Chapter 15
Practice Test 2
Click here to download a PDF of Practice Test 2.
PRACTICE SAT SUBJECT TEST IN CHEMISTRY–TEST 2
You are about to take the second practice SAT Subject Test in Chemistry. The bubble sheet can be found near the back of the book; feel free to tear it out for use. (Just don’t lose it!)
After answering questions 1–23, which constitute Part A, you’ll be directed to answer questions 101–116, which constitute Part B. Then, begin again at question 24. Questions 24–70 constitute Part C.
When you’re ready to score yourself, refer to the answer key and scoring instructions on this page and this page. Full explanations regarding the correct answers to all questions start on this page.
SAT SUBJECT TEST IN CHEMISTRY
MATERIAL IN THE FOLLOWING TABLE MAY BE USEFUL IN ANSWERING THE QUESTIONS IN THIS EXAMINATION.
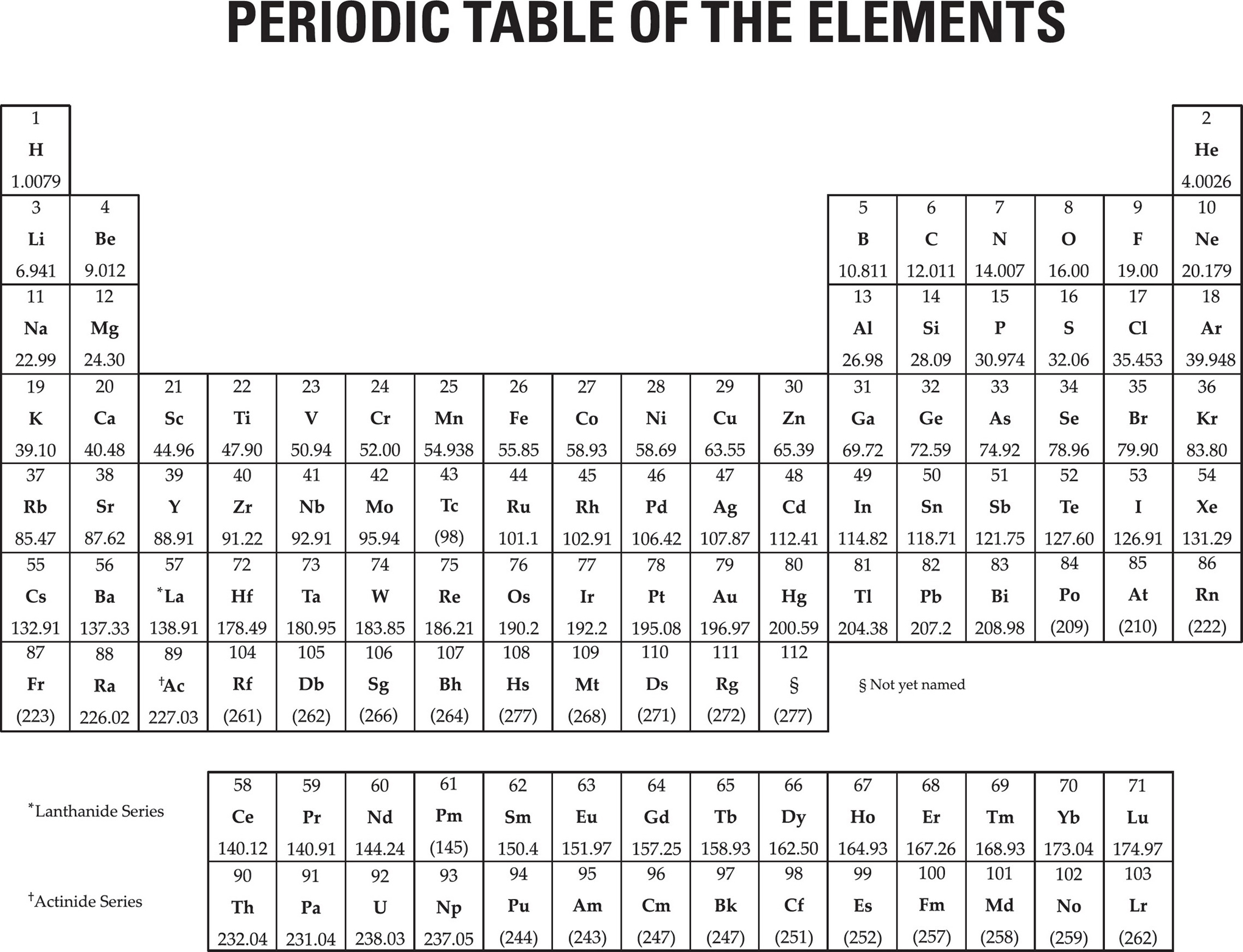
SAT SUBJECT TEST IN CHEMISTRY—TEST 2
Note: For all questions involving solutions and/or chemical equations, assume that the system is in pure water unless otherwise stated.
Part A
Directions: Each set of lettered choices below refers to the numbered statements or questions immediately following it. Select the one lettered choice that best fits each statement or answers each question, and then fill in the corresponding oval on the answer sheet. A choice may be used once, more than once, or not at all in each set.
Questions 1-4 refer to the following.
(A) Molarity
(B) Molality
(C) Mole fraction
(D) Density
(E) Partial pressure
1. Is measured in units of atmospheres or millimeters of mercury
2. Is measured in units of moles/kilogram
3. Is a measure of mass per unit volume
4. Is the quantity used in the calculation of boiling point elevation
Questions 5-9 refer to the following.
(A) Hydrogen bonding
(B) Ionic bonding
(C) Network bonding
(D) London dispersion force
(E) Metallic bonding
5. Chiefly responsible for the relatively high boiling point of water
6. Is present in liquid oxygen
7. Is primarily responsible for the hardness of diamond
8. Allows copper to conduct electricity
9. Is present in solid KCl
Questions 10-13 refer to the following.
(A) Na+
(B) Al
(C) F
(D) Ti
(E) Br–
10. Has 7 valence electrons
11. Has the electron configuration 1s22s22p63s23p1
12. Has the same electron configuration as a neon atom
13. Has valence electrons in d orbitals
Questions 14-17 refer to the following.
