3D Printing and Nanomanufacturing
Abstract
The rapid development of 3D printing and nanomanufacturing techniques has attracted tremendous attention within the field of bioengineering, in particular biomaterials and tissue engineering. Typical 3D printing and nanomanufacturing techniques use laser or UV light to fabricate 3D objects in a point-by-point or layer-by-layer fashion corresponding to 3D computer-aided design models. In this chapter, we give an overview of the existing 3D printing and nanomanufacturing techniques, particularly laser-assisted direct writing techniques and stereolithography systems. We describe the basic setups and processing principles of each system. We also introduce a variety of biomaterials, including their properties, applications, and the processing by the previously mentioned manufacturing systems. Furthermore, we emphasize the application of these 3D printing and nanomanufacturing techniques in the field of biomedical engineering with detailed examples of the state-of-the-art research that has been done worldwide. The cell lines, design guidelines, and parameters are reviewed as well.
Keywords
2.1. Introduction
2.2. 3D Printing and Nanomanufacturing Techniques
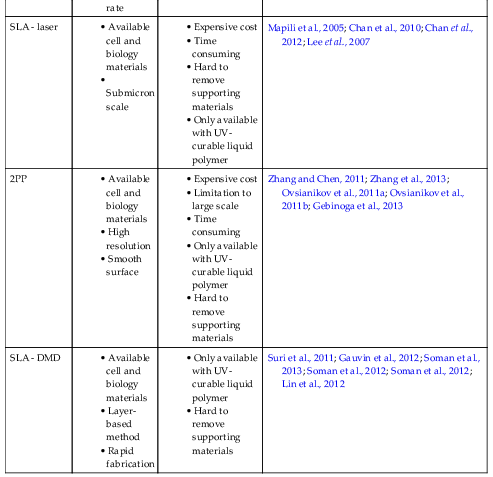
2.2.1. Selective Laser Sintering
