INTRODUCTION
In this chapter, we continue a discussion about events at the cellular level and explore what happens at receptors after neurotransmitters make contact.
Electrochemical communication continues at the receptor on the postsynaptic membrane. Without a receptor, the neurotransmitter is like a tree falling in the woods with no one to hear it. The binding of the neurotransmitter with the receptor initiates a series of events that change the postsynaptic cell in some way. Receptors are protein units embedded in the lipid layer of the cell membrane. There are two basic types of receptors activated by neurotransmitters (more on this below), and the response generated depends on what type of receptor is engaged.
Agonists and Antagonists
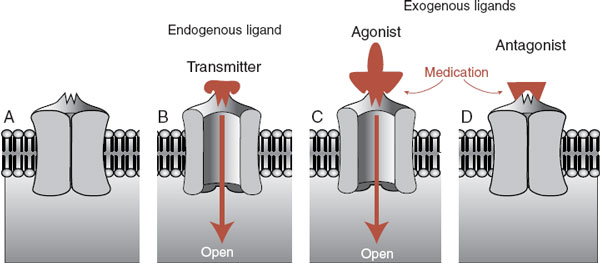
Most neuropsychiatric medications work their magic by enhancing or limiting the effects of the neurotransmitter at the receptor. Figure 5.1 shows a schematic representation of a receptor, a neurotransmitter, and the opposing effects of contrasting medications. Pharmacologists and neuroscientists use the terms ligand, agonist, and antagonists, but in this book we also use the more self-explanatory terms such as transmitter, drug/medication, stimulate, and block.
FAST RECEPTORS: CHEMICAL
There are two basic types of receptors for neurotransmitters. The one we usually think of is shown in Figure 5.1—an ion channel (also called transmitter-gated ion channel). A neurotransmitter or medication stimulates the opening of the pore inside the receptor and ions rapidly flow into the cell. Receptors that allow the entry of positive ions such as Na+ or Ca2+ result in an excitatory postsynaptic potential (EPSP). Acetylcholine (ACh) and glutamate result in such activation and are considered excitatory.
Receptors permeable to negative ions, such as Cl–, will result in inhibitory postsynaptic potentials. γ-Aminobutyric acid (GABA) and glycine, both considered inhibitory, cause this kind of activation. The essential point regarding these transmitter-gated ion channels is that they are fast. These receptors are magnificent little machines that rapidly allow the entry of large currents with great precision. The ions pour into the cell and the signal from the proceeding neuron, whether excitatory or inhibitory, is quickly propagated along the membrane of the target cell. As we reviewed in Chapter 3, an action potential is only generated if enough EPSPs bring the resting potential of the postsynaptic cell above the threshold at the axon hillock.
Amino Acid Receptors
The amino acid receptors mediate most of the fast transmitter-gated channels in the brain. The two prominent ones are glutamate and GABA, which are reviewed in the subsequent text.
POINT OF INTEREST
The receptors, unlike the transmitter, come in a variety of styles—a relationship that is much like feet and shoes. You only have two feet, but many shoes. Serotonin is one example: there are 14 different receptor subtypes for this one neurotransmitter. Some receptors are categorized as different classes, whereas others are just different subtypes within a class. It is not entirely clear why some differences constitute a new class and others just warrant a new subtype. Most likely a committee decided.
FIGURE 5.1  An unbound receptor in closed state (A). A natural ligand (neurotransmitter) stimulates the receptor, which then opens to allow entry of ions (B). Medication simulates the action of the natural ligand and the receptor opens (C). An antagonist blocks the action of the ligand so the receptor cannot be opened (D).
An unbound receptor in closed state (A). A natural ligand (neurotransmitter) stimulates the receptor, which then opens to allow entry of ions (B). Medication simulates the action of the natural ligand and the receptor opens (C). An antagonist blocks the action of the ligand so the receptor cannot be opened (D).
