Chapter 17
Practice Test 3
Click here to download a PDF of Practice Test 3.
PRACTICE SAT SUBJECT TEST IN CHEMISTRY–TEST 3
You are about to take the third practice SAT Subject Test in Chemistry. The bubble sheet can be found near the back of the book; feel free to tear it out for use. (Just don’t lose it!)
After answering questions 1–23, which constitute Part A, you’ll be directed to answer questions 101–116, which constitute Part B. Then, begin again at question 24. Questions 24–70 constitute Part C.
When you’re ready to score yourself, refer to the answer key and scoring instructions on this page and this page. Full explanations regarding the correct answers to all questions start on this page.
SAT SUBJECT TEST IN CHEMISTRY
MATERIAL IN THE FOLLOWING TABLE MAY BE USEFUL IN ANSWERING THE QUESTIONS IN THIS EXAMINATION.

Note: For all questions involving solutions and/or chemical equations, assume that the system is in pure water unless otherwise stated.
Part A
Directions: Each set of lettered choices below refers to the numbered statements or questions immediately following it. Select the one lettered choice that best fits each statement or answers each question, and then fill in the corresponding oval on the answer sheet. A choice may be used once, more than once, or not at all in each set.
Questions 1-4 refer to the following.
(A) Carbon
(B) Nitrogen
(C) Oxygen
(D) Neon
(E) Argon
1. Is the third most abundant gas in Earth’s atmosphere
2. At standard conditions, has an allotrophic form that is a good electrical conductor
3. The key element delivered in soil fertilizer
4. Allotrope of this element is the primary absorber of UV solar radiation in Earth’s atmosphere
Questions 5-8 refer to the following.
(A) Standard voltaic potential
(B) Entropy
(C) Enthalpy
(D) Reaction rate
(E) Gibbs free energy
5. Increased with the addition of a catalyst
6. A property that must decrease when a gas condenses into a liquid
7. Is always positive for a spontaneous chemical reaction
8. Is zero for a crystalline solid that is elementally pure at 0 K
Questions 9-12 refer to the following.
(A) Alkali metals
(B) Alkaline earth metals
(C) Noble gases
(D) Halogens
(E) Transition metals
9. The most unreactive family of elements
10. Form negative ions in an ionic bond
11. Consist of atoms that have valence electrons in a d subshell
12. Members possess the lowest first ionization energy in their respective period
Questions 13-16 refer to the following.
(A) O2
(B) KI
(C) CCl4
(D) AgNO3
(E) CaCO3
13. A product of a neutralization of a strong acid with a strong base
14. A volatile covalent liquid at 25°C and 1 atm
15. Releases a gas with the addition of dilute acid
16. Is a strong oxidizing agent
Questions 17-19 refer to the following.
(A) Gamma decay
(B) Nuclear fusion
(C) Alpha decay
(D) Positron emission
(E) Nuclear fission
17. Is the principle reaction responsible for the energy output of the Sun
18. Is a nuclear process that results in no change in the mass number and atomic number of a nuclide
19. The nuclear process that transmutes uranium-238 into thorium-234
Questions 20-23 refer to the following.
(A) 0.1 M MgCl2
(B) 0.1 M HClO4
(C) 0.1 M NH4OH
(D) 0.1 M KOH
(E) 0.1 M LiNO3
20. Has a pH of 13
21. The solution with the lowest freezing point temperature
22. The solution with the highest boiling point temperature
23. Indicates a red flame when ionized with a Bunsen burner
PLEASE GO TO THE SPECIAL SECTION LABELED CHEMISTRY AT THE LOWER RIGHT-HAND CORNER OF THE ANSWER SHEET YOU ARE WORKING ON AND ANSWER QUESTIONS 101-116 ACCORDING TO THE FOLLOWING DIRECTIONS.