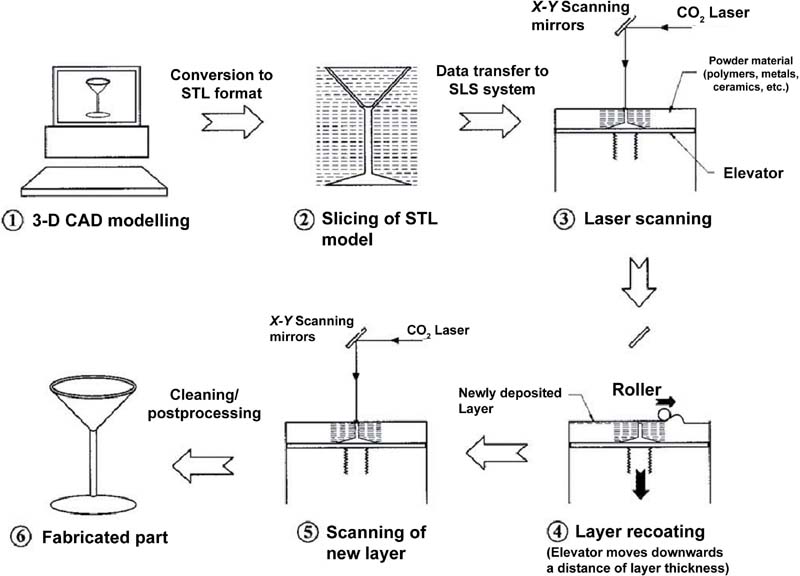
2.2.2. Laser-Guided Direct Writing
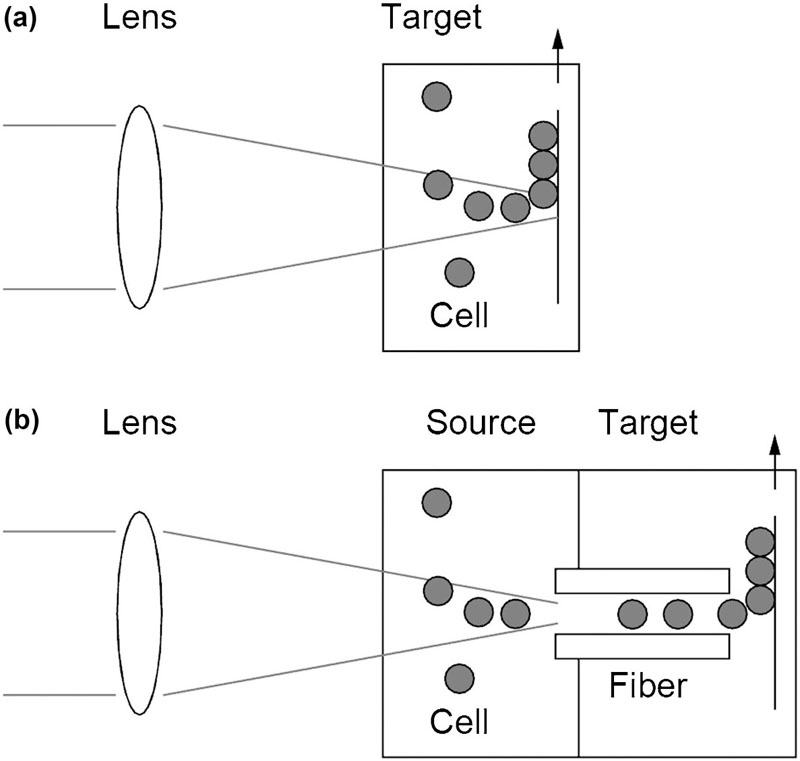
2.2.3. Laser-Induced Forward Transfer
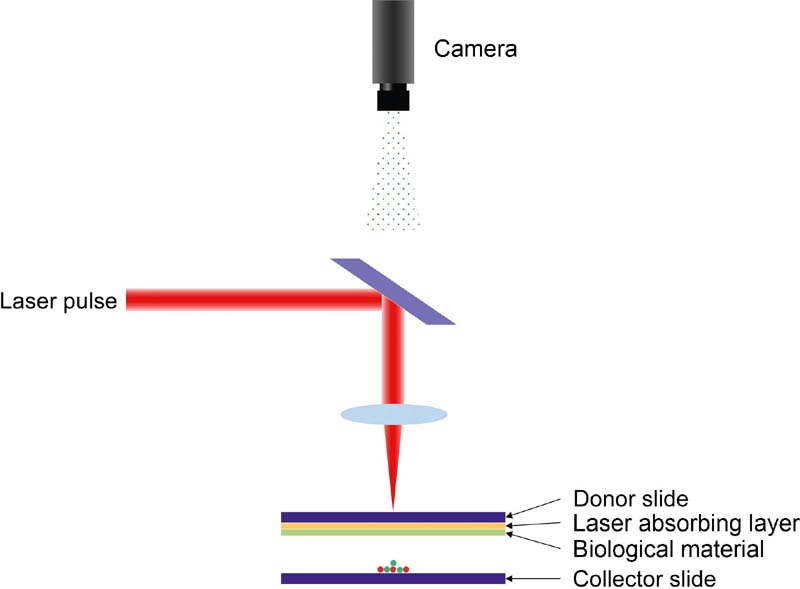
2.2.4. Matrix-Assisted Pulsed Laser Evaporation Direct Writing
2.2.5. Biological Laser Printing
2.2.6. Stereolithography Techniques
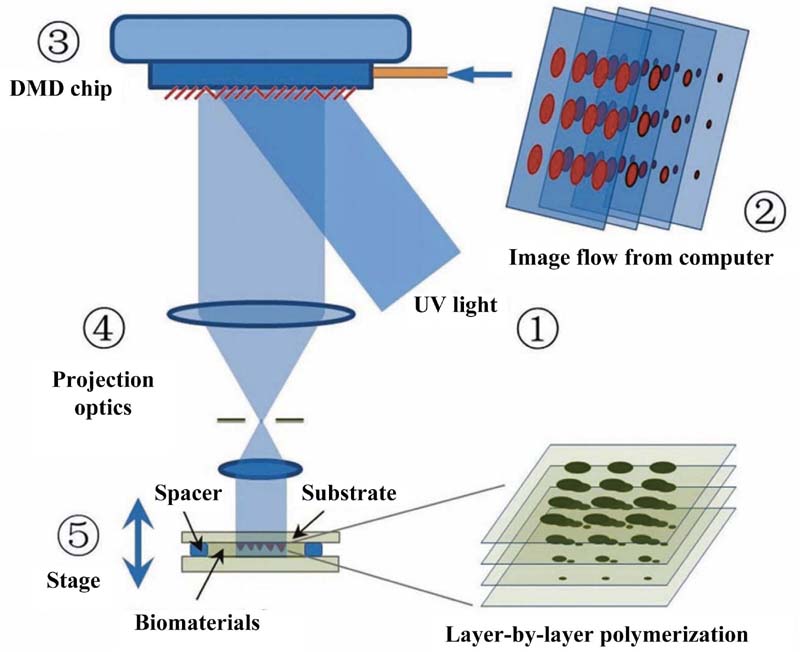
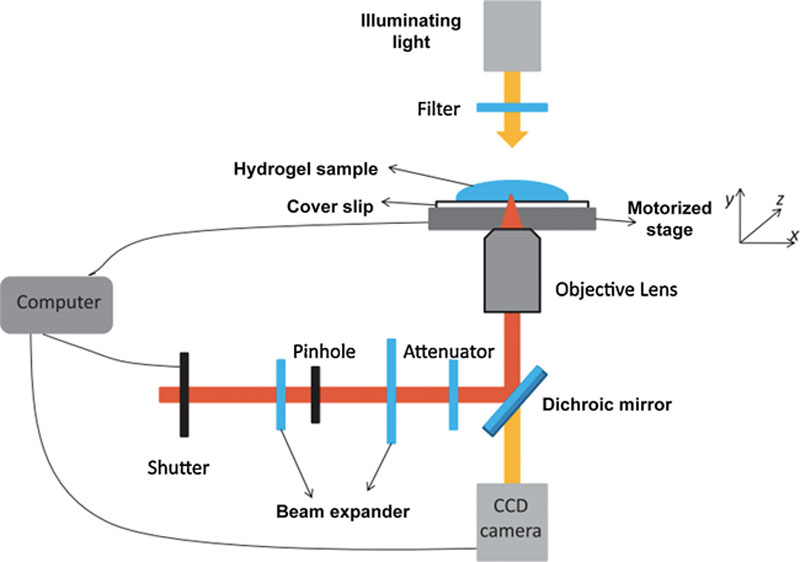
2.2.7. Classification of Additive Biomanufacturing Techniques
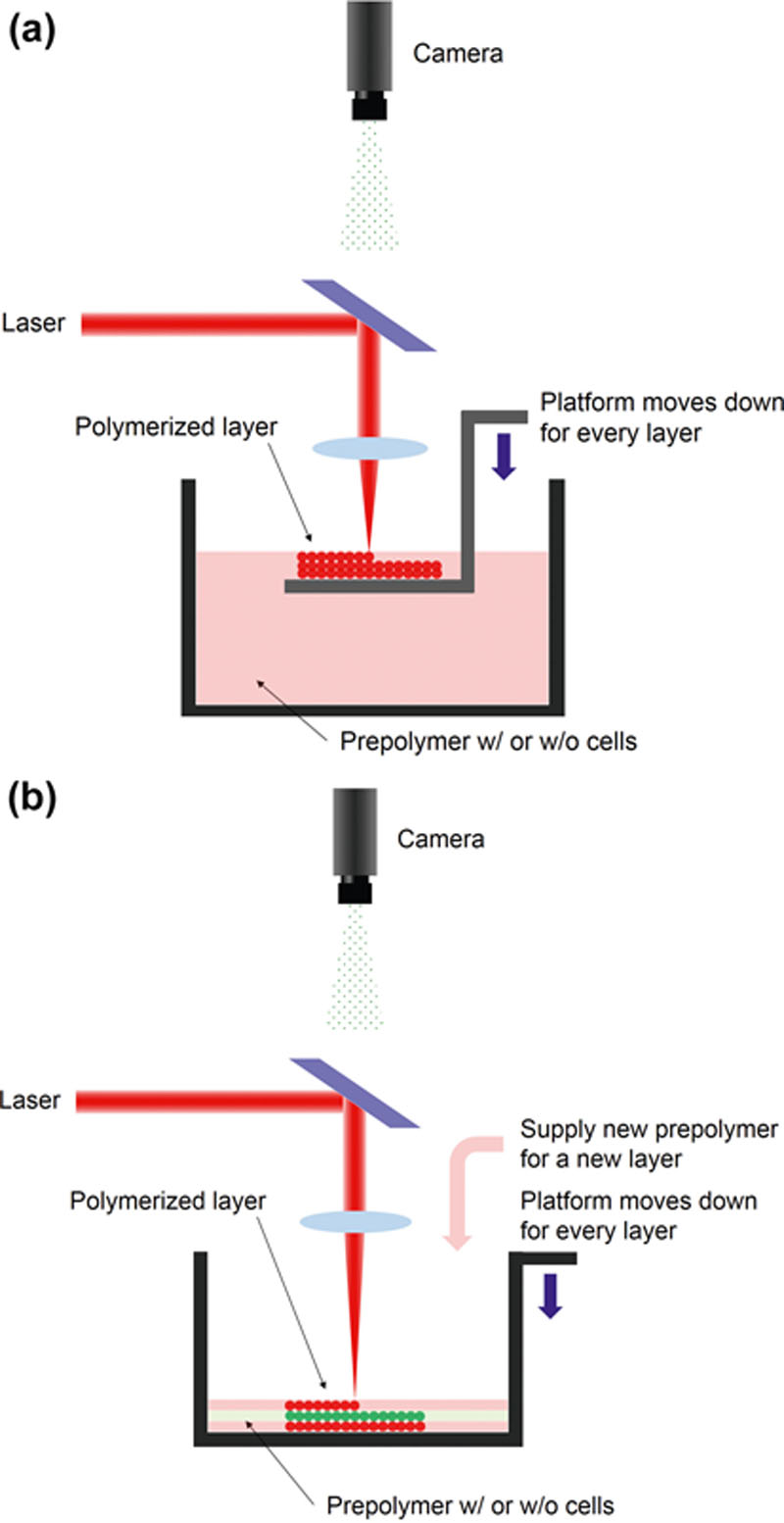
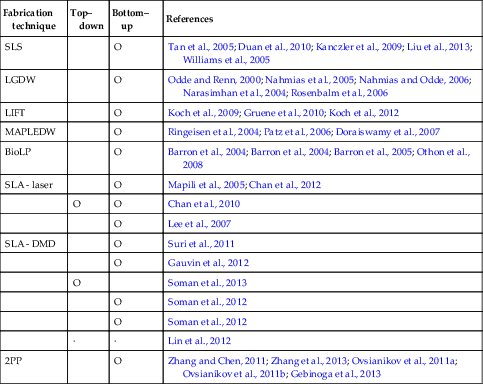
Table 2.1
Types of additive manufacturing techniques using laser
2.3. Biomaterials Used with Additive Biomanufacturing Techniques
Table 2.2
Additive manufacturing technique using laser
| Fabrication technique | Unit element | Polymer type | Laser type | Dimension | References |
| SLS | PEEK, PVA, PCL, PLLA, HA | Powder | CO2 laser | 3D | Tan et al., 2005 |
| Ca-P/PHBV CHA/PLLA | Powder (nanocomposite) | CO2 laser | 3D | Duan et al., 2010 | |
| PLA + Carbon Black | Powder | Fiber diode laser | 3D | Kanczler et al., 2009 | |
| Titanium | Powder | Nd:YAG laser | 3D | Liu et al., 2013 | |
| PCL | Powder | CO2 laser | 3D | Williams et al., 2005 | |
| LGDW | Laser guided cell | Unit cell | Diode laser | 3D | Odde and Renn, 2000; Nahmias et al., 2005; Nahmias and Odde, 2006 |