(A) A 0.01-molar solution of HNO3
(B) A 0.01-molar solution of HC2H3O2
(C) A 0.01-molar solution of Cu(NO3)2
(D) A 0.01-molar solution of NaNO3
(E) A 0.01-molar solution of NaOH
14. Will be colored blue
15. Will have a pH of 2
16. Will have the lowest freezing point
17. Will contain undissociated aqueous particles
Questions 18-20 refer to the following.
(A) Enthalpy change
(B) Entropy change
(C) Gibbs free energy change
(D) Activation energy
(E) Specific heat capacity
18. Is the amount of energy that must be added to raise the temperature of 1 gram of a substance 1°C
19. Its value indicates the spontaneity of a reaction
20. Its value indicates whether a reaction is endothermic or exothermic
Questions 21-23 refer to the following.
(A) Ionization energy
(B) Electronegativity
(C) Atomic radius
(D) Atomic number
(E) Mass number
21. Is the measure of the pull of the nucleus of an atom on the electrons of other atoms bonded to it
22. Is the energy required to remove an electron from an atom
23. Is equal to the number of protons in an atom
PLEASE GO TO THE SPECIAL SECTION LABELED CHEMISTRY AT THE LOWER RIGHT-HAND CORNER OF THE ANSWER SHEET YOU ARE WORKING ON AND ANSWER QUESTIONS 101-116 ACCORDING TO THE FOLLOWING DIRECTIONS.
Part B
Directions: Each question below consists of two statements, I in the left-hand column and II in the right-hand column. For each question, determine whether statement I is true or false and whether statement II is true or false, and fill in the corresponding T or F ovals on your answer sheet. Fill in oval CE only if statement II is a correct explanation of statement I.
|
EXAMPLES: |
||
|
I |
II |
|
|
EX 1. H2SO4 is a strong acid |
BECAUSE | H2SO4 contains sulfur |
|
EX 2. An atom of oxygen is electrically neutral |
BECAUSE | an oxygen atom contains an equal number of protons and electrons. |
|
SAMPLE ANSWERS |
||
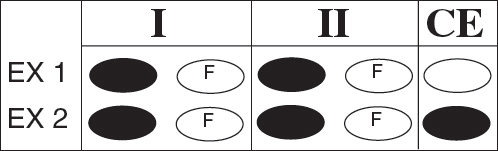
|
||
|
I |
II |
|
|
101. An ionic solid is a good conductor of electricity |
BECAUSE | an ionic solid is composed of positive and negative ions joined together in a lattice structure held together by electrostatic forces. |
|
102. The bond in an O2 molecule is nonpolar |
BECAUSE | the oxygen atoms in an O2 molecule share the bonding electrons equally. |
|
103. When a sample of water freezes, the process is exothermic |
BECAUSE | ice is at a lower potential energy state than water. |
|
104. At 25°C, an aqueous solution with a pH of 5 will have a pOH of 9 |
BECAUSE | the pH of a buffered solution is not greatly affected by the addition of a relatively small amount of acid or base. |
|
105. When a chlorine atom gains an electron, it becomes a positively charged ion |
BECAUSE | a neutral atom has equal numbers of protons and electrons. |
|
106. Lithium has a larger first ionization energy than oxygen |
BECAUSE | oxygen atoms have larger atomic radii than lithium atoms. |
|
107. Potassium chloride dissolves readily in water |
BECAUSE | water is a polar solvent. |
108. Ammonia is a Lewis base |
BECAUSE | ammonia can donate an electron pair to a bond. |
|
109. Elemental fluorine is more reactive than elemental neon |
BECAUSE | neon has a larger atomic weight than fluorine. |
|
110. The addition of a catalyst will decrease the ∆H for a reaction |
BECAUSE | a catalyst provides an alternate reaction pathway with a lower activation energy. |
|
111. The oxygen atom in a water molecule has a –2 oxidation state |
BECAUSE | water molecules exhibit hydrogen bonding. |
|
112. When a salt sample dissolves in water, ∆S for the process is positive |
BECAUSE | for a salt sample, aqueous ions have greater entropy than ions in a solid. |
|
113. When the temperature of a reaction at equilibrium is increased, the equilibrium will shift to favor the endothermic direction |
BECAUSE | at equilibrium, all reactants have been converted into products. |
|
114. An atom of 12C contains 12 protons |
BECAUSE | the identity of an element is determined by the number of protons in the nuclei of its atoms. |
|
115. Water boils at a lower temperature at high altitude than at low altitude |
BECAUSE | the vapor pressure of water is lower at higher altitude. |
|
116. Elemental sodium is a strong reducing agent |
BECAUSE | an atom of elemental sodium gives up its valence electron readily. |
RETURN TO THE SECTION OF YOUR ANSWER SHEET YOU STARTED FOR CHEMISTRY AND ANSWER QUESTIONS 24–70.
Part C
Directions: Each of the questions or incomplete statements below is followed by five suggested answers or completions. Select the one that is best in each case and then fill in the corresponding oval on the answer sheet.
24. What is the oxidation state of bromine in HBrO3 ?
(A) –3
(B) –1
(C) +1
(D) +3
(E) +5
25. What is the percent by mass of silicon in a sample of silicon dioxide?
(A) 21%
(B) 33%
(C) 47%
(D) 54%
(E) 78%
26. How many electrons does a 37Cl ion with a charge of –1 contain?
(A) 16
(B) 17
(C) 18
(D) 37
(E) 38
CH4(g) + 2O2(g) → CO2(g) + 2H2O(g) + 800 kJ
27. If 1 mole of O2(g) is consumed in the reaction given above, how much energy is produced?
(A) 200 kJ
(B) 400 kJ
(C) 800 kJ
(D) 1,200 kJ
(E) 1,600 kJ
28. Which of the following is NOT true of the element sodium?
(A) It takes the oxidation state +1.
(B) It reacts with water to form a basic solution.
(C) It forms metallic bonds in its solid uncombined form.
