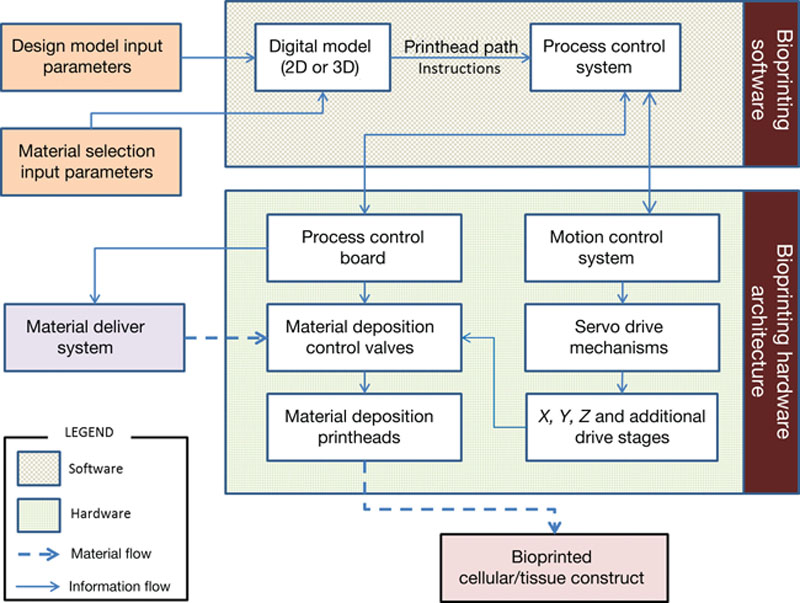
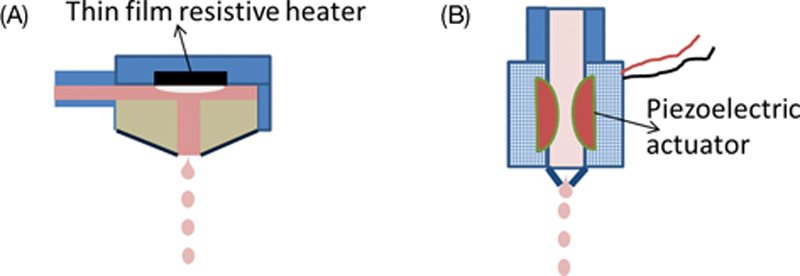
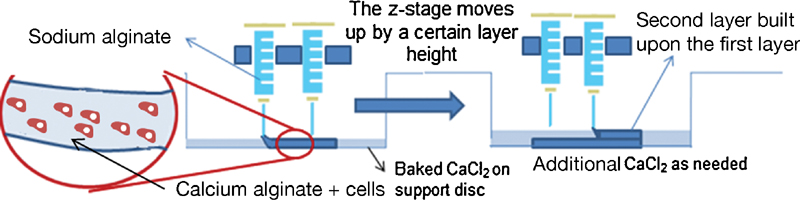
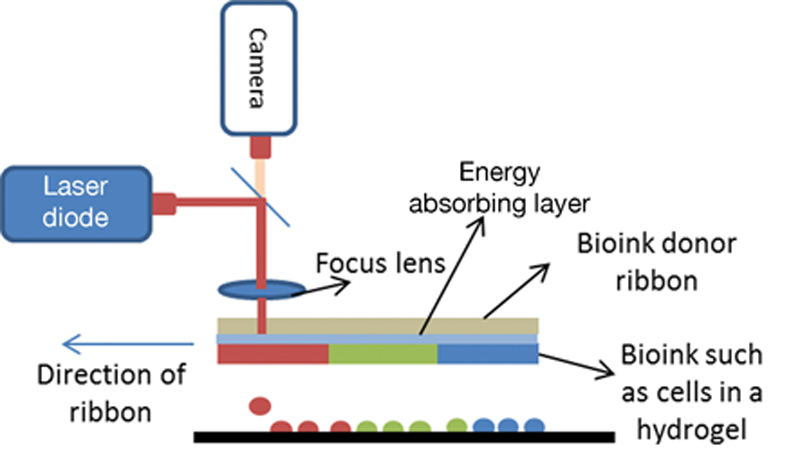
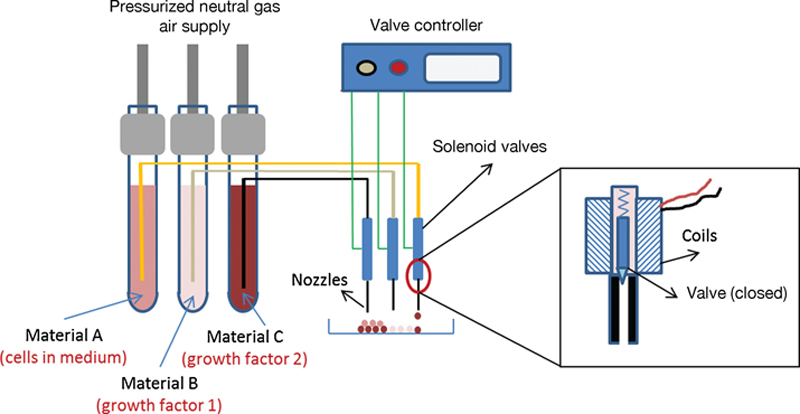
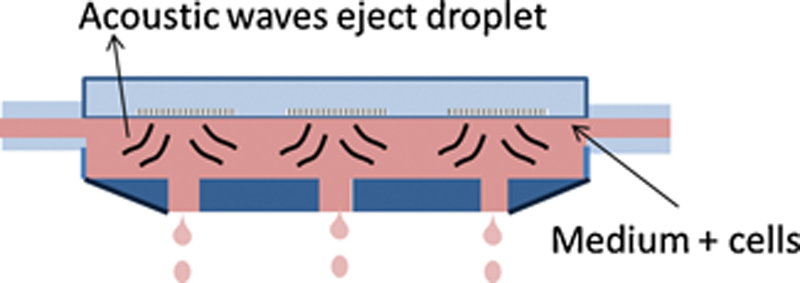
References
Barron JA, Ringeisen BR, Kim H, Spargo BJ, Chrisey DB. Application of laser printing to mammalian cells. Thin Solid Films. 2004;1:383–387.
Barron J, Wu P, Adouceur H, Ringeisen B. Biological laser printing: a novel technique for creating heterogeneous 3-dimensional cell patterns. Ann Biomed Eng. 2005;6(2):121–130.
Binder KW, Allen AJ, Yoo JJ, Atala A. Drop-on-demand inkjet bioprinting: a primer. Gene Therapy and Regulation. 2011;6(1):33–49.
Boland T, Mironov V, Gutowska A, Roth EA, Markwald RR. Cell and organ printing 2: fusion of cell aggregates in three-dimensional gels. Anatomical record Part A, Discoveries in Molecular, Cellular, and Evolutionary Biology. 2003;272:497–502.
Bu R, Wu P CJ, Brooks M, Bubb D, Wu H, Piqué A, B S, R M, Chrisey DB. The deposition, structure, pattern deposition, and activity of biomaterial thin-films by matrix-assisted pulsed-laser evaporation (MAPLE) and MAPLE direct write. Thin Solid Films. 2001:607–614.
Chahal D, Ahmadi A, Cheung KC. Improving piezoelectric cell printing accuracy and reliability through neutral buoyancy of suspensions. Biotechnology and Bioengineering. 2012;109:2932–2940.
Cheng J, Lin F, Liu H, Yan Y, Wang X, Zhang R, Xiong Z. Rheological properties of cell-hydrogel composites extruding through small-diameter tips”. Journal of Manufacturing Science and Engineering. 2008;130:021014.