| Laser guided cell | Unit cell | Ti: Sapphire laser | 2D | Narasimhan et al., 2004; Rosenbalm et al., 2006 | |
| LIFT | Alginate + NaCl | Gel-type layer | Nd:YAG laser | 3D | Koch et al., 2009; Gruene et al., 2010; Koch et al., 2012 |
| MAPLEDW | Matrigel® | Gel-type layer | ArF excimer laser | 3D | Ringeisen et al., 2004; Patz et al., 2006 |
| Hydroxyapatite and zirconia / glycerol-water solution | Solution layer | ArF excimer laser | 3D | Doraiswamy et al., 2007 | |
| BioLP | Protein/ cell solution with glycerol | Solution layer | Nd:YAG laser | 3D | Barron et al., 2004; Barron et al., 2004 |
| Cell solution with glycerol | Solution layer | Multigas excimer laser | 2D | Barron et al., 2005 | |
| Cell solution with methyl-cellulose | Solution layer | Nd:YAG laser | 3D | Othon et al., 2008 | |
| SLA - laser | PEGDMA | Liquid | Nd:YAG laser | 3D | Mapili et al., 2005 |
| PEGDA | Liquid | HeCd laser | 3D | Chan et al., 2010; Chan et al., 2012 | |
| PPF/ DEF | Liquid | Nd:YVO4 laser | 3D | Lee et al., 2007 | |
| SLA - DMD | GMHA | Liquid | UV light DMD | 3D | Suri et al., 2011 |
| GelMA | Liquid | UV light DMD | 3D | Gauvin et al., 2012; Soman et al., 2013 | |
| PEG | Liquid | UV light DMD | 3D | Soman et al., 2012 | |