Glutamate
There are three prominent glutamate receptors: N-methyl-d-aspartate (NMDA), α-amino-3-hydroxy-5-methyl-4-isoxazole propionate (AMPA), and kainate, each with several subtypes. They are named after the artificial agonist that selectively activates them. For example, NMDA activates the NMDA receptor, but not the AMPA or kainate receptors. NMDA and AMPA constitute the bulk of fast excitatory synaptic transmission in the brain. The role of kainate is not clearly understood.
Both NMDA and AMPA receptors, which often coexist on the same postsynaptic receptor, allow the rapid entry of Na+ into the cell (and the simultaneous exit of K+) that generates the depolarization of the postsynaptic cell. NMDA receptors are unique in that they also allow the entry of Ca2+ that can act as a second messenger inside the cell. This can have a profound impact on the cell resulting in lasting changes, as will be shown at the end of this chapter when we discuss long-term memory.
The NMDA receptor is further unique in that it requires both the glutamate transmitter and a change in the voltage to open before it will allow the entry of Na+ and Ca2+. This property is due to the presence of Mg2+ ions, which clog the NMDA receptor at resting voltage. Figure 5.2 shows how the AMPA receptor works in conjunction with the NMDA receptor to depolarize the cell and bring Ca2+ into the cell. This property has a significant impact on the capacity of the neurons to change.
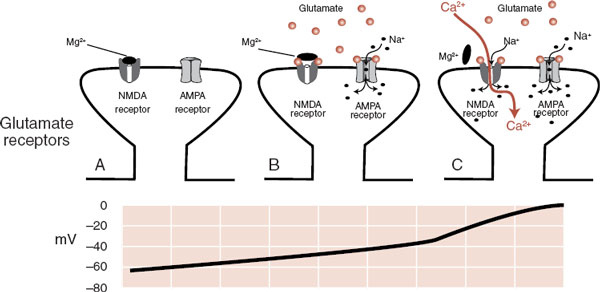
FIGURE 5.2  A. A postsynaptic glutamate terminal with both receptors closed. B. The presence of glutamate transmitters opens the AMPA receptor but not the NMDA receptor. C. When the voltage in the postsynaptic neuron gets above –35 mV, the magnesium ion blocking the receptor falls off and Na+ as well as Ca2+ ions pour into the cell. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate; NMDA, N-methyl-D-aspartate.
A. A postsynaptic glutamate terminal with both receptors closed. B. The presence of glutamate transmitters opens the AMPA receptor but not the NMDA receptor. C. When the voltage in the postsynaptic neuron gets above –35 mV, the magnesium ion blocking the receptor falls off and Na+ as well as Ca2+ ions pour into the cell. AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate; NMDA, N-methyl-D-aspartate.
The glutamate receptor is increasingly being explored as a novel target for psychiatric conditions. Preliminary studies have shown that a single IV injection of ketamine (an NMDA antagonist) can rapidly and temporarily reduce depression. With schizophrenia, glutamate has long been suspected as a possible culprit, and Eli Lilly is conducting clinical trials with a medication that reduces glutamate release. Figure 15.3 shows the benefits of a partial NMDA agonist that, in conjunction with exposure therapy, reduces fear.
γ-Aminobutyric Acid
GABA and glycine are the primary inhibitory neurons in the brain; inhibition is a process that must be tightly regulated. Too much inhibition causes the brain to slow down—even lose consciousness. Not enough inhibition and the electrical activity can get out of control—evoke a seizure. GABA is the most common inhibitory receptor.
The GABA receptor is made up of five protein subunits, which vary for different subclasses of the receptor. The GABAA receptor (Figure 5.3) is the focus of much pharmacological interest. There are several other sites on the GABAA receptor where chemicals can modulate its function. For example, barbiturates and benzodiazepines have their own distinct sites on the GABA receptor. These medications by themselves do not open the GABA channel, but they can enhance the strength or frequency of the opening. Benzodiazepines plus GABA results in more Cl– entering the cell and a greater inhibitory effect.
