The Diabetic Foot
Introduction
The prevalence of diabetes mellitus worldwide has reached epidemic proportions and the myriad of complications that result from this disease cannot be overstated. In the United States alone, the diabetic population continues to grow exponentially, affecting an estimated 24 million people. Approximately 15% of patients with this disease will develop some form of foot complication and despite ongoing improvements in care, the annual cost associated with diabetic foot infections, tissue loss, and amputations exceeds $10 billion.
Up to 10% of patients with diabetes will develop a foot ulcer or tissue loss on the foot in their lifetime. In those patients who have other specific risk factors, such as peripheral arterial occlusive disease and altered foot architecture, the percentage rises to nearly 30%. The ideal management of patients with diabetes involves preemptive education and an awareness of the clinical manifestations of diabetes as well as routine surveillance of the foot surfaces. Patients at high risk for development of foot pathology are best cared for in a multidisciplinary setting that includes medicine, surgery, wound care, and rehabilitation specialists. Should a patient with diabetes mellitus experience a break in the integument of the skin, referral to a surgical specialist for evaluation is appropriate for optimal results.
Pathophysiology
There are three categories of foot ulcers that can occur in those with diabetes mellitus: neuropathic, ischemic, and those that arise from mixed neuropathic and ischemic components. Understanding these categories and recognizing the etiology of a given ulcer is necessary for efficient and effective management. Conversely, failure to understand and recognize the categories of foot ulceration may lead to unnecessary tests and procedures or delays in recognition and treatment.
The first category is a result of diabetic peripheral neuropathy affecting the surfaces of the toes and feet of patients with long-standing diabetes. Even in the setting of normal arterial perfusion, diabetic neuropathy represents a frequent cause of foot injury, infection, and subsequent tissue loss. Neuropathic ulcers are also referred to as traumatic and originate because of abnormal or absent sensation to the toes and feet. In the setting of neuropathy, activity or injury that would otherwise be uncomfortable and prompt examination, behavioral adjustment, or treatment goes unnoticed or neglected. An example is repetitive irritation or abrasion from poor fitting shoes, which may go unrecognized in patients with neuropathy until the trauma results in a break of the skin integument and initiation of an ulcer. Similarly, a puncture wound or laceration to the foot, which would normally prompt examination and medical attention, may go unnoticed in patients with diabetic neuropathy. In addition to abnormal sensation, chronic diabetic neuropathy may lead to malformation of the joints of the foot and toes, which, in turn, leads to a condition termed neuropathic arthropathy or Charcot's foot (Figure 1). In this condition, joints and surfaces of the foot not otherwise prone to injury become abnormally positioned and exposed to surface trauma and ulceration. Finally, chronic diabetic neuropathy affects autonomic innervation of the skin of the foot and toes, which leads to abnormally dry surfaces. Dry skin associated with chronic neuropathy leads to scaling, cracking, or other breaks in the integument and predisposition to ulceration, infection, or both.
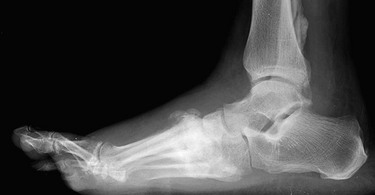
FIGURE 1 Lateral foot film demonstrating loss of foot architecture resulting in classic neuropathic arthropathy.
Chronic lower extremity ischemia from arterial occlusive disease may also affect those with diabetes who have normal sensation to the surfaces of the foot and toes. In these instances, the ulceration is referred to as ischemic tissue loss or ischemic ulceration. Ischemic ulcers of the foot or toes arise from multilevel arterial occlusive disease, meaning that more than one arterial level between the aorta and the foot is affected by significant stenosis or occlusion. Most commonly, patients with chronic diabetes have severe occlusive disease of one or more of the tibial arteries in combination with aortoiliac occlusive disease, femoral-popliteal artery occlusive disease, or both (Figure 2). Finally, depending on the duration and severity of diabetes, patients may have foot or toe ulcerations that are of a mixed neuropathic and ischemic component. These cases are typically the most severe and prone to significant tissue loss that leads to amputation.

FIGURE 2 Tibial occlusive disease showing proximal anterior tibial artery and posterior tibial artery occlusion with peroneal artery runoff. At the level of the ankle, the anterior tibial artery reconstitutes and retrograde fills via perforating branch of the peroneal artery.