| PEGDA | Liquid | UV light DMD | 3D | Soman et al., 2012 | |
| PEGDA | Liquid | SLA machine | 3D | Lin et al., 2012 | |
| 2PP | PEGDA | Liquid | Ti: Sapphire laser | 3D | Zhang and Chen, 2011; Zhang et al., 2013 |
| GelMOD | Liquid | Ultrafast infrared laser | 3D | Ovsianikov et al., 2011a; Ovsianikov et al., 2011b | |
Collagen Fibrinogen | Liquid | Femtosecond pulsed laser | 3D | Gebinoga et al., 2013 |

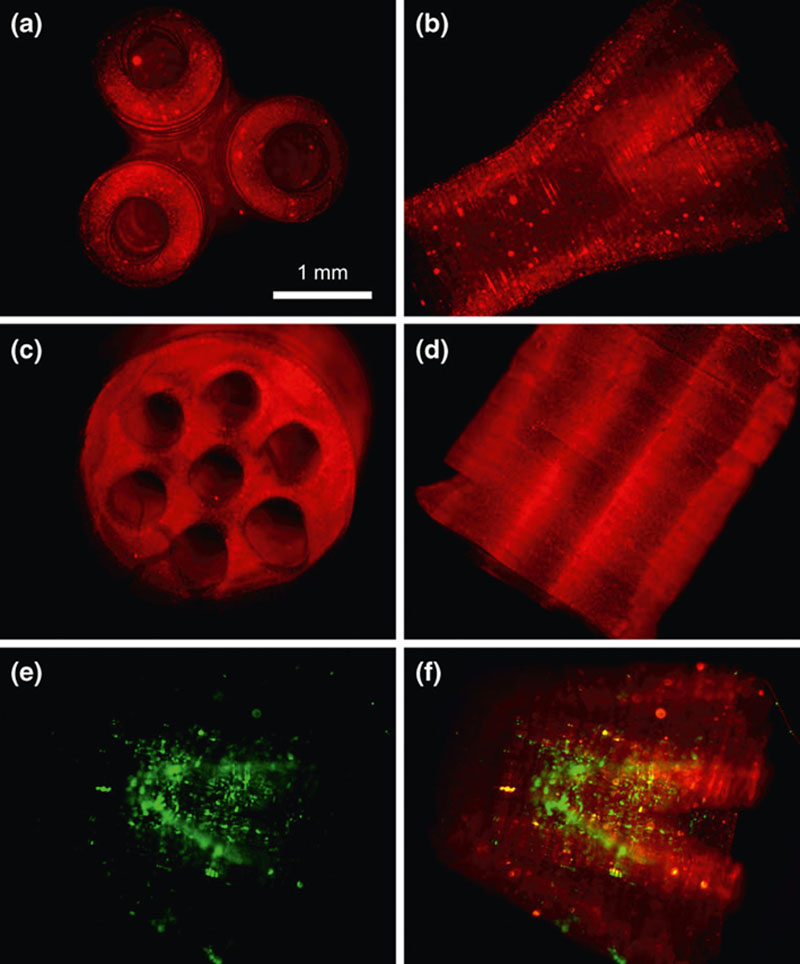
2.3.1. Powder-Type Materials
2.3.2. Gel-Based Biomaterials
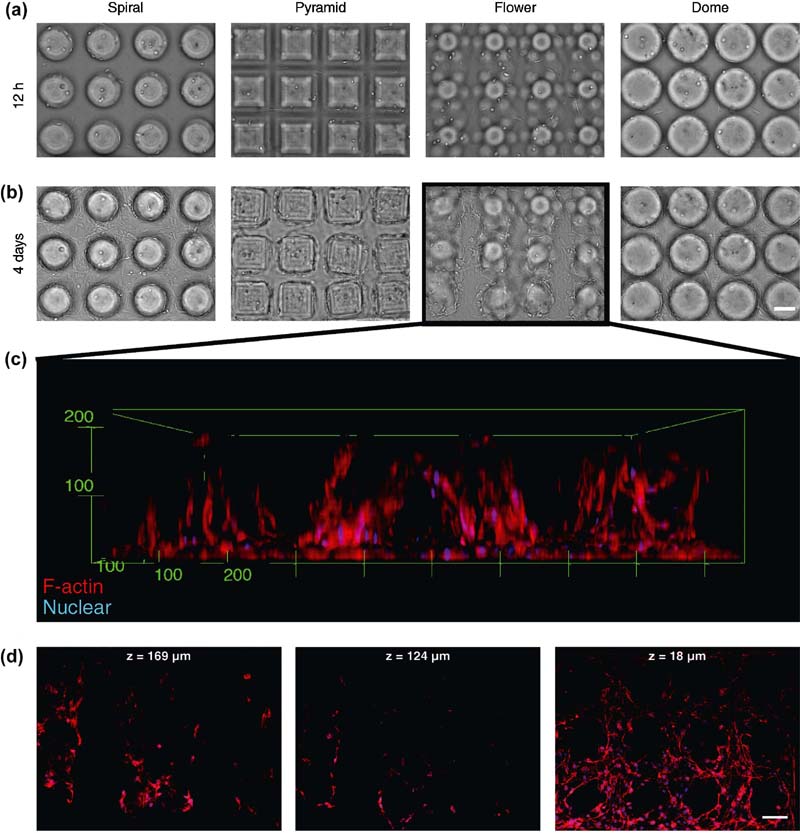
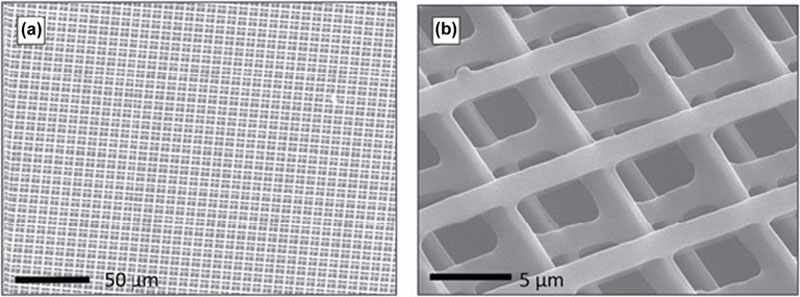
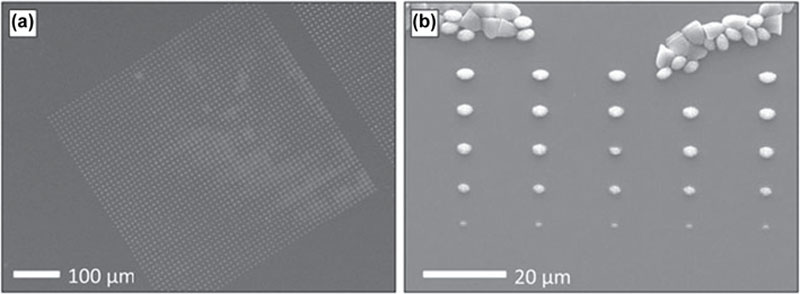
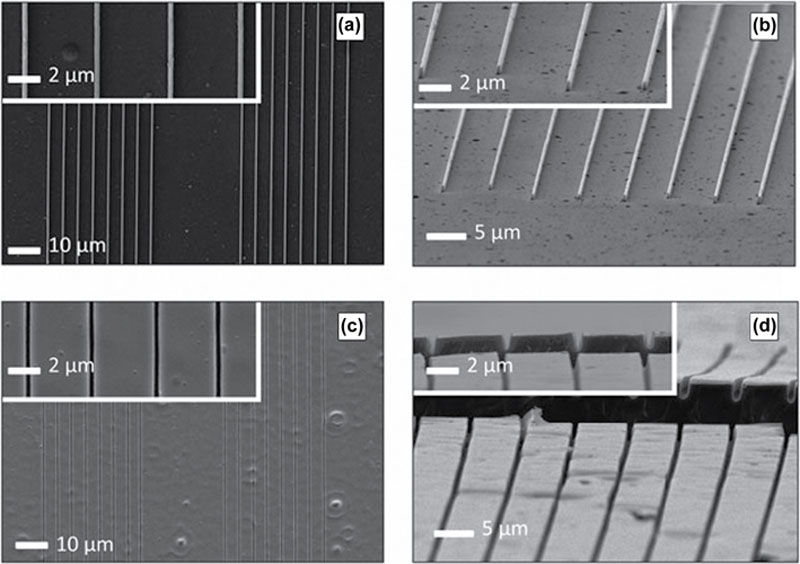
2.3.3. Photocurable Polyesters
2.4. Cells and Bioapplications
Table 2.3
Cells and bioapplications
| Fabrication technique | Cells | Bioprinting | Application | References |
| SLS | · | No | 3D scaffold | Tan et al., 2005 |
| Human osteoblast-like cell | No | 3D scaffold | Duan et al., 2010 | |
| Fetal femur-derived cell | No | Bone replacement scaffold | Kanczler et al., 2009 | |
| Human osteogenic sarcoma | No | 3D scaffold | Liu et al., 2013 | |
| Human gingival fibroblast (HGF) | No | 3D scaffold | Williams et al., 2005 | |
| LGDW | Embryonic chick Spinal cord cell | Yes | Cell patterned array | Odde and Renn, 2000 |