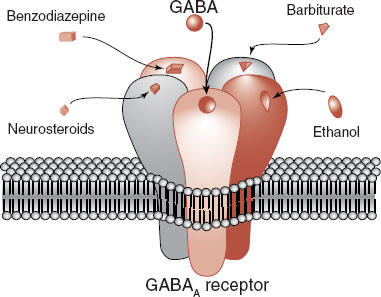
FIGURE 5.3  The GABA receptor showing other drugs that can modify and enhance the inhibitory effect of these receptors. GABA, γ-aminobutyric
The GABA receptor showing other drugs that can modify and enhance the inhibitory effect of these receptors. GABA, γ-aminobutyric
Ethanol is another popular drug that enhances the function of the GABA receptor. Long-term use of ethanol decreases the expression of the GABA receptor, which may explain the tolerance that develops with alcoholism. Whether the receptor alterations contribute to the propensity for seizures when the alcohol is withdrawn remains unclear.
The steroid hormones can also modulate GABA receptors (sometimes called neurosteroids when they have effects on neurons) (see Figure 6.1). This may explain the psychiatric symptoms that develop at times when the sex hormones are reduced, for example, premenstrual syndrome, menopause, and chemical castration for men. Additionally researchers have found that some steroid hormone levels drop in patients who panic during an attack. Others have shown that medications such as olanzapine and fluoxetine—known to decrease anxiety—increase steroid hormone levels.
SLOW RECEPTORS: METABOLIC
Starting in the 1950s, researchers teased out the details of a second type of receptor—one that activates a cascade of biochemical events in the cytosol of the receptor cell that ultimately modifies the function of target proteins or the DNA. This type of receptor, called a G-protein–coupled receptor, is perhaps even more relevant to the effect of psychiatric medications than the transmitter-gated ion channel. G-protein is the short form for guanosine triphosphate–binding protein. Although there are many types of G-protein–coupled receptors, the basic style involves three steps:
1. A neurotransmitter binds to the receptor.
2. The receptor activates the G-protein, which moves along the intracellular membrane.
3. The G-protein activates the “effector” protein.
The activated “effector” protein can have a variety of functions including just opening traditional ion channels (not shown). However, the more interesting effector proteins are enzymes that trigger a process called a secondary messenger cascade (Figure 5.4). The neurotransmitter activates the G-protein, which slides along the membrane and stimulates the effector protein. The activated effector protein then converts adenosine triphosphate into cyclic adenosine monophosphate (cAMP): the secondary messenger, which will diffuse away into the cytosol where it can change the neuronal operations. With the exception of serotonin type 3 receptor (5-hydroxytryptamine3 [5-HT3]), all the monoamine receptors belong to the G-protein– coupled family—which means that most psychiatric medications deliver their punch through secondary messengers.
TREATMENT
ANTIEPILEPTIC DRUGS
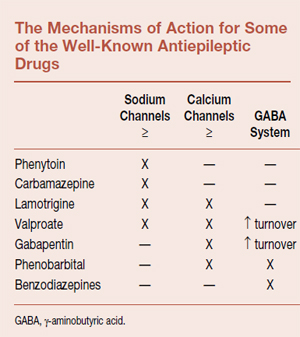
The background about inhibitory and excitatory receptors helps one understand the major mechanisms of action of the antiepileptic drugs (AEDs). The goal of treatment with these medications is to modify the aberrant bursting properties, synchronization, and spread of abnormal firing without affecting ordinary electrical activity. These effects, although intended to control seizure disorders, have wide-ranging applications to other neuropsychiatric disorders such as bipolar affective disorder, anxiety, pain, and alcohol dependence, to name a few.
The major effects of the well-known AEDs fall into three categories and are shown in the table below. The first involves the voltage-gated sodium channel discussed in Chapter 3. Remember that these are the pores that allow rapid entry of sodium into the cell to propagate the action potential along the axon. Modulation of these channels is believed to account for some of the effectiveness of several AEDs. The second category involves the voltage-gated calcium channels, which are located on the terminals of the neuron and instigate the release of neurotransmitters into the synaptic cleft. Blockade of these channels decreases the neurotransmitter release and ultimately decreases excitability.