Part B
Directions: Each question below consists of two statements, I in the left-hand column and II in the right-hand column. For each question, determine whether statement I is true or false and whether statement II is true or false, and fill in the corresponding T or F ovals on your answer sheet. Fill in oval CE only if statement II is a correct explanation of statement I.
|
EXAMPLES: |
||
|
I |
II |
|
|
EX 1.H2SO4 is a strong acid |
BECAUSE | H2SO4 contains sulfur |
|
EX 2. An atom of oxygen is electrically neutral |
BECAUSE | an oxygen atom contains an equal number of protons and electrons. |
|
SAMPLE ANSWERS |
||
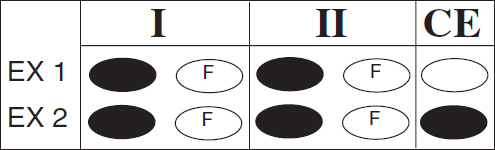
|
||
|
I |
II |
|
|
101. Transition metal compounds are often colored |
BECAUSE | they frequently possess partially filled d orbitals. |
|
102. Chemical reactions slow down with lower temperature |
BECAUSE | the energy barrier for the formation of products decreases with decreasing temperature. |
|
103. Exothermic reactions absorb heat |
BECAUSE | breaking covalent bonds always requires energy. |
|
104. The solubility of gases in liquids does not depend upon pressure |
BECAUSE | the vapor pressure of a substance is independent of external pressure. |
|
105. MgO has a high melting point |
BECAUSE | highly charged ions result in strong ionic forces and high lattice energies. |
|
106. The ground state electron configuration orbitals of elemental Cu is [Ar] 4s13d10 |
BECAUSE | completely half-filled and filled d bestow special electronic stabilization. |
|
107. Isotopes of a particular element have nearly identical chemical behavior |
BECAUSE | they have identical electron configurations. |
108. The addition of acid to a solution buffered to pH 7 slightly lowers the pH |
BECAUSE | the addition of acids to any neutral solution always lowers the pH. |
|
109. Saltwater boils at a higher temperature than pure water |
BECAUSE | the presence of salt increases the vapor pressure of water. |
|
110. BF3 has a tetrahedral geometry |
BECAUSE | the central B atom does not have a complete stable octet. |
|
111. Hydrogen peroxide, H2O2, is a good oxidizing agent |
BECAUSE | the hydrogen in H2O2 has a +1 oxidation number. |
|
112. Hydrogen gas (H2) is considered a perfectly ideal gas |
BECAUSE | hydrogen atoms interact with each other via hydrogen bonds. |
|
113. Gas particles have a wider range of velocity distributions as temperature increases |
BECAUSE | the velocity for all of the particles in the gas will increase. |
|
114. By mass, oxygen is the most abundant element in the human body |
BECAUSE | it is principally found as O2 in the bloodstream. |
|
115. LiOH is considered a strong base |
BECAUSE | it undergoes neutralization reactions with acids. |
RETURN TO THE SECTION OF YOUR ANSWER SHEET YOU STARTED FOR CHEMISTRY AND ANSWER QUESTIONS 24–70.
Part C
Directions: Each of the questions or incomplete statements below is followed by five suggested answers or completions. Select the one that is best in each case and then fill in the corresponding oval on the answer sheet.
24. Given the following scientists and their contributions to atomic theory, which of the following concepts do we now recognize as incorrect?
(A) Rutherford’s ideas about all atoms having an extremely dense nucleus
(B) Thomson’s estimates for the mass and charge of an electron
(C) Bohr’s hypothesis that electrons orbit the nucleus at fixed distances
(D) Planck’s theory that all electromagnetic energy is quantized
(E) De Broglie’s postulate that electrons behave like electromagnetic radiation
25. Choose the answer below that accurately describes the correct molecular shape for the molecule XeOF4.
(A) Tetrahedral
(B) Trigonal pyramidal
(C) Trigonal bipyramidal
(D) Square pyramidal
(E) Flat
26. For the radioactive atom 99Tc, what is the correct number of protons and neutrons?
(A) 43 protons and 56 neutrons
(B) 43 protons and 99 neutrons
(C) 56 protons and 43 neutrons
(D) 56 protons and 99 neutrons
(E) Cannot be determined
27. 4Fe + 3O2 → 2Fe2O3
4.0 moles of iron react with 4.0 moles of oxygen to create iron(III) oxide via the reaction above. If 1.5 moles of Fe2O3 are created, what is the percent yield for the reaction?