(D) It is found in nature as a diatomic gas.
(E) It reacts with a halogen to form an ionic salt.
29. What volume of a 0.200-molar solution of sodium hydroxide is required to neutralize 40 liters of a 0.300-molar hydrochloric acid solution?
(A) 10 liters
(B) 20 liters
(C) 40 liters
(D) 60 liters
(E) 120 liters
…PH3 +…O2 →…P2O5 +…H2O
30. When the equation above is balanced and the coefficients are reduced to the lowest whole numbers, the coefficient for H2O is
(A) 1
(B) 2
(C) 3
(D) 4
(E) 5
H2SO4(aq) + Ba(OH)2(aq) →
31. Which of the following are products of the reaction shown above?
I. O2(g)
II. H2O(l)
III. BaSO4(s)
(A) I only
(B) III only
(C) I and II only
(D) I and III only
(E) II and III only
2Mg(s) + O2(g) → 2MgO(s)
32. If 48.6 grams of magnesium are placed in a container with 64 grams of oxygen gas and the reaction above proceeds to completion, what is the mass of MgO(s) produced?
(A) 15.4 grams
(B) 32.0 grams
(C) 80.6 grams
(D) 96.3 grams
(E) 112 grams
33. An ideal gas in a closed inflexible container has a pressure of 6 atmospheres and a temperature of 27oC. What will be the new pressure of the gas if the temperature is decreased to –73oC ?
(A) 2 atm
(B) 3 atm
(C) 4 atm
(D) 8 atm
(E) 9 atm
34. Equal molar quantities of hydrogen gas and oxygen gas are present in a closed container at a constant temperature. Which of the following quantities will be the same for the two gases?
I. Partial pressure
II. Average kinetic energy
III. Average molecular velocity
(A) I only
(B) I and II only
(C) I and III only
(D) II and III only
(E) I, II, and III
35. Which of the following is a nonpolar molecule?
(A) CO2
(B) H2O
(C) NH3
(D) NO
(E) HI
36. What is the molar concentration of a 500-milliliter solution that contains 20 grams of CaBr2 (formula weight = 200) ?
(A) 0.1 molar
(B) 0.2 molar
(C) 0.5 molar
(D) 1 molar
(E) 5 molar
37. The structure of BeCl2 can best be described as
(A) linear
(B) bent
(C) trigonal
(D) tetrahedral
(E) square
2NO(g) + 2H2(g) → N2(g) + 2H2O(g)
38. Which of the following statements is true regarding the reaction given above?
(A) If 1 mole of H2 is consumed, 0.5 mole of N2 is produced.
(B) If 1 mole of H2 is consumed, 0.5 mole of H2O is produced.
(C) If 0.5 mole of H2 is consumed, 1 mole of N2 is produced.
(D) If 0.5 mole of H2 is consumed, 1 mole of NO is consumed.
(E) If 0.5 mole of H2 is consumed, 1 mole of H2O is produced.
Questions 39-40 pertain to the reaction represented by the following equation.
…Cu(s) +…NO3–(aq) +…H+(aq) →…Cu2+(aq) +…NO2(g) +…H2O(l)
39. When the equation above is balanced with lowest whole number coefficients, the coefficient for H+(aq) will be
(A) 1
(B) 2
(C) 3
(D) 4
(E) 5
40. Which of the following takes place during the reaction above?
(A) Cu(s) is oxidized.
(B) Cu(s) is reduced.
(C) H+(aq) is oxidized.
(D) H+(aq) is reduced.
(E) NO3–(aq) is oxidized.
41. Which of the following could be the molecular formula for a molecule with an empirical formula of CH2 ?
(A) CH
(B) CH4
(C) C2H2
(D) C2H6
(E) C3H6
42. When CO2 is bubbled through distilled water at 25°C, which of the following is most likely to occur?
(A) Solid carbon will precipitate.
(B) An electrical current will be produced in an oxidation-reduction reaction.
(C) The pH of the solution will be reduced.
(D) The water will boil.
(E) Methane (CH4) gas will be formed.
43. In which of the following processes is entropy increasing?
(A) N2(g) + 3Cl2(g) → 2NCl3(g)
(B) H2O(g) → H2O(l)
(C) 2H2O(l) → 2H2(g) + O2(g)
(D) CO(g) + 2H2(g) → CH3OH(l)
(E) 2NO2(g) → N2O4(g)
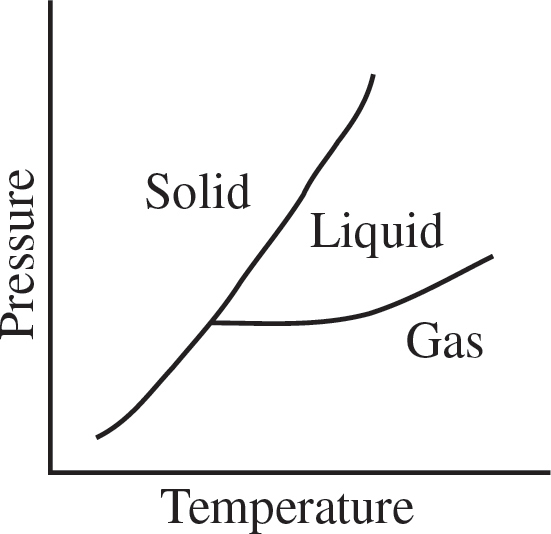
44. Based on the phase diagram above, which series of phase changes could take place as pressure is decreased at a constant temperature?
(A) Solid to liquid to gas
(B) Solid to gas to liquid
(C) Gas to liquid to solid
(D) Gas to solid to liquid
(E) Liquid to gas to solid
45. Which of the following forms of radioactive decay has (have) no electrical charge?
I. Alpha decay
II. Beta decay
III. Gamma decay
(A) II only
(B) III only
(C) I and II only
(D) I and III only
(E) II and III only
46. Based on the solubility products given below, which of the following salts is the most soluble?
(A) BaCO3 Ksp = 5.1 × 10–9
(B) PbCrO4 Ksp = 2.8 × 10–13
(C) AgCl Ksp = 1.8 × 10–10
(D) CaSO4 Ksp = 9.1 × 10–6
(E) ZnC2O4 Ksp = 2.7 × 10–8
HCN(aq) → H+(aq) + CN–(aq)
47. Hydrocyanic acid dissociates according to the reaction given above. Which of the following expressions is equal to the acid dissociation constant for HCN?