The final mechanism of action involves the GABA receptor and increasing inhibition, for example, barbiturates and benzodiazepines as mentioned above. Additionally, valproate and gabapentin increase GABA synthesis and turnover, with the net effect of increased activity of these inhibitory neurons. It is easy to understand the rationale for trying medications with different mechanisms of action when one medication fails to control the disorder.
Serotonin Receptors
The original discovery of the serotonin receptor led to two subtypes: 5-HT1 and 5-HT2. Further discoveries, especially the application of molecular cloning techniques, has resulted in multiple subdivisions of these two receptors and the addition of several more for a total of 14. Although the prospect of activating or blocking the various receptors for further refinement of psychopharmacologic treatment is an enticing possibility, the clinical results with a few exceptions have been limited.
POINT OF INTEREST
Autoreceptors reside on the cell body or terminal of the presynaptic neuron. They sense the presence of the neurotransmitter and provide negative feedback to the neuron. That is, if too much of the neurotransmitter is present, these receptors activate a second messenger and turn off further release of the neurotransmitter—and in some cases reduce synthesis of the neurotransmitter. The figure shows an example of this type of receptor using the 5-HT1A (anxiety-related) and 5-HT1D (migraine-related) receptors. Similar autoreceptors are known for other transmitters such as dopamine and norepinephrine. Blocking these specific receptors may provide unique ways to alleviate specific symptoms.
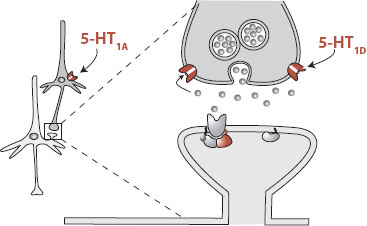
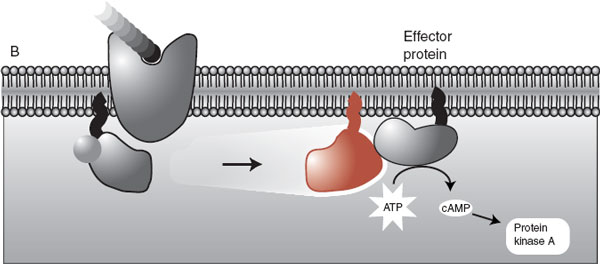
FIGURE 5.4  The initiation of the second messenger cascade starts with a neurotransmitter binding with the G-protein receptor and ends with the conversion of ATP into cAMP. ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; GTP, guanosine triphosphate; GDP, guanosine diphosphate.
The initiation of the second messenger cascade starts with a neurotransmitter binding with the G-protein receptor and ends with the conversion of ATP into cAMP. ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; GTP, guanosine triphosphate; GDP, guanosine diphosphate.
The 5-HT1 receptors make up the largest subtype with 5-HT1A, 5-HT1B, 5-HT1D, 5-HT1E, and 5-HT1F. 5-HT1A has received the most interest and seems to play a prominent role in depression and anxiety. It is an autoreceptor on the cell body. Stimulation of this receptor reduces cell firing and curtails the release of serotonin. How this would improve mood is unclear, but blocking this receptor has decreased the effectiveness of tricyclic antidepressants in rat models of depression. The anxiolytic buspirone (Buspar) is a partial 5-HT1A agonist, which suggests that 5-HT1A has some role in anxiety. The development and distribution of buspirone is an example of how a specific serotonin receptor is targeted for the development of a medication.
The 5-HT1D receptor is also an autoreceptor but is located on the nerve terminal at the synapse. Here it appears to function to sense the serotonin in the synaptic cleft and turn off release of more serotonin when stimulated. The 5-HT1D receptor is stimulated by the antimigraine drug sumatriptan (Imitrex), although the importance of this effect in the overall efficacy of the medication is unclear. Some researchers are exploring the effectiveness of a 5-HT1D receptor antagonist for the treatment of depression. The goal is to block the negative feedback mediated through the 5-HT1D receptor so that more serotonin is released into the synapse.