From the standpoint of pathophysiology, it is important to keep in mind that a common pathway to ulceration and infection in diabetic patients is reduced local and systemic immune defense. This phenomenon increases significantly the propensity for any breaks in the skin integument, whether due to trauma or ischemia, to progress to complications. The abnormal local inflammatory response in patients with diabetes is due to alterations at the cellular level, including thickening of the endothelial basement membrane of the microvessels of the foot. Thickening of the basement membrane impairs leukocyte migration to the site of injury and the local cellular capability to defend against pathogens. Diabetes mellitus also impairs the function of circulating inflammatory cells through variations in blood glucose levels and as a result of chronic insulin administration, which interacts with circulating hormones (e.g., growth hormone) to negatively affect the immune response.
Presentation
Whether from neuropathy, ischemia, or both, skin breakdown typically occurs on the pressure-bearing surfaces of the foot, such as hard calluses or bony prominences. The chronicity of any foot ulceration is important to discern, as is the history of other healing or nonhealing ulcers on the same foot. These factors often provide insight as to whether or not there is a significant ischemic component to the situation at hand. For example, a patient who presents with a chronic nonhealing ulcer that has been stagnant or indolent over a number of weeks or months is less likely to have a profound ischemic component. Likewise, a patient with a recurrent ulcer or a new ulcer on a foot on which healing has occurred in the recent past is less likely to have severe ischemia. In these cases, the fact that the ulcer has not progressed or may have healed in the past suggests that there is adequate blood flow to support tissue stabilization and ward off gangrene. In these scenarios, the ulcer most likely is caused by recurrent trauma to an area of surface pressure or injury. In contrast, first-time foot ulcers that show significant necrosis, deep space infection, or both, are more likely to have a significant ischemic component with or without neuropathy.
The presentation of foot ulcerations range from superficial blisters or chronic wounds to painful, erythematous or necrotic ulcerations with deep space infection. As mentioned previously, the intrinsic muscles of the foot may atrophy as a result of chronic neuropathy, resulting in a condition known as neuropathic arthropathy or Charcot's foot. This condition is recognized easily on examination and creates abnormal pressure points on the foot. The degenerative arthropathy associated with Charcot's foot also leads to loss of normal foot architecture and collapse of the arch, also referred to as a “rocker bottom” deformity. Examination of the foot frequently reveals dry and cracked skin and forefoot; erythema, edema, or tenderness is suggestive of a more extensive deep space infection. Of particular importance in this patient population is the presence of unexplained hyperglycemia. Elevation in circulating blood glucose levels in patients with diabetes, and especially those with foot ulceration, should prompt an aggressive search for infection. In these instances, one should suspect undrained fluid collections or osteomyelitis or deep space forefoot infections.
The contribution of ischemia to a foot ulcer can be gauged quickly by performing an assessment of perfusion to the extremity, beginning with an assessment of peripheral pulses. This aspect of the physical exam begins with palpation of the femoral pulse, which may be compared in strength to that of the pulse in the opposite groin. Similarly, palpation for the popliteal and pedal pulses should be performed comparing their strength with those in the opposite extremity. Completing the pulse exam is most useful in determining the ischemic component to a foot ulcer at the two ends of the clinical spectrum: normal versus severe ischemia. A patient who has normal palpable pulses throughout an extremity, including the foot, on the initial examination is much more likely to have an isolated neuropathic ulcer. Conversely, a patient who has absent femoral, popliteal, and pedal pulses on the initial exam has an ischemic component and should be managed accordingly. Patients in whom the pulse exam is difficult or equivocal require further noninvasive testing, beginning with use of the continuous wave Doppler.
Continuous wave Doppler is an extension of the physical exam and used to determine the presence and quality of arterial signals in the foot. Normal signals are strong and biphasic, whereas weak monophasic signals are indicative of arterial occlusive disease and ischemia. The ankle brachial index (ABI) is the ratio of the occlusion pressure of the arterial signal at the ankle or foot compared with that in the arm. This aspect of the exam is performed using a manual blood pressure cuff and a continuous wave Doppler to assess the pressure at which the signals of the dorsalis pedis and posterior tibial arteries of the foot and the brachial artery of the arm occlude as the cuff is inflated. The normal index is 0.9 or greater while an index of 0.4 or less is diagnostic of critical limb ischemia.