Human Umbilical-Vein endothelial cell (HUVEC) Hepatocytes Multipotent adult progenitor cell (MAPC) | Yes | Tissue architecture | Nahmias et al., 2005 | |
Endothelial cell Hepatocyte | Yes | Cell patterning and self-assembly | Nahmias and Odde, 2006 | |
Fibroblast Cardiomyocyte | Yes | Cell patterned array | Narasimhan et al., 2004 | |
| Enbryonic day 7 (E7) chick forebrain neuron | Yes | Cell patterned array | Rosenbalm et al., 2006 | |
| LIFT | Fibroblast Keratinocyte Human mesenchymal stem cell (hMSC) | Yes | Skin substitute using cell patterning | Koch et al., 2009 |
| Porcine mesenchymal stem cell (MSC) | Yes | Autologous graft | Gruene et al., 2010 | |
Murine fibroblast Human skin keratinocyte | Yes | Skin substitute using cell patterning | Koch et al., 2012 | |
| MAPLEDW | Pluripotent embryonal carcinoma cell | Yes | 3D cell-seeded scaffold | Ringeisen et al., 2004 |
| B35 neuronal cell | Yes | 3D cell-seeded scaffold | Patz et al., 2006 | |
| Human osteosarcoma | Yes | 3D cell-seeded scaffold | Doraiswamy et al., 2007 | |
| BioLP | Human osteosarcoma cell | Yes | 3D cell-seeded scaffold | Barron et al., 2004; Barron et al., 2004; Barron et al., 2005 |
| Olfactory ensheathing cell | Yes | 3D cell-seeded scaffold | Othon et al., 2008 | |