Some other important serotonin receptors for the psychiatrist are 5-HT2A and 5-HT2C. The 5-HT2A receptor has been identified as playing an important role in the “atypicalness” of the second-generation antipsychotic agents (clozapine, risperidone, and olanzapine). These newer agents have a greater capacity to block 5-HT2A than the traditional agents such as haloperidol, and it is speculated that this results in the observed decrease in extrapyramidal symptom (EPS) and mediation of the negative symptoms of schizophrenia.
Dopamine Receptors
The dopamine receptors are involved in a wide range of functions including locomotion, cognition, psychosis, and even neuroendocrine secretion. It became clear after 1979 that there was more than one dopamine receptor: D1 and D2. More recently with molecular cloning they have identified three more receptors: D3, D4, and D5. The D3 and D4 receptors are “D2-like” and the D5 receptor is “D1-like.” However, the D1 and D2 receptors remain the most important.
“D1-like” = D1 and D5
“D2-like” = D2, D3 and D4
The D1 and D2 receptors have been distinguished by their differing affinity for binding with traditional antipsychotic agents such as haloperidol: The D2 receptor has high affinity, whereas the D1 receptor has low affinity. Increased D2 receptor antagonism correlates with the therapeutic efficacy and EPS side effect with the traditional antipsychotics. There is great interest in the “D2-like” receptors (D3 and D4) as possible alternative sites for therapeutic potentiation with antipsychotic agents, but as yet these receptors have failed to translate into clinically significant benefits.
The psychostimulants (cocaine, amphetamine, and methylphenidate) work in part by blocking the reuptake of dopamine and leaving more dopamine in the synapse to stimulate the dopamine receptors. The effects are increased energy, improved cognition, and even psychosis. The effects on reward and cognition seem to be more prominently mediated by the D1 receptor. Augmenting D1 and D2 receptors (agonists) is the mainstay of treatment for Parkinson’s disease.
Adrenergic Receptors
The adrenergic receptors are divided into three main subtypes α1, α2, and β. Each one of these has three subtypes: α1a, α1b, α1d, α2a, and so on. We focus on the three main subtypes. The α1 receptor is believed to play a role in smooth muscle contraction and has been implicated in effecting blood pressure, nasal congestion, and prostate function. Although widely expressed in the central nervous system (CNS), the central role of the α1 receptor remains to be determined; locomotor activation and arousal have been suggested by some studies. Stimulation of the α1 receptor may synergistically increase the activity of the serotonin neurons in the raphe nucleus, although stimulation of the α2 receptor may have just the opposite effect.
The α2 receptor subtypes in the CNS inhibit the firing of the norepinephrine neurons through autoreceptors. This mechanism of action is believed to mediate the sedative and hypotensive effects of the α2 receptor agonist clonidine. Additionally, stimulation of the α2 receptors decreases sympathetic activity that may explain the therapeutic utility of clonidine for suppressing the heightened sympathetic state for patients in opiate withdrawal.
The β receptor subtypes are more famous for their part in slowing cardiac rhythm and lowering blood pressure. The functions of the β receptors in the CNS, although widely distributed, are not well understood. It is not uncommon to use a β blocker, for example, propranolol, for treating performance anxiety or antipsychotic-induced akathisia. Whether these benefits come from a central or peripheral blockage of the β receptor, or both, is not known.
Histamine Receptors
There are now four histamine receptors although H4 is predominately in the periphery and only recently discovered. The H1 receptor is the target for the classic antihistamines, which highlights its role in sedation and conversely arousal. Of great interest to psychiatrists is the role of H1 in weight gain. Recent analysis has shown that the potential to gain weight with antipsychotic agents correlates with the antagonism for the H1 receptor, for example, clozapine and olanzapine have the most affinity for weight gain, whereas aripiprazole and ziprasidone have the least.
The H2 receptor is more traditionally associated with the gut. Blockade of the H2 receptor has been a widely used treatment for peptic ulcer disease. The H3 receptor functions as an inhibitory receptor on the histamine neurons as well as other nonhistamine nerve terminals. The role of this receptor is not clearly understood, but it may be involved in appetite, arousal, and cognition.