Cui X, Boland T. Human microvasculature fabrication using thermal ink-jet printing technology. Biomaterials. 2009;30:6221–6227.
Cui X, Dean D, Ruggeri ZM, Boland T. Cell damage evaluation of thermal ink-jet printed Chinese hamster ovary cells. Biotechnology and Bioengineering. 2010;106:963–969.
Cui X, Boland T, D’Lima DD, Lotz MK. Thermal inkjet printing in tissue engineering and regenerative medicine. Recent Patents on Drug Delivery & Formulation. 2012;6(2):149–155.
Delaney Jr JT, Smith PJ, Schubert US. Inkjet printing of proteins. Soft Matter. 2009;5:4866–4877.
Derby B. Bioprinting: ink-jet printing proteins and hybrid cell-containing materials and structures. Journal of Materials Chemistry. 2008;18:5717–5721.
Derby B. Printing and prototyping of tissues and scaffolds. Science. 2012;338:921.
Duocastella M, Colina M, Fernandez-Pradas JM, Serra P, J.L M. Study of the laser-induced forward transfer of liquids for laser bioprinting. Applied Surface Science. 2007;253:7855–7859.
Fedorovich NE, Wijnberg HM, Dhert WJ, Alblas J. Distinct tissue formation by heterogeneous printing of osteo- and endothelial progenitor cells. Tissue Engineering Part A. 2011;17:2113–2121.
Fedorovich NE, Schuurman W, Wijnberg HM, Prins HJ, Van Weeren PR, Malda J. J. Alblas and W. J. Dhert, Biofabrication of osteochondral tissue equivalents by printing topologically defined, cell-laden hydrogel scaffolds. Tissue Engineering Part C: Methods. 2011;18(1):33–44.
