Piperidine, the “enchanted ring”
Big Pharma’s search for the holy grail of pain control—a drug with all the power of opiates but none of the addiction—has led us not to perfect pain control, but to the some of the highest levels of addiction and the worst epidemic of overdoses in American history.
The difference is that we’ve now moved from natural opiates—based on the sap of the poppy—to entirely new, totally synthetic substances, made to order in laboratories. These newer drugs (which fall under the larger heading of opioids rather than poppy-based opiates) are far more powerful and potentially more addictive than any of the opiates used by our great-grandparents. Designed in part to help cure opiate addiction, they’ve only made the problem worse.
The first was discovered, once again, in Germany, at the Hoechst labs in the late 1930s, just before World War II. The company wasn’t looking for it. It was found, once again, by accident. And the reason was the tail of a mouse.
Instead of a painkiller, the Hoechst chemists were looking for a drug to ease muscle spasms. Their starting point was a family of molecules completely unlike opium. The chemists were deep into the usual grind, starting with a candidate molecule, then making variation after variation, testing each one on mice to see what happened. That was when one sharp-eyed researcher noticed something odd: The mice that got one of these experimental drugs were raising their tails in an S shape. Most scientists would have ignored it. But this particular researcher had done work with opium-related drugs, and he knew what mice did when they were high on opiates. They raised their tails in an S shape. If he hadn’t known better, he would have said this new drug was morphine.
So the Hoechst team did more tests. And it quickly became clear that they had discovered something entirely new: a powerful painkiller that did not resemble, in its molecular structure, morphine or codeine or any other alkaloid. True, this new drug wasn’t as strong as morphine, but it did provide significant pain relief. Instead of putting test animals into the usual opiate dreamy state, it seemed to jack them up, like cocaine. Most important—and here the Hoechst researchers probably crossed their fingers—early tests hinted that it might be far less addictive than morphine.
Maybe they had stumbled across the holy grail. They named it pethidine (in the United States, it’s better known as meperidine), did some quick tests on humans, deemed it good, and put it on the market in Germany. The ads said it was a powerful painkiller with fewer side effects than morphine and no risk of addiction.
Wrong, it turned out, on both counts. Pethidine—sold after the war under the trade name Demerol—has a slew of side effects, can be dangerous because of drug–drug interactions, and is anything but nonaddictive. It was attractive as a drug of abuse because it not only killed pain, it made users feel energized. Because of the combination of side effects and abuse potential—plus the appearance of newer painkillers—pethidine doesn’t get used much anymore.
But it opened the door to something new: the promise of molecules completely unlike morphine or heroin that just might, with a bit more work, be made nonaddictive. This had what one historian called “a tremendously stimulating effect on drug research.”
The years around World War II were a great time to be in the pharmaceutical business. New drugs were arriving at a record pace. There were a number of reasons for the flowering of big drug companies just after the war. The government had put a lot of money into medical research during the war, looking for better ways to treat wounds and prevent disease among soldiers, to understand how high altitudes affected fliers and high pressures affected submarine crews, how oxygen levels could be more accurately measured and blood plasma might be made in a laboratory. All that money helped scientists develop new tools and improved methods for testing and analyzing the human body. The victory over Germany yielded more riches for research, opening up laboratories, unveiling patents, and bringing German scientists to the United States. The post-war economic boom helped fund an enormous expansion of scientific research at universities and public laboratories, which in turn fueled further improvements in chemistry. Freed from wartime priorities and handsomely funded, drug science leaped forward.
Much of the excitement in medical research centered around molecular biology, the new ability to study life in finer and finer detail down to the level of individual molecules involved in digestion, say, or hormonal processes, or nerve conduction. This shift downward in focus, deeper and deeper into the workings of individual cells, was capped, in a way, in 1953, when the unlikely trio of a gawky American grad student named James Watson, a talkative young British researcher named Francis Crick, and research done by a talented female scientist named Rosalind Franklin, uncovered the molecular structure of DNA, opening up a new era of genetics research.
The more that was known about the molecules of life, the more opportunities came up for finding drugs that might have an effect. This built a sense of optimism that there might be a drug for every disease. All we had to do was understand the diseases well enough at the level of molecules, and then we could make the right drugs to treat them.
So first, there were powerful new tools; second, there was a growing understanding of the molecules of life; and third, there was a lot of money. With every successful new drug came another infusion of cash into the industry. Drug companies were growing fast. This private-sector growth was complemented after the war by a massive influx of funding in the United States from the federal government, which started funneling tens of millions of dollars into basic medical research through the new National Institutes of Health. Those drug companies that best understood the new dynamic—were most up-to-date on the latest findings, had the best lobbyists, and were most innovative in their in-house research—prospered. Smaller firms, those without the resources to compete, went belly up or were bought up.