Cholinergic Receptors
It was with the cholinergic receptor that scientists first realized that one neurotransmitter (ACh) could have different receptors. The initial subtypes were identified and named after the drug that distinguished its effect. For example, nicotine will stimulate cholinergic receptors in skeletal muscle, but not in the heart. Conversely, muscarine will stimulate the heart, but has no effect on skeletal muscle. Therefore, the two receptors can be identified by the actions of different drugs and the receptors were named after those drugs: nicotinic and muscarinic. Unfortunately, it has been hard to find a drug with unique action on each receptor subtype, so we are stuck with designations such as 1A and 2B.
Many more subtypes of the nicotinic and muscarinic receptors have been identified since the early days of receptor delineation, but the significance of these various subtypes for the psychiatrist remains obscure. Clearly, ACh is important in cognition and memory as noted by the benefits of inhibiting acetylcholinesterase as a treatment for Alzheimer’s disease. Likewise, the blockage of the muscarinic receptor by tricyclic antidepressants and antipsychotic medications results in troublesome dry mouth, constipation, and urinary hesitancy (which we generically call the anticholinergic side effects). However, the importance of one receptor subtype over another has not been shown.
DISORDER
Myasthenia gravis is an autoimmune disease in which the body produces antibodies to the nicotinic ACh receptor. Patients complain of weakness and fatigue in the voluntary muscles resulting from the interruption of the chemical signal at the neuromuscular junction. Muscarinic receptors in the heart and CNS are unaffected.
ACh, unlike the monoamines, is cleared from the synaptic cleft by an enzyme, acetylcholinesterase. One treatment for myasthenia gravis is with the acetylcholinesterase inhibitors such as edrophonium (Tensilon) that prolong the life of the released ACh.
SIGNALING THE NUCLEUS
After the neurotransmitter has stimulated the G-protein to slide across the membrane and activate an enzyme (which is the case with most of the catecholamines), a cascade of events with second messengers transpires to modify neuronal function. Neurons use many different second messengers as signals within the cytosol—two of which we have discussed: Ca2+ and cAMP. The secondary messengers regulate neuronal function by activating enzymes that will add a phosphate group (phosphorylation) to other proteins in the cell. The protein kinases, of which there are wide varieties, are the enzymes that add a phosphate group to other proteins. Protein kinase A and calcium/calmodulin protein kinase are two examples of this type of enzyme. Once proteins are phosphorylated, they are “turned on” and can execute a broad range of cellular functions such as regulating enzyme activity or ion channels.
Additionally, the protein kinases can induce our two favorite words—gene expression. The protein kinases can “turn on” the DNA and start the synthesis of messenger ribonucleic acid. This process takes longer but can have large and relatively stable effects on the cell, for example, upregulation of receptors or the production of growth factor proteins. Figure 5.5 shows an example of how two different types of receptors—a G-protein–coupled receptor and a transmittergated ion channel—can activate a second messenger that will stimulate the production of new proteins.
LONG-TERM POTENTIATION: A SUMMARY EXAMPLE
Understanding long-term potentiation (LTP) is a way to apply what has been discussed in the past few chapters to a topic of great relevance to neuroscience: learning and memory. LTP is a laboratory example of learning that was discovered accidentally. As we have seen, applying a single stimulus to a neuron generates an excitatory impulse (EPSP) in the cell. Applying high-frequency stimuli—hundreds of impulses within a second—generates a higher EPSP in the cell. What was found accidentally was that once a neuron has been exposed to high-frequency stimulation, something changes, and now a single stimulus will generate a high EPSP. This sort of change has been shown to last for several months if not longer. Figure 5.6 shows these three steps.
FIGURE 5.5  Signaling the nucleus. The neurotransmitters stimulate a cascade of events that ultimately leads to activation of the DNA (gene expression) and the synthesis of proteins that can modify the function of the cell. ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CaMK, calcium/calmodulin protein kinase; CREB, cyclic adenosine monophosphate response element binding; mRNA, messenger ribonucleic acid.