(A) 20%
(B) 33%
(C) 50%
(D) 75%
(E) 100%
28. Which one of the following acids is NOT strong?
(A) HCl
(B) HBr
(C) HNO3
(D) H3PO4
(E) H2SO4
29. Identify the equation used to determine the amount of heat required to melt 10 grams of ice.
(A) Q = mCspΔT
(B) Q = nΔH
(C) KE =  mv2
mv2
(D) PE = mgh
(E) PV = nRT
30. Which of the following options is a viable set of quantum numbers for an electron in a 3d orbital?
(A) (3, 2, –1,  )
)
(B) (3, 1, 0, – )
)
(C) (3, 2, 3,  )
)
(D) (2, 2, 1,  )
)
(E) (4, 1, 0, – )
)
31. Identify the correct ground state electron configuration for Cr.
(A) [Ar] 3s23d4
(B) [Ar] 3s23d5
(C) [Ar] 4s23d5
(D) [Ar] 4s23d4
(E) [Ar] 4s13d5
32. What is the hydroxide concentration for a solution with a pH of 10 at 25ºC?
(A) 10−14 M
(B) 10−10 M
(C) 10−7 M
(D) 10−4 M
(E) 10−1 M
33. The correct name of the compound CrCO3 is:
(A) chromium carbonate
(B) chromium(I) carbonate
(C) chromium(II) carbonate
(D) chromium(III) carbonate
(E) chromium(IV) carbonate
34. Five hundred milliliters of solution of 0.1 M NaBr has how many milligrams of bromine?
(A) 200 mg
(B) 400 mg
(C) 2,000 mg
(D) 4,000 mg
(E) 20,000 mg
35. According to the ideal gas law, what is the approximate volume that will be occupied by 0.5 mole of an ideal gas at 30°C and 3 atm pressure (gas constant R = 0.0821 L•atm/mol•K)?
(A) Less than 1 L
(B) 5 L
(C) 10 L
(D) 15 L
(E) More than 20 L
36. The correct formula for the compound diphosphorus tetroxide would be:
(A) PO4
(B) P5O2
(C) P2O5
(D) P4O7
(E) P2O4
37. Given that ΔG = ΔH – TΔS, how is the spontaneity of an endothermic reaction expected to change with decreasing T ?
(A) Becomes less spontaneous
(B) Becomes more spontaneous
(C) Does not change
(D) Decreases at first but then increases
(E) Insufficient information to make a conclusion
38. Identify the element with the greatest first ionization energy.
(A) Ce
(B) C
(C) Cl
(D) Ca
(E) Cs
39. Which of the following correctly lists the strength of the three primary types of intermolecular forces in compounds of similar size from strongest to weakest?
(A) London dispersion > H-bonding > Permanent dipoles
(B) London dispersion > Permanent dipoles > H-bonding
(C) Permanent dipoles > H-bonding > London dispersion
(D) H-bonding > Permanent dipoles > London dispersion
(E) H-bonding > London dispersion > Permanent dipoles
40. When a sample of liquid H2S boils, which of the following is the strongest type of force that is being neutralized?
(A) Hydrogen bonding
(B) Permanent dipoles
(C) London dispersion forces
(D) Covalent bonds
(E) Ionic bonds
2Ca3(PO4)2 + 6SiO2 + 10C → P4 +…CaSiO3 + 10CO
41. Which coefficient balances the reaction given above?
(A) 2
(B) 4
(C) 5
(D) 6
(E) 8
42. What is the percent by mass of NaCl in a solution created when 10.0 g of NaCl is dissolved in 40.0 mL of water?
(A) 10%
(B) 20%
(C) 25%
(D) 33%
(E) 40%
43. A 100-milliliter solution containing AgNO3 was treated with excess NaCl to completely precipitate the silver as AgCl. If 5.7 g AgCl was obtained, what was the concentration of Ag+ in the original solution?
