for themselves.
Section 8. Evolution
8.6. Adaptive Radiation: The Beaks of Darwin’s Finches
Prior to this activity, cut pieces of yarn into three-inch lengths. Place about fifty pieces of yarn in a cup. Each student will need a cup of this yarn, which will represent bird food.
Section 10. Ecology
10.3. Food Web: Piecing Together a Food Web Puzzle
Prior to this activity, find and photocopy several pictures of food webs. Cut the food webs into puzzle pieces and place the pieces of one food web into an envelope. Repeat this process for each student in the class. You may consider laminating the pieces so you can reuse them each year.
10.5. The Importance of Niches: Extinction and the Paper Clip Niche
Prior to doing the activity for the first time, prepare two envelopes (envelope A and B) for each student. Place ten regular paper clips in each A envelope. In each B envelope, place ten paper clips that have been elongated or straightened so there is no longer a curved section.
10.7. Human Pollution: Plastic Killers
Prior to starting the activity, collect the plastic rings that hold together six-packs of cans. Cut the rings apart so that you have six complete rings from each six-pack set. Each student will need one ring.
Section 12. Structure of Earth Systems
12.6. Mineral Hardness: Mineral Ranks
Prior to doing this activity, prepare a cup of five minerals for each student or pair of students. Label each mineral as A, B, C, D, or E. Minerals that could be used in this experiment include talc, gypsum, fluorite, calcite, and quartz.
Section 13. Earth’s History
13.1. Inferences from Fossils: Who Was Here?
Before class, fill a reusable or paper bag with some baby items. Suggestions include a diaper, a pacifier, a bootie, a jar of baby food, ribbons, and a baby spoon. If these items are not available, fill the bag with items that are typical of a specific type of individual. Other ideas include a teenager, a plumber, an accountant, or a teacher.
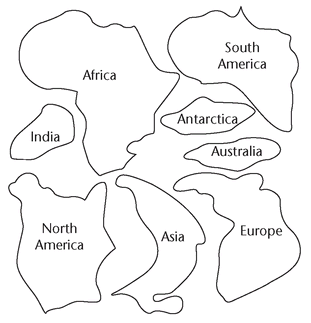
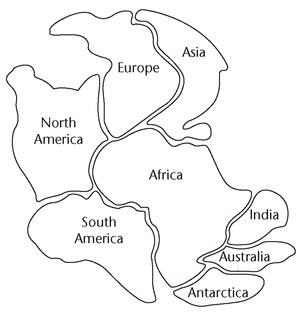
13.4. Continental Drift: Puzzling Over the Continents
Make enlarged copies of the following figure for each student. Cut out the continents and put them in an envelope. The figure on the next page shows how the pieces should look after students have assembled them.
Section 14. Meteorology
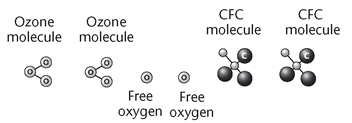
14.6. The Loss of Ozone: Oxygen Is Not Just for Breathing
Make enlarged copies of the following figure and cut out the individual molecules. Place the molecules in envelopes for the students. If class time permits, you can gives students copies of the figure to cut out.
Section 15. The Universe
15.9. Infrared Light: Feel the Heat
Place an infrared bulb in a gooseneck lamp and position it so that the bulb is 30 cm above the tabletop.
15.11. Inertia in Space: Objects Keep Moving
For each group of students, provide one piece of foam. Prepare the foam by cutting lengths of tubular foam pipe insulation into sections that are about four feet long. Cut each tubular section of foam in half lengthwise.