Signaling the nucleus. The neurotransmitters stimulate a cascade of events that ultimately leads to activation of the DNA (gene expression) and the synthesis of proteins that can modify the function of the cell. ATP, adenosine triphosphate; cAMP, cyclic adenosine monophosphate; CaMK, calcium/calmodulin protein kinase; CREB, cyclic adenosine monophosphate response element binding; mRNA, messenger ribonucleic acid.
LTP sounds like one of those topics that neuroscientists discuss ad nauseam, but which has little relevance to practicing clinicians. Oh contraire! LTP is a demonstration—although artificial—that neurons can incorporate lasting changes, which is an essential step to developing memories and skills. And what is life without memories? Unfortunately, some people are haunted by memories. LTP is an example, at the cellular level, of what may happen for people who experience overwhelming trauma and develop posttraumatic stress disorder.
Furthermore, the changes brought about with LTP can be analyzed at the molecular level. Researchers have shown that both glutamate receptors (NMDA and AMPA) must be operational for the process to work. Likewise others have shown that calcium (a second messenger) and the kinases are required for LTP. Of even greater interest is the demonstration that protein synthesis (gene expression!) is an essential part of the development of LTP. All the processes shown in Figure 5.5 are involved in LTP.
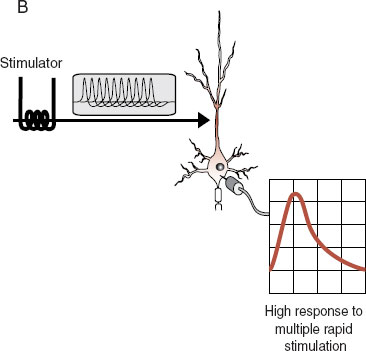
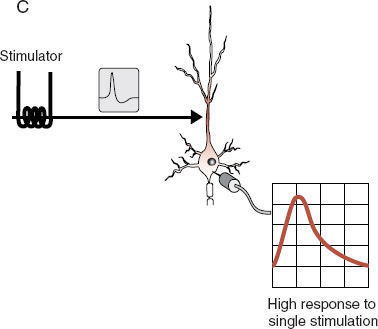
FIGURE 5.6  Long-term potentiation. In (A) the neuron receives a single stimulus that generates a small impulse in the cell body. In (B) a high-frequency stimulus generates a big response in the cell body. This process changes something in the cell, and following this high-frequency testing, the original single stimulus (C) generates a big response in the cell body.
Long-term potentiation. In (A) the neuron receives a single stimulus that generates a small impulse in the cell body. In (B) a high-frequency stimulus generates a big response in the cell body. This process changes something in the cell, and following this high-frequency testing, the original single stimulus (C) generates a big response in the cell body.
What happens inside the cell with the induction of LTP? Clearly there is some strengthening of the connection between the two cells that are communicating when LTP occurs. Figure 5.7 depicts the events that transpire when the presynaptic cell is hyperstimulated, the postsynaptic cell signals its nucleus, and the connection between the two cells is enhanced by the insertion of more glutamate receptors. Additional evidence has shown that a retrograde signal such as nitric oxide diffuses back across the synaptic cleft and induces the presynaptic neuron to release more transmitters. The combined result is increased sensitivity and responsiveness at the synapse: more transmitters and more receptors resulting in a stronger signal.
Amazingly, the structural changes to the synapse can actually be seen under the right experimental circumstances. Engert and Bonhoeffer filled neurons from the hippocampus with fluorescent dye and then induced LTP. They captured the development of new spines on the postsynaptic dendrite as shown in Figure 5.8. This research is consistent with other data that suggest that the development of spines is possibly the structural manifestation of learning and memory.
FIGURE 5.7  LTP inducing a change at the synapse. A. Hyperstimulation of the presynaptic neuron. B. The NMDA and AMPA receptors open, allowing the entry of the second messenger, calcium, which signals the nucleus to synthesize more receptors. C. The new AMPA receptors are inserted into the membrane, which has the effect of increasing the cell’s responsiveness to future stimulation. LTP, long-term potentiation; NMDA, N-methyl-D-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate.