(A) 0.03 M
(B) 0.05 M
(C) 0.12 M
(D) 0.30 M
(E) 0.40 M
44. Identify which of the following statements is FALSE.
(A) The vapor pressure of a liquid decreases with increasing atmospheric pressure.
(B) The value of an equilibrium constant is dependent on temperature.
(C) The rate of a spontaneous reaction cannot be determined solely by its Gibbs free energy.
(D) During a phase transition, the temperature of a substance must be constant.
(E) The addition of a catalyst to a reaction at equilibrium has no net effect on the system.
45. In which of the following gas laws does the temperature NOT have to be measured in Kelvins?
(A) Charles’ Law
(B) Boyle’s Law
(C) Gay-Lussac’s Law
(D) Combined Gas Law
(E) Ideal Gas Law
46. Which of the following compounds would be expected to have the greatest lattice binding energy?
(A) LiNO3
(B) LiF
(C) KI
(D) NH4Br
(E) CsNO3
47. The daughter nucleus formed when 18F undergoes positron emission is
(A) 14N
(B) 16O
(C) 18O
(D) 19F
(E) 20Ne
48. Which of the following elements would be the strongest reducing agent?
(A) Fluorine
(B) Nickel
(C) Xenon
(D) Phosphorus
(E) Sodium
49. Which of the following reactions produces a yellow precipitate?
(A) NaOH(aq) + HCl(aq) → NaCl(s) + H2O
(B) NaOH(aq) + BaCl(aq) → BaOH(s) + NaCl(aq)
(C) Pb(NO3)2(aq) + 2KI(aq) → 2KNO3(aq) + PbI2(s)
(D) CuO(s) + Mg(s) → Cu(s) + MgO(s)
(E) 4Fe + 3O2 → 2Fe2O3
Zn(s)|ZnCl2(aq)||Cl–(aq)|Cl2(g)|C(s)
50. When mixed with water, which of the following acids will react and cause the formation of bubbles?
(A) H2CO3
(B) H2SO4
(C) HF
(D) HC2H3O2
(E) HCl
51. Given the reaction A → B + C, where ΔHrxn is negative, what effect would increasing the temperature (at constant pressure) have on the system at equilibrium?
(A) No change
(B) Cannot be determined
(C) Shift to the right
(D) Shift to the left for K < 1 and to the right for K >1
(E) Shift to the left
52. An unknown acid solution was presumed to be either HCl or H2SO4. Which one of the following salt solutions would produce a precipitate when added to H2SO4 but not when added to HCl?
(A) LiNO3
(B) NH4NO3
(C) CsNO3
(D) Ba(NO3)2
(E) AgNO3
Ca3(PO4)2(s) ⇋ 3Ca2+(aq) + 2PO43-(aq)
53. What is the equilibrium expression for the dissolution of Ca3(PO4)2 where the above is true?
(A) Ksp = [Ca2+]3[PO43-]2
(B) Ksp = [Ca2+]2[PO43-]3
(C) Ksp = [Ca2+][PO43-]/[Ca3(PO4)2]
(D) Ksp = [Ca2+]3[PO43-]2/[Ca3(PO4)2]
(E) Ksp = [Ca2+]2[PO43-]3/[Ca3(PO4)2]
54. Which of the following represents a conjugate acid/base pair?
(A) Na+/Cl–
(B) HCl/H+
(C) H2CO3/CO32-
(D) NH3/NH4+
(E) K+/OH–
55. An unknown solution having a pH of 3.5 was titrated with 0.1 M NaOH. Analysis of the resulting titration curve showed a single equivalence point at pH 7. Therefore, which of the following could be the unknown solute in the initial solution?