(A) [H+][CN−]
(B) [H+][CN−][HCN]
(C) 
(D) 
(E) 
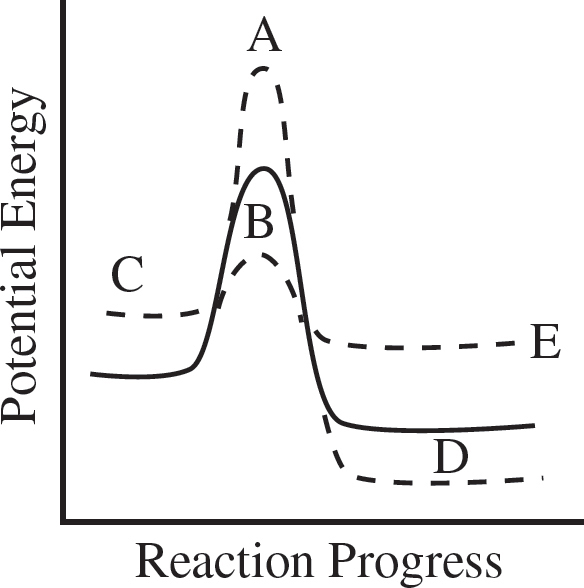
48. The reaction progress diagram of an uncatalyzed reaction is shown by the solid line. Which dotted line presents the same reaction in the presence of a catalyst?
49. In a hydrogen atom, when an electron jumps from an excited energy state to a more stable energy state,
(A) electromagnetic radiation is emitted by the atom
(B) electromagnetic radiation is absorbed by the atom
(C) the atom becomes a positively charged ion
(D) the atom becomes a negatively charged ion
(E) the atom undergoes nuclear decay
Questions 50-52 pertain to the following situation.
A closed 5-liter vessel contains a sample of neon gas. The temperature inside the container is 25°C, and the pressure is 1.5 atmospheres. (The gas constant, R, is equal to 0.08 L•atm/mol•K.)
50. Which of the following expressions is equal to the molar quantity of gas in the sample?