It is important to note that chronic diabetes may result in medial calcinosis of the tibial arteries, also referred to as Monckeberg's arteriosclerosis. In patients with this condition, occlusion of the tibial vessels with the cuff is not possible, even at high pressures; thus, it is impossible to measure ABI. Noncompressible ABI occurs mostly in patients with long-standing, insulin-dependent diabetes, and this condition necessitates other types of noninvasive vascular testing to assess perfusion.
One test that is similar to ABI and that can be used to assess perfusion to the foot in the diabetic patient is the toe brachial index (TBI). Interestingly, the digital vessels of the toes are spared from medial calcinosis and are therefore compressible with small specialized cuffs and a form of plethysmography. While the upper values do not correlate well with ABI, a TBI of less than 0.4 is indicative of critical limb ischemia. Whether it is ABI or TBI, measured values of less than 0.4 is indicative of severe ischemia. Patients with indices of 0.4 or less are unlikely to heal a foot or toe ulceration or any débridement of the affected area.
Treatment
Treatment of the diabetic foot ulcer and any associated infection should be accomplished with a multidisciplinary approach. An important initial step that should not be overlooked is blood glucose control with an hemoglobin A1c target level of less than 6.5% to 7.0%. Local infection control is also paramount and requires débridement of nonviable tissue and opening of any deep space or forefoot infection. In the case of toe ulceration with gangrene or infection, amputation of the digit with or without proximal extension to include the metatarsal head (i.e., Ray amputation) may be necessary. If more than one toe is affected or there is suspicion for a more extensive forefoot infection, a transmetatarsal amputation may be required. In cases involving digit, Ray, or transmetatarsal amputations, we favor starting the incision distally at the infected ulcer and extending proximally with the goal of exposing all of the relevant plantar spaces. The surrounding tissues should be carefully palpated for unrecognized and undrained abscess, especially along tendon sheaths, which can disguise and facilitate spread of infection (Figure 3).

FIGURE 3 Left medial forefoot deep space infection necessitating wide débridement with first and second toe amputation.
Chronic or indolent ulcers without soft tissue or deep space infection can be treated without an extensive operation. These ulcers may have elements of surrounding cellulitis and often have necrotic tissue with polymicrobial colonization. In these cases, initial antibiotic therapy should be broad-spectrum, based on institutional culture and sensitivities and include coverage for methicillin-resistant Staphylococcus aureus. The antibiotic regimen should be modified and focused based on the results of the cultures obtained during the initial evaluation and débridement. Antibiotic therapy for chronic ulcers is continued until the cellulitis or superficial infection is resolved (7-10 days) and is not necessary until the wound is healed. Long-term intravenous antibiotic therapy is no longer recommended for diabetic foot ulcers with osteomyelitis given the high recurrence rate in this patient population.
Offloading of or minimizing trauma to the affected area of the foot is also paramount. This strategy may include bed rest, foot elevation, and restricted weight bearing to decrease edema and prevent further mechanical damage of tenuous tissue. Specialized orthotics are available to assist the provider and patient in this strategy, including custom-fit shoes for neuropathic or malformed feet. Although offloading and surface protection should be started during the initial evaluation of any foot ulcer, this strategy should be approached in combination with comprehensive foot care measures as a life-long requirement for patients with diabetes mellitus.
Correction of arterial ischemia will be required for ulcers that fail to heal despite conservative and local wound care and for those with critical ischemia (i.e., ABI or TBI <0.4). Diabetic patients with nonhealing ischemic foot ulcers should be evaluated first for the merit of limb salvage, as some already immobile or debilitated patients may be better served with a primary amputation. This overall assessment for limb salvage should be made in patients with acutely septic ulcers as well as those with more chronic or indolent, nonhealing ulcers.
If a patient is deemed a candidate for limb salvage, a vascular operation should be undertaken after control of associated foot infection and performance of vascular imaging. Revascularization for ischemic ulceration or tissue loss should be undertaken with the goal of restoring in-line or pulsatile flow to the foot. In other words, direct arterial flow is required, through recanalization of an existing artery or with a bypass conduit, to a tibial artery below the extent of occlusion. The arterial target in these cases is free of distal stenosis or occlusion and therefore provides direct flow to the affected foot. In most cases, distal bypass or recanalization for ischemic tissue loss is performed to the anterior or posterior tibial artery, either of which provides direct flow to the plantar arch of the foot. In contrast, the peroneal artery ends at the ankle and provides perfusion to the foot only through collateral branches. While collateral perfusion to the leg and foot may be adequate to relieve claudication or even ischemic rest pain, in-line pulsatile flow is typically required to meet the metabolic demands needed to heal an ischemic foot ulcer.