(A) HF
(B) HCl
(C) LiOH
(D) NH3
(E) H2SO4
56. Acid/base titration experiments could be used to determine all of the following directly EXCEPT
(A) the acid concentration of an acidic solution
(B) the alkalinity of a basic solution
(C) the pKa of an unknown weak acid
(D) whether an unknown acid is monoprotic or polyprotic
(E) the molecular weight of an unknown acid or base
57. What is the correct term for the phase change from gas directly to solid?
(A) Deposition
(B) Sublimation
(C) Liquefaction
(D) Fusion
(E) Vaporization
58. What is the correct name for a straight-chained organic compound with the molecular formula C3H8?
(A) Methane
(B) Ethane
(C) Methylethane
(D) Propane
(E) Isopropane
59. If the pH of a solution is changed from 1 to 3 with the addition of an antacid, what percentage of [H+] was neutralized?
(A) 2%
(B) 10%
(C) 20%
(D) 90%
(E) 99%
60. Which of the following statements is the most accurate with regard to the significance of Avogadro’s number, 6.02 × 1023?
(A) It is the conversion factor between grams and atomic mass units.
(B) It is a universal physical constant just as the speed of light.
(C) It is the number of particles that is required to fill a 1-liter container.
(D) It is the inverse diameter of an H atom.
(E) It is the number of electrons in the universe.
Questions 61–64 refer to the following data at standard conditions.

61. Unknown metal #1 could be
(A) mercury
(B) copper
(C) zinc
(D) iron
(E) silver
62. Unknown metal #2 could be
(A) carbon
(B) copper
(C) zinc
(D) sodium
(E) silver
63. The addition of dilute HCl to unknown metal #1 produced a transparent gas. What is the likely identity of this gas?
(A) Cl2
(B) H2
(C) O2
(D) CO2
(E) NO2
64. The addition of dilute HNO3 to unknown metal #2 produced an orange gas. What is the likely identity of this gas?
(A) Cl2
(B) H2
(C) O2
(D) CO2
(E) NO2
65. Which of the following solutions is the product of the neutralization reaction between 10 ml 0.2 M KOH and 10 ml 0.2 M HI?
(A) 0.1 M KI3
(B) 0.1 M KI
(C) 0.2 M KI
(D) 0.4 M KI
(E) 0.4 M HOH
66. Which of the following is true regarding an Ne atom with a mass number of 20 and an O2– ion with a mass number of 16?
(A) They contain the same number of protons.
(B) They contain the same number of neutrons.
(C) They contain the same number of protons plus neutrons.
(D) They are isoelectronic.
(E) They are isomers.
67. Which of the following statements is NOT correct regarding chemical catalysts?
(A) They are not consumed during the chemical reaction.
(B) They cannot make nonspontaneous reactions occur.
(C) They do not have to be the same phase as the reactant molecules.
(D) They shift equilibrated reactions to the product’s side.
(E) Enzymes are biological catalysts.
68. Most elements are solids at 25°C and 1 atm pressure, the exception being the 11 elements that are gases and 2 that are liquids. What 2 elements are liquids?
(A) Hg and Br
(B) Hg and I
(C) Ag and Kr
(D) Au and Kr
(E) Pt and Co
69. A student conducted an experiment and obtained three values during three repetitive trials: 1.65, 1.68, 1.71. Later, the student discovered that the true value was 2.37. In contrast to the real value, the experimental results should be characterized as
(A) not accurate and not precise
(B) accurate but not precise
(C) not accurate but precise
(D) accurate and precise
(E) accurate, precise, but unreliable
70. Sulfurous acid, H2SO3, is a weak diprotic acid. In a solution of H2SO3, which of the following species would be present in the lowest concentration?
(A) H2SO3
(B) HSO3–
(C) SO32–
(D) H+
(E) H2O
STOP
If you finish before time is called, you may check your work on this section only. Do not turn to any other section in the test.