(A) 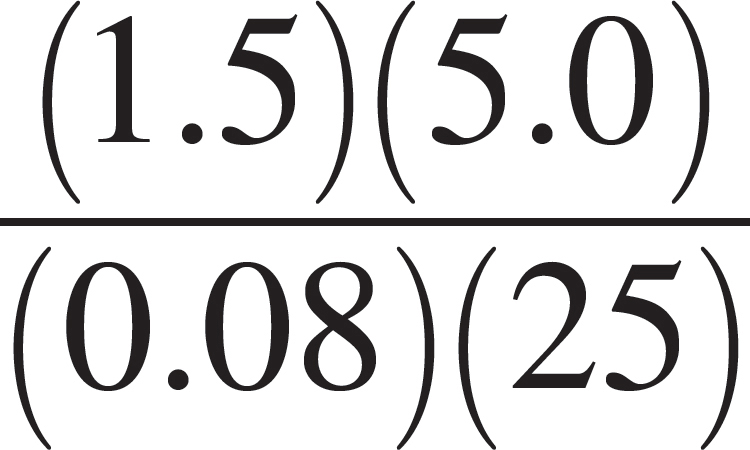 moles
moles
(B) 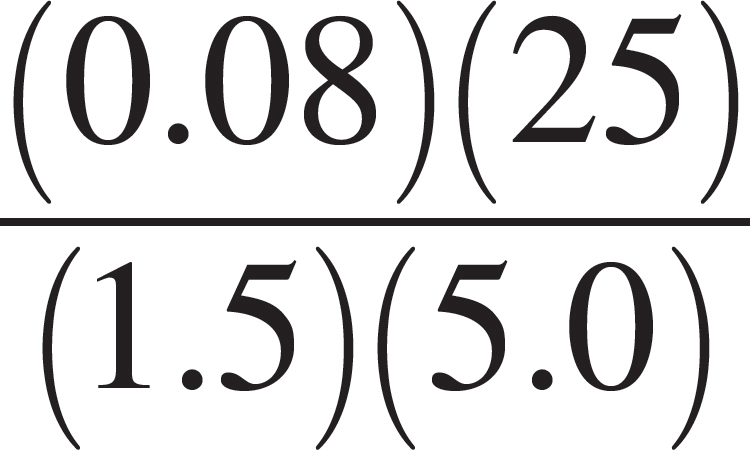 moles
moles
(C) 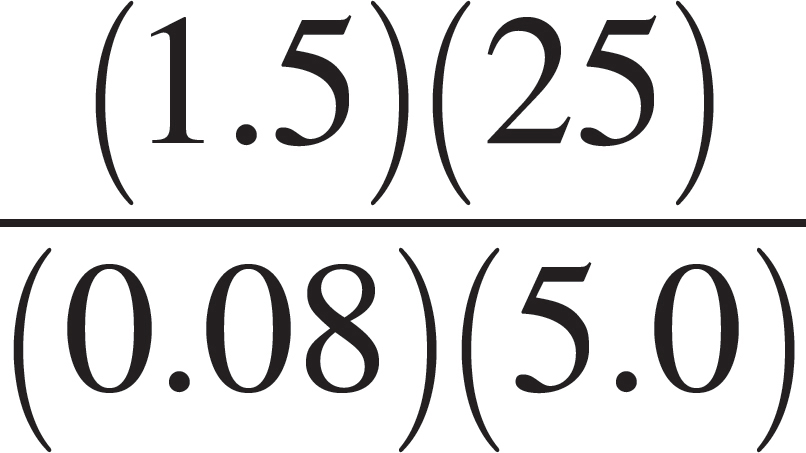 moles
moles
(D) 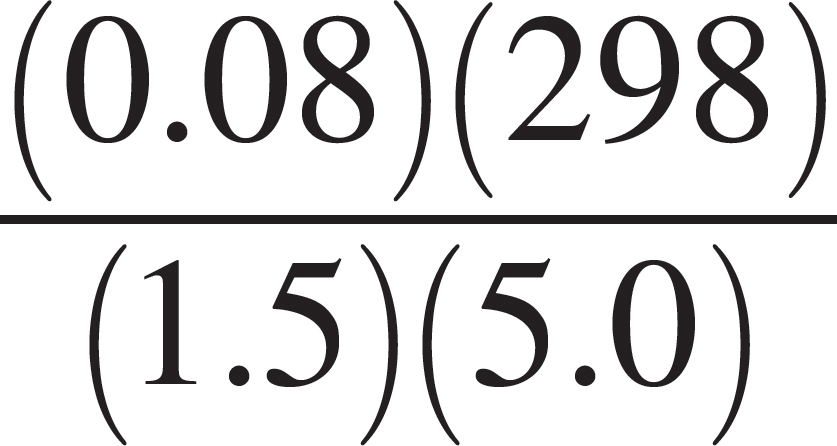 moles
moles
(E) 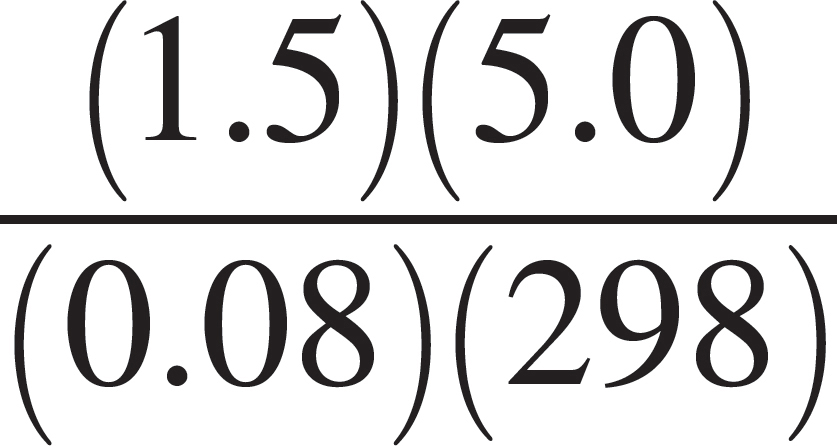 moles
moles
51. If the neon gas in the vessel is replaced with an equal molar quantity of helium gas, which of the following properties of the gas in the container will be changed?
I. Pressure
II. Temperature
III. Density
(A) I only
(B) II only
(C) III only
(D) I and II only
(E) II and III only
52. The volume of the vessel was gradually changed while temperature was held constant until the pressure was measured at 1.6 atmospheres. Which of the following expressions is equal to the new volume?
(A) 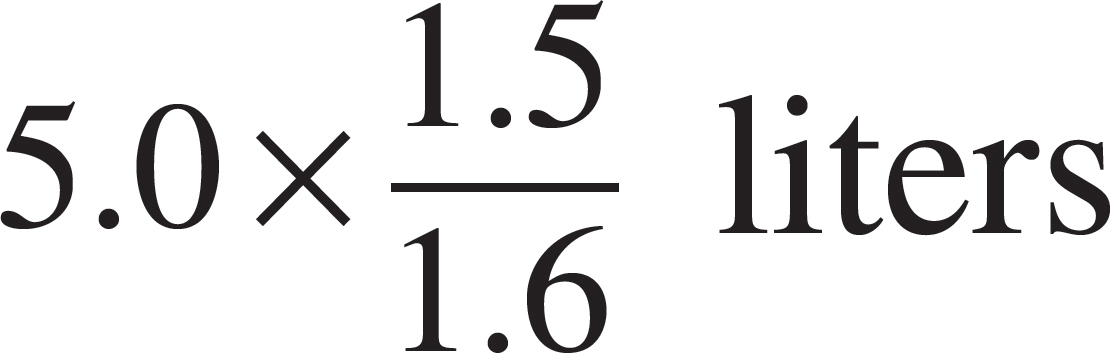
(B) 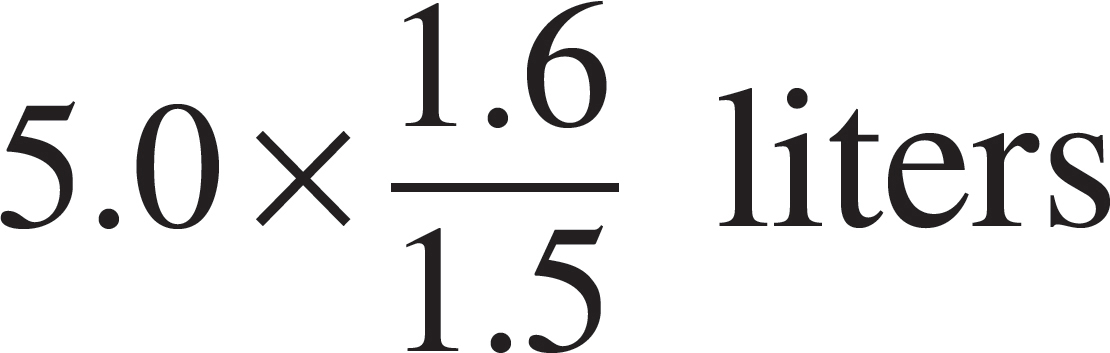
(C) 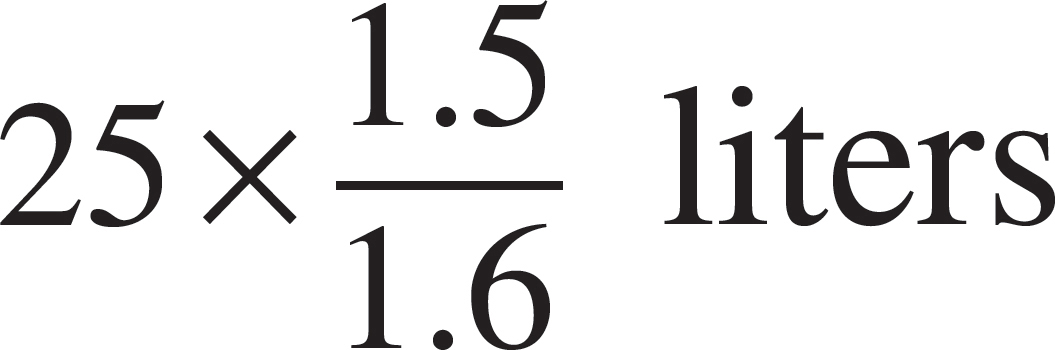
(D) 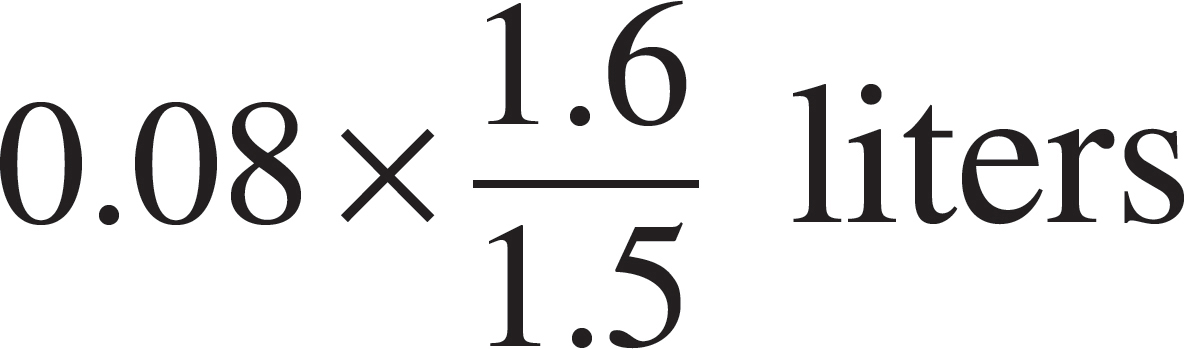
(E) 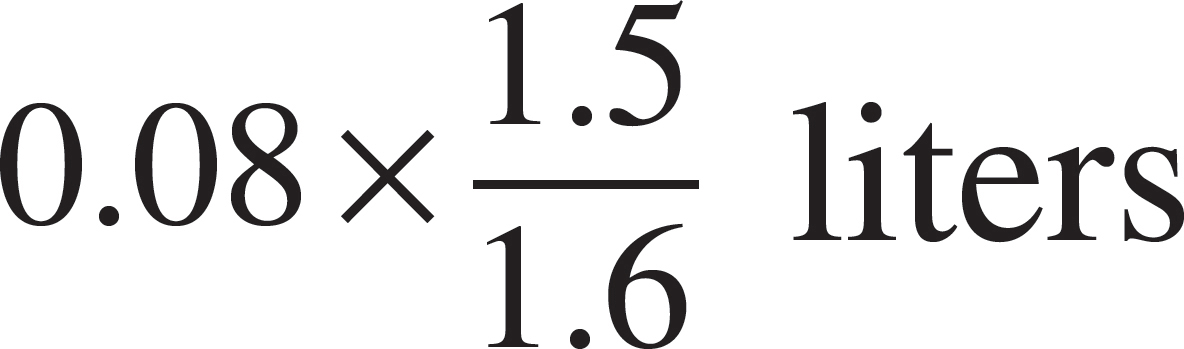
53. Which of the following list of atoms is ranked from greatest to least in terms of chemical reactivity?
(A) Ne > S > Co > Fr
(B) Fr > S > Co > Ne
(C) Co > Fr > S > Ne
(D) S > Ne > Fr > Co
(E) Fr > Co > Ne > S
54. A solution containing which of the following pairs of species could be a buffer?
(A) H+ and Cl–
(B) H2CO3 and HCO3–
(C) Na+ and NO3–
(D) Na+ and OH–
(E) HNO3 and NO3–
55. Which of the following species is the conjugate acid of ammonia (NH3) ?
(A) N2
(B) H2
(C) NH2–
(D) NH2–
(E) NH4+
56. A solution of H2SO3 is found to have a hydrogen ion concentration of 1 × 10–3 molar at 25°C. What is the hydroxide ion concentration in the solution?
(A) 1 × 10–13 molar
(B) 1 × 10–11 molar
(C) 1 × 10–7 molar
(D) 1 × 10–4 molar
(E) 1 × 10–3 molar
57. Which of the following expressions is equal to the number of iron (Fe) atoms present in a pure sample of solid iron with a mass of 10 grams? (The atomic mass of iron is 55.9.)