Lower extremity revascularization options can be considered in two categories: open surgical and endovascular. Regardless of the chosen approach, revascularization must begin with assuring or establishing adequate inflow to the extremity at the level of the common and deep femoral arteries. Options to establish flow from the femoral artery level to the foot will depend on the extent of occlusive disease. As previously discussed, nearly all diabetic patients with ischemic foot ulcers have multilevel arterial occlusive disease that includes the tibial vessels. Because endovascular procedures are yet to be established as effective or durable in the tibial vessels, bypass to a reconstituted tibial artery is most common in this scenario. In most instances, this is performed using autologous saphenous vein as the conduit and either the common femoral or popliteal artery as the inflow vessel. Prosthetic grafts (i.e., expanded polytetrafluoroethylene) can be used for conduit in distal bypass procedures, but they have not been shown to be as durable as saphenous vein. Although distal bypass with saphenous vein as a conduit is superior to endovascular therapy in terms of primary and secondary patency, there is no difference in limb savage rates between the two modalities. In some cases, endovascular therapy and bypass surgery are complementary techniques (i.e., hybrid procedures) for the treatment of ischemic, nonhealing diabetic foot ulcers.
Numerous adjunctive methods are available to aid healing of diabetic ulcers. These methods include hyperbaric oxygen therapy, which has been shown to facilitate wound granulation, contraction, and healing but has not been shown to be cost-effective given the lack of evidence for optimal duration of therapy. One adjunct that has been shown to result in faster rates of wound healing in diabetic patients with adequate perfusion is the negative pressure wound therapy device or VAC. Appropriately applied, closed negative pressure wound therapy results in fewer dressing changes and outpatient wound care visits and improved quality of life. The vacuum-assisted wound closure (VAC) dressing adjunct has also been shown to improve local wound oxygenation, granulation, and contraction.
One of the most difficult decisions in managing a chronic, nonhealing, or septic diabetic foot ulcer is whether to use leg amputation as the primary procedure. Certain patients will not be candidates for staged surgical revascularization and attempted limb salvage because of their preexisting immobility or severe comorbidities. In general, bedridden and wheelchair-bound patients with little potential for ambulation should have an amputation as the initial procedure to reduce complications and speed any chance for recovery. Patients with significant medical comorbidities such as advanced chronic kidney disease may also benefit from primary amputation instead of revascularization and attempted limb salvage. Finally, it should be noted that diabetic foot ulcers heal at a slow rate, even after revascularization. Ulcers over the calcaneus are particularly slow to contract and close because of a relatively lower arterial perfusion and their exposure to pressure or trauma. On average, only one quarter of foot ulcers treated with revascularization are healed within 6 months of surgery. Two thirds of such ulcers are healed within a year of operation, and freedom from amputation ranges from 80% at 1 year to 70% at 3 years.
Conclusion
The number of people with diabetes mellitus has reached epidemic proportions, and therefore a firm understanding of the etiology, presentation, and management of foot ulcers is increasingly important. If present, gangrene or forefoot infection must be débrided or drained in the acute setting along with pursuit of tight glucose control. Chronic or indolent diabetic foot ulcers may be managed more conservatively initially, although failure to heal should prompt a detailed examination of arterial perfusion. The presence of critical limb ischemia will press a decision on the merits of limb salvage, including the potential for revascularization or preference for primary leg amputation.
Disclaimer
The views expressed in this chapter are those of the authors and do not reflect official policy of the Department of Defense.
Hobizal, KB, Wukich, DK. Diabetic foot infections: current concept review. Diabet Foot Ankle. 2012; 3.
Lipsky, BA, Berendt, AR, Cornia, PB, et al. 2012 Infectious Diseases Society of America clinical practice guidelines for the diagnosis and treatment of diabetic foot infections. J Am Podiatr Med Assoc. 2013; 103(1):2–7.
Malhotra, S, Bello, E, Kominsky, S. Diabetic foot ulcerations: biomechanics, Charcot foot, and total contact cast. Semin Vasc Surg. 2012; 25(2):66–69.
Pinzur, MS. Diabetic peripheral neuropathy. Foot Ankle Clin. 2011; 16(2):345–349.
Zgonis, T, Stapleton, JJ, Roukis, TS. A stepwise approach to the surgical management of severe diabetic foot infections. Foot Ankle Spec. 2008; 1(1):46–53.