| SLA - laser | Murine bone-marrow stromal cell | No | 3D scaffold | Mapili et al., 2005 |
| Cardiomyocyte | No | Cell-based biohybrid actuator | Chan et al., 2012 | |
| Murine embryonic fibroblast cell | Yes | 3D cell-seeded scaffold | Chan et al., 2010 | |
| · | No | 3D scaffold | Lee et al., 2007 | |
| 2PP | · | No | 3D scaffold | Zhang and Chen, 2011 |
| Clonal mouse embryo cell | No | 3D scaffold | Zhang et al., 2013 | |
| Human adipose-derived stem cell | No | 3D scaffold | Ovsianikov et al., 2011a | |
| Porcine mesenchymal stem cell | No | 3D scaffold | Ovsianikov et al., 2011b | |
| Fibroblast | Yes | 3D scaffold (cell glued) | Gebinoga et al., 2013 | |
| SLA - DMD | Schwann cell | No | 3D scaffold | Suri et al., 2011 |
| Human umbilical vein endothelial cell | No | 3D scaffold | Gauvin et al., 2012 | |
Murine embryonic fibroblast Murine mesenchymal progenitor cell | Yes | Cell-laden high-throughput platform | Soman et al., 2013 | |
| Human mesenchymal stem cell | No | Biomedical application | Soman et al., 2012; Soman et al., 2012 | |
| Human adipose-derived stem cell | Yes | 3D cell-seeded scaffold | Lin et al., 2012 |

2.4.1. Scaffold-Based Approach to Tissue Constructs
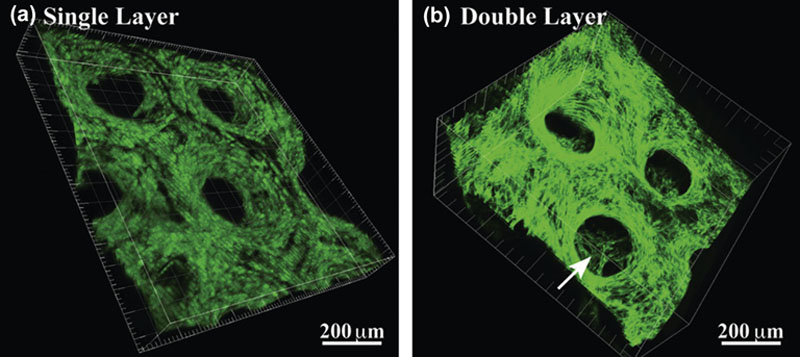
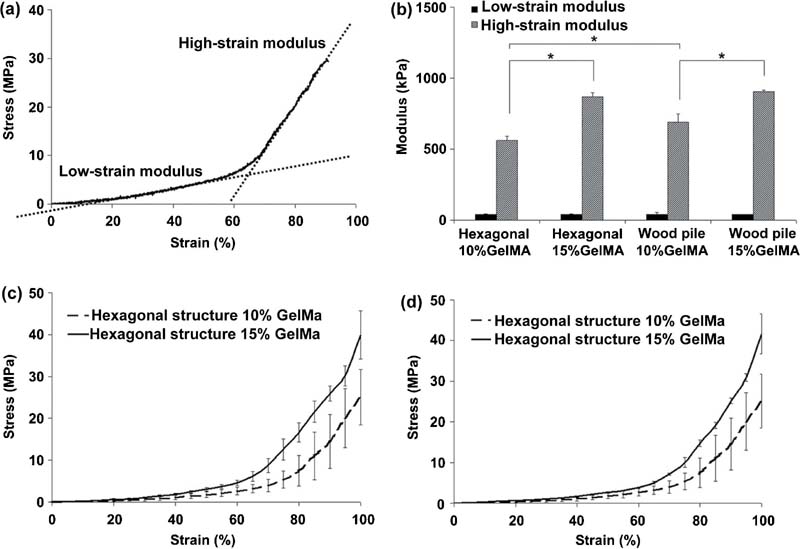