(A) (10.0)(55.9)(6.02 × 1023) atoms
(B) 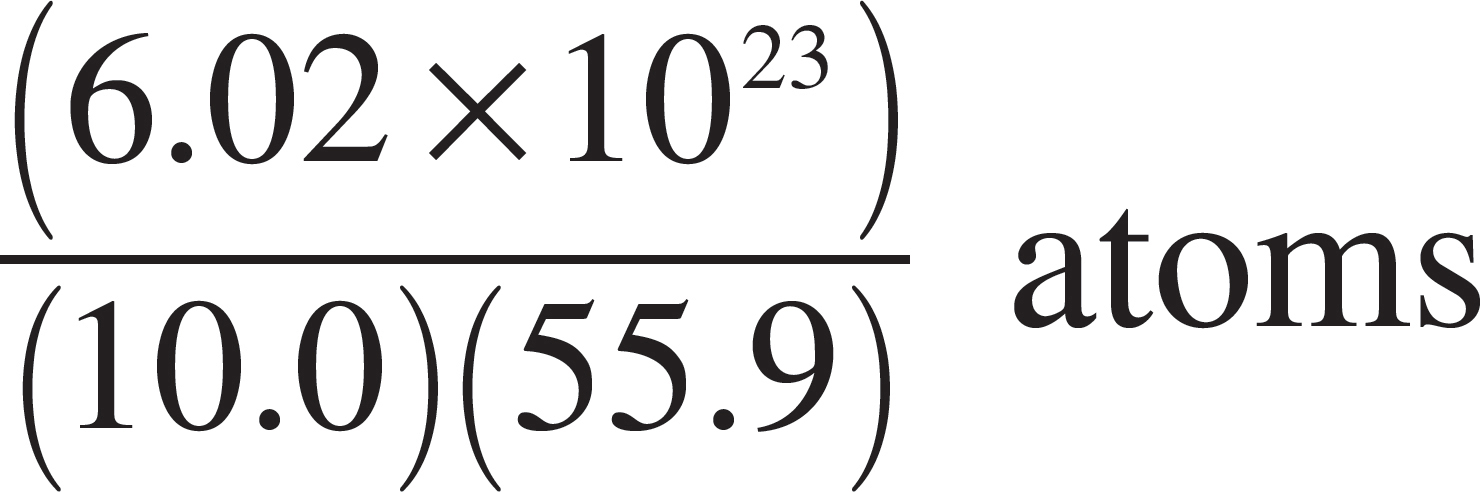
(C) 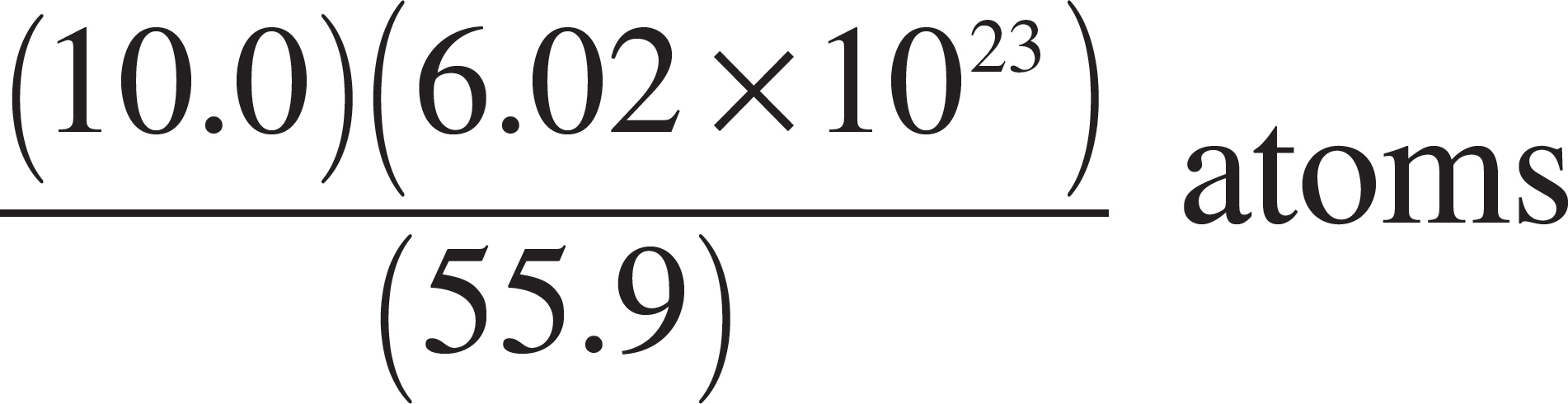
(D) 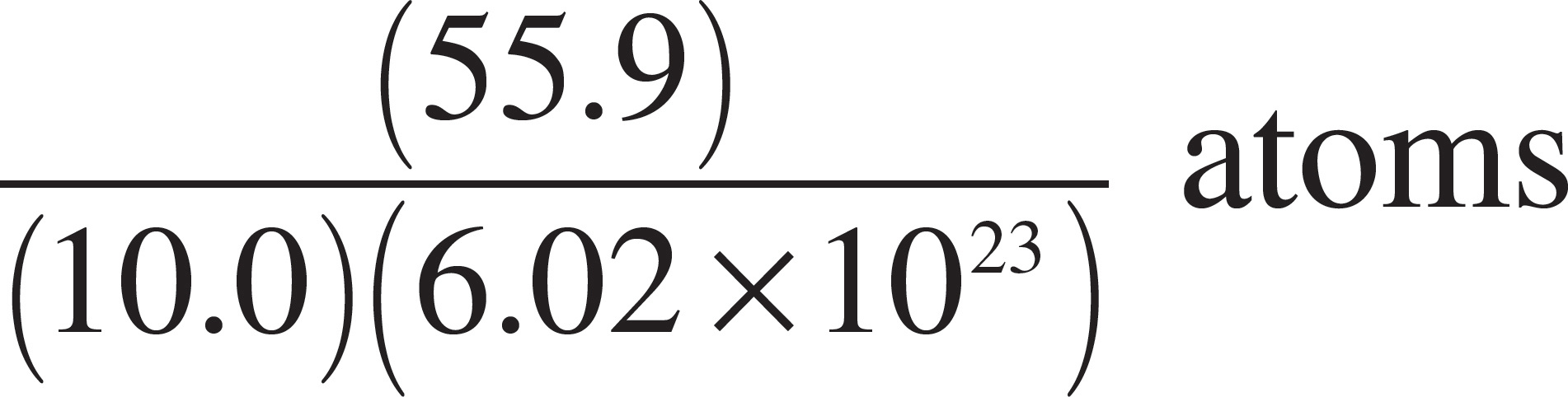
(E) 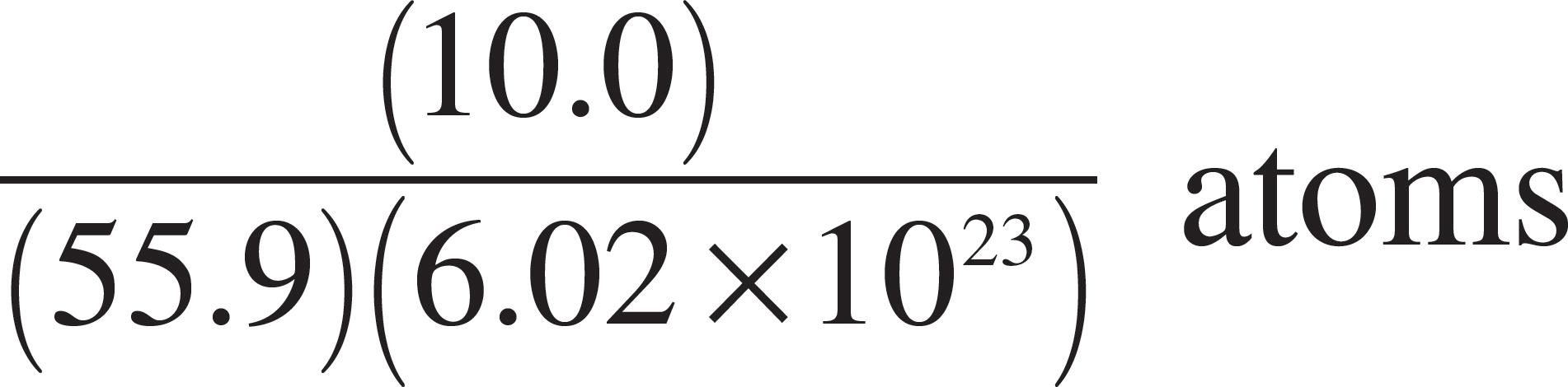
58. A radioactive material is undergoing nuclear decay. After 40 minutes, 25 percent of the sample remains. What is the half-life of the sample?