Guillemot F, Souquet A, S C, B G. Laser-assisted cell printing: principle, physical parameters versus cell fate and perspectives in tissue engineering. Nanomedicine. 2010;5(3):507–515.
Gurkan UA, Assal RE, Yildiz SE, Sung Y, Alexander WPK, Trachtenberg J, Demirci U. Engineering anisotropic biomimetic fibrocartilage microenvironment by bioprinting mesenchymal stem cells in nanoliter gel droplets. Mol. Pharmaceutics. 2014.
Hopkins TR. In: Seetharam R, Sharma SK, eds. Purification and analysis of recombinant proteins. New York: Marcel Dekker; 1991:69.
Hopp B, Smausz T, Antal Z, Kresz N, Bor Z, D.B C. Absorbing film assisted laser induced forward transfer of fungi (Trichoderma conidia). Journal of Applied Physics. 2004;96:3478–3481.
Landers R, Mulhaupt R. Desktop manufacturing of complex objects, prototypes, and biomedical scaffolds by means of computer-assisted design combined with computer-guided 3D plotting of polymers and reactive oligomers. Macromolecular Materials and Engineering. 2000;282:17–21.
Lee Y-B, Polio S, Lee W, Dai G, Menon L, Carrol RS, Yoo S-S. Bioprinting of collagen and VEGF-releasing fibrin gel scaffolds for neural stem. Experimental Neurology. 2010;223:645–652.
Lee J-S, Hong JM, Jung JW, Shim J-H, Oh J-H, Cho D-W. 3D printing of composite tissue with complex shape applied to ear regeneration. Biofabrication. 2014;6:024103.
Lee V, Singh G, Trasatti J, Bjornsson C, Xu X, Tran TN, Yoo S-S, Dai G, Karande P. Design and fabrication of human skin by 3D bioprinting. Tissue Engineering Part C: Methods. 2014;20(6):473–484.
Li S, Xiong Z, Wang X, Yan Y, Liu H, Zhang R. Direct fabrication of a hybrid cell/hydrogel construct by a double nozzle assembling technology. Journal of Bioactive and Compatible Polymers. 2009;24:249–265.
Lipson H. Fabricated: The New World of 3D Printing. Wiley; 2013.
Mannoor MS, Jiang Z, James T, Kong YL, Malatesta KA, Soboyejo WO, Verma N, Gracias DH, McAlpine MC. 3D printed bionic ears. Nano Letters. 2013;13:2634–2639.
Moon S, Hasan SK, Song YS, Khademhosseini A, Demirci U. Layer by layer three-dimensional tissue. Tissue Engineering: Part C. 2010;16(1):157–165.
Nahmias Y, Schwartz RE, Verfaillie CM, D.J O. Laser-guided direct writing for three-dimensional tissue engineering. Biotechnology and Bioengineering. 2005;92:129–136.
Okamoto T, Suzuki T, Yamamoto N. Microarray fabrication with covalent attachment of DNA. Nature Biotechnology. 2000;18:438–441.
Park SA, Lee SH, Kim WD. Fabrication of porous polycaprolactone/hydroxyapatite (PCL/HA) blend scaffolds using a 3D plotting system for bone tissue engineering. Bioprocess and Biosystems Engineering. 2011;34(4):505–513.
Patz TM, Doraiswamy A, Narayan RJ, He W, Zhong Y, Bellamkonda R, Modi R, Chrisey DB. Three-dimensional direct writing of B35 neuronal cells. Journal of Biomedical Materials Research Part B: Applied Biomaterials. 2006;78(1):124–130.
Pepper ME, Seshadri V, Burg TC, Burg KJ, Groff RE. Characterizing the effects of cell settling on bioprinter output. Biofabrication. 2012;4:011001.
Pepper ME, Groff RE, Cass CA, Mattimore JP, Burg T, Burg KJ. A quantitative metric for pattern fidelity of bioprinted cocultures. Artificial Organs. 2012;36:E151–162.
Reis N, Ainsley C, Derby B. Ink-jet delivery of particle suspensions by piezoelectric droplet ejectors. Journal of Applied Physics. 2005;97(9):094903.
Ringeisen KH, Barron BRJ, Krizman D, Chrisey D, Jackman S ARSB. Laser printing of pluripotent embryonal carcinoma cells. Tissue Eng. 2004;10(3–4):483–491.
Roth EA, Xu T, Das M, Gregory C, Hickman JJ, Boland T. Inkjet printing for high-throughput cell patterning. Biomaterials. 2004;25:3707–3715.
Saunders RE, Bosworth L, Gough JE, Derby B, Reis N. Selective cell delivery for 3D tissue culture and engineering. European Cells and Materials. 2004;7(S1):84–85.