LTP inducing a change at the synapse. A. Hyperstimulation of the presynaptic neuron. B. The NMDA and AMPA receptors open, allowing the entry of the second messenger, calcium, which signals the nucleus to synthesize more receptors. C. The new AMPA receptors are inserted into the membrane, which has the effect of increasing the cell’s responsiveness to future stimulation. LTP, long-term potentiation; NMDA, N-methyl-D-aspartate; AMPA, α-amino-3-hydroxy-5-methyl-4-isoxazole propionate.
TREATMENT
TRANSCRANIAL MAGNETIC STIMULATION
LTP has not been established in humans for obvious ethical reasons. Repetitive transcranial magnetic stimulation offers a possible noninvasive method of stimulating conscious human subjects that can mimic the effects of LTP. The prospect of inducing long-lasting changes to the human cortex through noninvasive stimulation offers the prospect of a new kind of treatment applicable to a wide range of mental disorders.
Preliminary studies have demonstrated changes in motor skills consistent with changes seen with LTP when subjects receive continuous stimulation at the motor cortex. The changes lasted up to 60 minutes beyond the period of stimulation. Although the utility of a change lasting only 60 minutes is of little clinical value, the prospect of altering the function of the cortex without breaching the blood–brain barrier is an exciting possibility.
LTP demonstrates that electrical, molecular, and structural changes are involved in the process of storing information. It is a small leap to conclude that highly charged events in our lives—the emotional equivalent of high-frequency stimulation—leave enduring changes to neuronal connections in a manner similar to LTP.
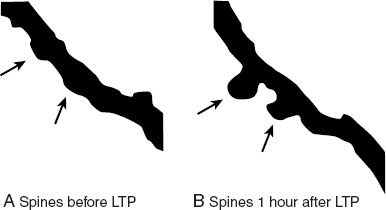
FIGURE 5.8  With the induction of LTP in neurons from the hippocampus, new spines developed on the dendrite within an hour as shown by the arrows. LTP, long-term potentiation. (Adapted from Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399(6731):66-70.)
With the induction of LTP in neurons from the hippocampus, new spines developed on the dendrite within an hour as shown by the arrows. LTP, long-term potentiation. (Adapted from Engert F, Bonhoeffer T. Dendritic spine changes associated with hippocampal long-term synaptic plasticity. Nature. 1999;399(6731):66-70.)
1. Which equation is correct?
a. Antagonist = stimulate.
b. Agonist = block.
c. Agonist = transmitter.
d. Antagonist = endogenous ligand.
2. Which is not a glutamate receptor?
a. NMDA.
b. cAMP.
c. AMPA.
d. Kainate.
3. The strength of the GABA inhibitory signal is enhanced by all of the following except
a. Ethanol.
b. Phenobarbital.
c. Carbamazepine.
d. Neurosteroids.
4. The AEDs exert their major effects through all of the following except
a. Decreasing glutamate activity.
b. Modulation of the voltage-gated sodium channels.
c. Blockade of the voltage-gated calcium channels.
d. Increasing GABA activity.
5. Which of the following is true?
a. Mg2+ is a common second messenger.
b. Most monoamine receptors are fast receptors.
c. Fast receptors typically lead to gene expression.
d. Autoreceptors give negative feedback to the neuron.
6. Gene expression stimulated by a neurotransmitter involves all of the following except
a. Phosphorylation.
b. Long-term potentiation.
c. Protein kinases.
d. G-protein–coupled receptor.
7. All of the following about LTP are true except
a. Enhanced EPSP.
b. Increased receptors.
c. Gene expression.
d. Enhanced ligand binding.
8. Which describes LTP best?
a. It was discovered by accident.
b. Neuroscientists love to pontificate on this topic.
c. It is an example of learning.
d. All of the above.
See Answers section at the end of the book.