(A) 10 minutes
(B) 20 minutes
(C) 40 minutes
(D) 80 minutes
(E) 160 minutes
| Element | First Ionization Energy (kJ/mol) |
| Lithium | 520 |
| Sodium | 496 |
| Rubidium | 403 |
| Cesium | 376 |
59. Based on the table above, which of the following is most likely to be the first ionization energy for potassium?
(A) 536 kJ/mol
(B) 504 kJ/mol
(C) 419 kJ/mol
(D) 391 kJ/mol
(E) 358 kJ/mol
Questions 60–62 pertain to the reaction represented by the following equation.
2NOCl(g) 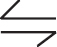 2NO(g) + Cl2(g)
2NO(g) + Cl2(g)
60. Which of the following expressions gives the equilibrium constant for the reaction above?
(A) 
(B) 
(C) 
(D) 
(E) 
61. Which of the following changes to the equilibrium above would serve to decrease the concentration of Cl2 ?
I. The addition of NOCl(g) to the reaction vessel
II. The addition of NO(g) to the reaction vessel
III. A decrease in the volume of the reaction vessel
(A) I only
(B) II only
(C) I and II only
(D) I and III only
(E) II and III only
62. Which of the following is true of the reaction above as it proceeds in the forward direction?
(A) NO(g) is produced at the same rate that NOCl(g) is consumed.
(B) NO(g) is produced at half the rate that NOCl(g) is consumed.
(C) NO(g) is produced at twice the rate that NOCl(g) is consumed.
(D) Cl2(g) is produced at the same rate that NOCl(g) is consumed.
(E) Cl2(g) is produced at twice the rate that NOCl(g) is consumed.
63. Which of the following is an organic molecule?
(A) SiO2
(B) NH3
(C) H2O
(D) CH4
(E) BeF2
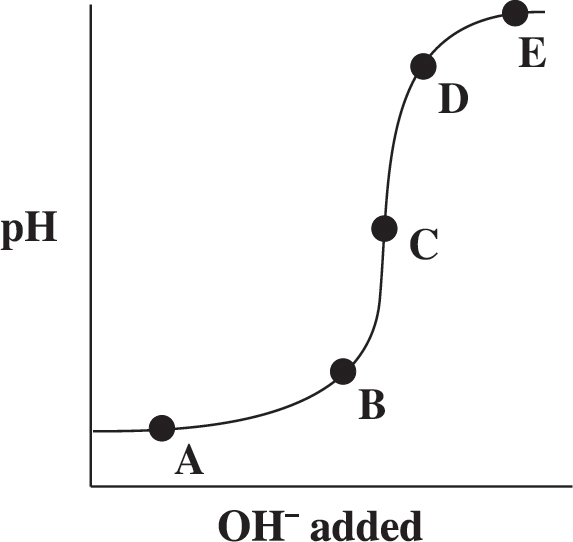
64. The graph above represents the titration of a strong acid with a strong base. Which of the points shown on the graph indicates the equivalence point in the titration?
(A) A
(B) B
(C) C
(D) D
(E) E
65. Which of the following statements about fluorine is NOT true?
(A) It is the most electronegative element.
(B) It contains 19 protons in its nucleus.
(C) Its compounds can engage in hydrogen bonding.
(D) It takes the oxidation state –1.
(E) It is found in nature as a diatomic gas.
66. The reactivity and chemical behavior of an atom is governed by many factors. The most important factor is
(A) the number of protons in the atom’s nucleus
(B) the number of neutrons in the atom’s nucleus
(C) the number of protons and neutrons in the atom’s nucleus
(D) the ratio of protons to neutrons in the atom’s nucleus
(E) the number of electrons in the atom’s valence shell
67. A beaker contains a saturated solution of copper(I) chloride, a slightly soluble salt with a solubility product of 1.2 × 10–6. The addition of which of the salts listed below to the solution would cause the precipitation of copper(I) chloride?
(A) Sodium chloride
(B) Potassium bromide
(C) Silver(I) nitrate
(D) Lead(II) acetate
(E) Magnesium iodide
68. Bromothymol blue is an acid/base indicator with a pKa of 6.8. Therefore, at approximately what pH will bromothymol blue undergo a color change during an acid/base titration?
(A) 1
(B) 3
(C) 5
(D) 7
(E) 13
69. Which of the following is necessarily true of a nonionic substance with a high boiling point?
(A) It has a large vapor pressure.
(B) It has strong intermolecular attractive forces.
(C) It has a low freezing point.
(D) It has a low heat of vaporization.
(E) It will be present in gas phase at very low temperatures.
70. Which of the following substances would have the highest melting point?
(A) CF4
(B) BaS
(C) MgCl2
(D) CaO
(E) NH3