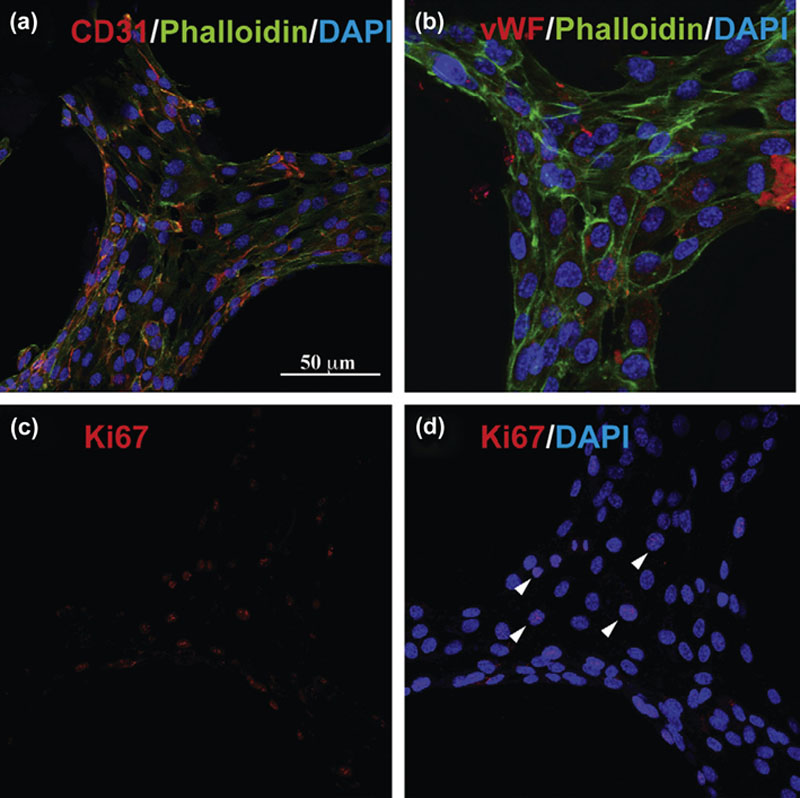
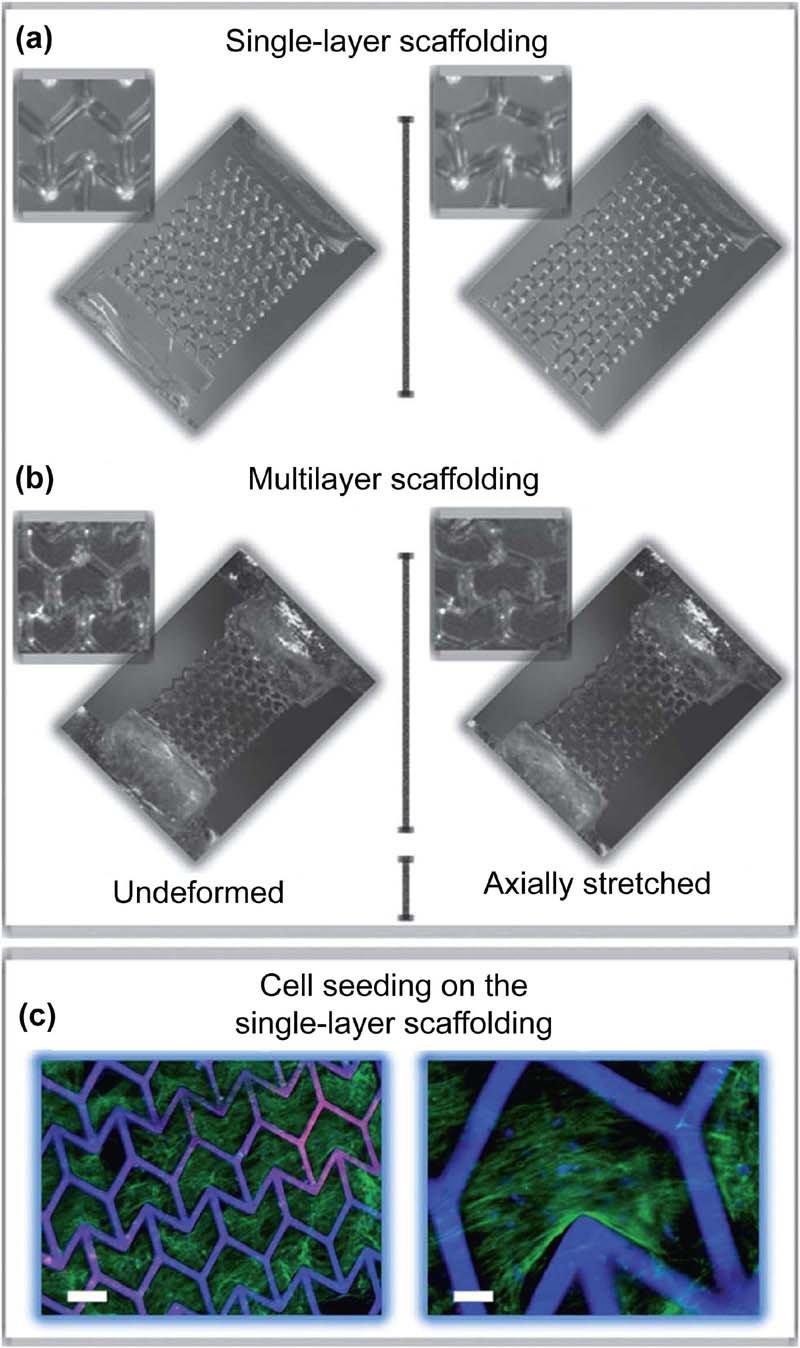
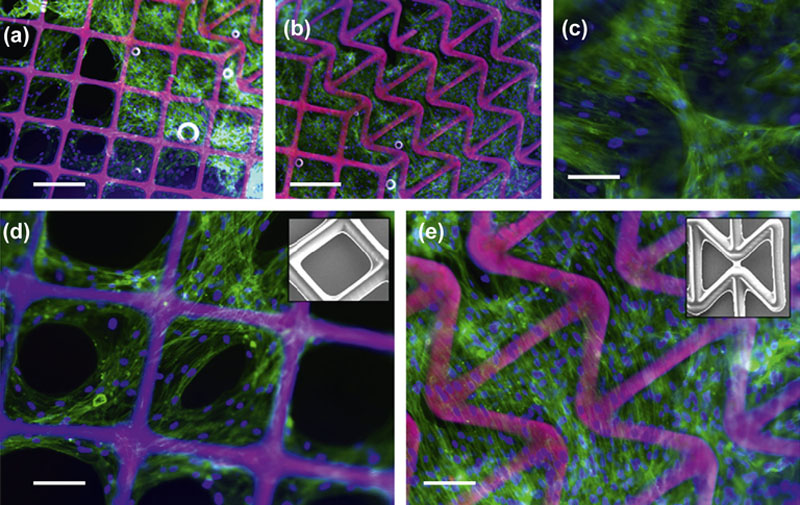
2.4.2. Bioprinting Approach to Tissue Constructs
2.5. Discussion: Pros and Cons of Each Technique
Table 2.4
Pros and cons of each technique
| Fabrication technique | Advantages | Disadvantages | References |
| SLS | • High-strength object • Self-support process • Variety of materials | • Limitation to powder form • Rough surface • Hard to remove supporting materials • Impossible to print cells or biological materials | Tan et al., 2005; Duan et al., 2010; Kanczler et al., 2009; Liu et al., 2013 |
| LGDW | • Available cell and biology materials • Single cell resolution • Precise cell printing | • Hard to build full 3D structure • Time consuming • Low cell viability (up to 95%) | Odde and Renn, 2000; Nahmias et al., 2005; Nahmias and Odde, 2006; Narasimhan et al., 2004; Rosenbalm et al., 2006 |
| LIFT | • 3D structure • Available cell and biology materials • High cell viability (∼95%) | • Weak structural support | Koch et al., 2009; Gruene et al., 2010; Koch et al., 2012 |
| MAPLEDW | • Available cell and biology materials • High cell viability (near 100%) • Simple donor slide | • Weak structural support • Low reproducibility | Ringeisen et al., 2004; Patz et al., 2006; Doraiswamy et al., 2007 |
| BioLP | • Available cell and biology materials • High efficiency (99% of incident) • High cell viability (∼ 95%) • Rapid printing rate | • Weak structural support • Metal or metal oxide layer inserted | Barron et al., 2004; Barron et al., 2004; Barron et al., 2005; Othon et al., 2008 |
| SLA - laser | • Available cell and biology materials • Submicron scale | • Expensive cost • Time consuming • Hard to remove supporting materials • Only available with UV-curable liquid polymer | Mapili et al., 2005; Chan et al., 2010; Chan et al., 2012; Lee et al., 2007 |
| 2PP | • Available cell and biology materials • High resolution • Smooth surface | • Expensive cost • Limitation to large scale • Time consuming • Only available with UV-curable liquid polymer • Hard to remove supporting materials | Zhang and Chen, 2011; Zhang et al., 2013; Ovsianikov et al., 2011a; Ovsianikov et al., 2011b; Gebinoga et al., 2013 |
| SLA - DMD | • Available cell and biology materials • Layer-based method • Rapid fabrication | • Only available with UV-curable liquid polymer • Hard to remove supporting materials | Suri et al., 2011; Gauvin et al., 2012; Soman et al., 2013; Soman et al., 2012; Soman et al., 2012; Lin et al., 2012 |