Saunders R, Gough J, Derby B. Inkjet printing of mammalian primary cells for tissue engineering applications. Boston: Nanoscale materials science in biology and medicine; 2005.
Saunders RE, Gough JE, Derby B. Delivery of human fibroblast cells by piezoelectric drop-on-demand ink-jet printing. Biomaterials. 2008;29(2):193–203.
Schiele NR, Chrisey DB, D.T T. Gelatin-based laser direct-write technique for the precise spatial patterning of cells. Tissue Engineering Part C: Methods. 2010;17(3):289–298.
Schiele NR, Corr DT, Huang Y, Raof NA, Y X, Chrisey DB. Laser-based direct-write techniques for cell printing. Biofabrication. 2010;2(3).
Schuurman W, Khristov V, Pot MW, Van Weeren PR, Dhert WJ, Malda J. Bioprinting of hybrid tissue constructs with tailorable mechanical properties. Biofabrication. 2011;3:021001.
Seerden KAM, Reis N, Evans JRG, Grant PS, Halloran JW, Derby B. Ink-jet printing of wax-based alumina suspensions. Journal of the American Ceramic Society. 2001;84(11):2514–2520.
Shim J-H, Kim JY, Park M. J. Park and D.-W. Cho, Development of a hybrid scaffold with synthetic biomaterials and hydrogel using solid freeform fabrication technology. Biofabrication. 2011;3:034102.
Simons KR, Morton RJ, Mosier DA, Fulton RW, Confer AW. Comparison of the Pasteurella haemolytica A1 envelope proteins obtained by two cell disruption methods. Journal of Clinical Microbiology. 1989;27(4):664–667.
Valerie Liu Tsang SNB. Three-dimensional tissue fabrication. Advanced Drug Delivery Reviews. 2004;56:1635–1647.
Vozzi G, Previti A, De Rossi D, Ahluwalia A. Microsyringe-based deposition of two-dimensional and three-dimensional polymer scaffolds with a well-defined geometry for application to tissue engineering. Tissue Engineering. 2002;8(6):1089–1098.
Wang F, Shor L, Darling A, Khalil S, Sun W, Güçeri S, Lau A. Precision extruding deposition and characterization of cellular poly- -caprolactone tissue scaffolds. Rapid Prototyping Journal. 2004;10(1):42–49.
-caprolactone tissue scaffolds. Rapid Prototyping Journal. 2004;10(1):42–49.
Wang X, Yan Y, Pan Y, Xiong Z, Liu H, Cheng J, Liu F, Wu R, Zhang R, Lu Q. Generation of three-dimensional hepatocyte/gelatin structures with rapid prototyping system. Tissue Engineering Part A. 2006;12:83–90.
Wilson Jr WC, Boland T. Cell and organ printing 1: protein and cell printers. The Anatomical Record Part A: Discoveries in Molecular, Cellular, and Evolutionary Biology. 2003;272A(2):491–496.
Xiong Z, Yan Y, Zhang R, Sun L. Fabrication of porous poly(l-lactic acid) scaffolds for bone tissue engineering via precise extrusion. Scripta Materialia. 2001;45(7):773–779.
Xu T, Jin J, Gregory C, Hickman JJ, Boland T. Inkjet printing of viable mammalian cells. Biomaterials. 2005;26(1):93–99.
Xu T, Gregory CA, Molnar P, Cui X, Jalota S, Bhaduri SB, Boland T. Viability and electrophysiology of neural cell structures generated by the ink-jet printing method. Biomaterials. 2006;27:3580–3588.
Xu W, Wang X, Yan Y, Zheng W, Xiong Z, Lin F, Wu R, Zhang R. Rapid prototyping three-dimensional cell/gelatin/fibrinogen constructs for medical regeneration. Journal of Bioactive and Compatible Polymers. 2007;22:363–377.
Xu M, Yan Y, Liu H, Yao R, Wang X. Controlled adipose-derived stromal cells differentiation into adipose and endothelial cells in a 3D structure established by cell-assembly technique. Journal of Bioactive and Compatible Polymers. 2009;24:31–47.
Yan Y, Wang X, Xiong Z, Liu H, Liu F, Lin F, Wu R, Zhang R, Lu Q. Direct construction of a three-dimensional structure with cells and hydrogel. Journal of Bioactive and Compatible Polymers. 2005;20:259–269.
Yan Y, Wang X, Pan Y, Liu H, Cheng J, Xiong Z, Ln F, Wu R, Zhang R, Lu Q. Fabrication of viable tissue-engineered constructs with 3D cell-assembly technique. Biomaterials. 2005;26(29):5864–5871.