Hoechst prospered. After pethidine, the firm made more variations of their synthetic painkiller through the war years. And after hundreds of failures, they finally found another effective painkiller—five times better than pethidine—that looked like it might be nonaddictive. They named it amidon. But this new drug, too, had downsides, particularly its tendency to cause nausea. It never got much use.
Until after World War II, when amidon made it to the United States and became better known under a new name: methadone.
It was a somewhat unusual opioid: a decent, but not great, painkiller; capable of being taken by mouth; slow to act, taking some time to get up to speed in the body; and less euphoria-inducing than other forms. Plus it made a lot of patients feel nauseated. Early U.S. tests seemed to confirm the German findings that it was nonaddictive. But as it went into wider use, it became clear that methadone patients, just like morphine patients, needed larger and larger doses to get relief, and many of them formed a dependence. In 1947 it was put on the U.S. list of controlled drugs.
Methadone never made much money as a painkiller. But there was something else: Because it was more unpleasant than euphoric, and because it could be taken without a syringe, physicians began playing around with the idea of using methadone as a way to get addicts off of heroin. Addicts didn’t like it much, but it did soothe some of the itch of withdrawal. By 1950, a few hospitals had started using it to treat heroin addiction.
Heroin had disappeared from American streets during WWII because opium supply lines were interrupted. The number of addicts in the United States dropped by 90 percent, from 200,000 before the war to around 20,000 in 1945. As Time magazine put it, “The war was probably the best thing that ever happened to drug addicts.”
But once the war was over, supply lines to Asia were reestablished (the most famous one running from Turkey through France to the United States—the “French Connection”) and heroin came back with a vengeance. In the 1950s, the drug moved from inner-city black neighborhoods to rich white suburbs, from jazz clubs to pool parties. Heroin was cool, hip, dangerous. And profitable. “Junk is the ideal product,” William S. Burroughs wrote in 1959, “the ultimate merchandise. No sales talk necessary. The client will crawl through a sewer and beg to buy.”
The bigger and whiter the heroin problem grew, the more the government became concerned. Tough-on-drugs types argued that the answers were harsher laws, zero tolerance, and more jail time, while many physicians and community activists made the case for detox and compassionate care. A 1963 President’s Advisory Commission on Narcotics and Drug Abuse split the difference, recommending both more treatment for addicts and tougher sentences for dealers. The emphasis was on getting junkies off the street and off the drug, into prison or in detox. Once they were clean, the thinking went, they could stay off the drug.
Except they didn’t. About three-quarters of heroin addicts, once they were out of detox and had access to the drug, relapsed within a few months. Serious heroin addiction is really, really hard to kick.
Then the drug-friendly sixties hit, and everything got worse. Between 1960 and 1970, the number of junkies in the United States shot from 50,000 to around 500,000.
That was when methadone made a comeback. While many doctors shied away from addiction treatment during the 1950s—remembering perhaps how doctors after the Harrison Act were jailed for prescribing morphine to treat addicts—a few were still treating addiction as a medical problem. U.S. Public Health Service hospitals, for instance, were steadfast in providing treatment. And it was there that an increasing number of doctors first tried methadone.
Replacing heroin with methadone offered several advantages: The synthetic drug lasted longer than morphine, so instead of shooting up four times a day, addicts got a single dose; there was no need for needles; and it often eased the physical craving for opiates without giving addicts the euphoric kick of heroin.
In 1963 a tough, thickset New York physician, Vincent Dole, won a grant to study drug treatments to fight heroin addiction. Just getting the grant wasn’t easy at the time because the drugs he wanted to study—morphine and methadone—were controlled. As a Federal Bureau of Narcotics agent told him, Dole was breaking drug laws just doing these studies, and if he persisted, they’d probably have to put him out of business. Dole didn’t back down, inviting the Feds to try and shut him down so he could sue them and get a proper court ruling.
Dole, his wife, the psychiatrist Marie Nyswander, and a newly minted young physician, Mary Jeanne Kreek, started their work. They quickly found that morphine didn’t work as a heroin substitute; the addicts simply wanted more morphine. That wasn’t true with methadone. First, the researchers could get patients on an effective dose, one that eased withdrawal and the craving for heroin, and then could keep them there. The addicts weren’t asking for more. And second, their methadone patients, unlike those on morphine, were not nodding out or sitting passively while they waited for their next dose. They were active and engaged. They might even be able to get a job.
Dole’s team tried slowly reducing the dose of methadone, seeing if they could wean their patients off the drug, get them completely clean. But it didn’t work. They could get the dose down to a certain point, but no lower. Hit that critical amount, and withdrawal symptoms would start.
The answer was to maintain patients on methadone for years—maybe for the rest of their lives. It was a trade-off, one drug for another. And methadone was the better choice. On methadone, addicts weren’t breaking the law to get money for a fix, weren’t shooting up with dirty needles, and weren’t overdosing. They could build a life.
In 1965, when Dole and Kreek first presented their results, heroin treatment entered a new era. The media picked up the story, inquiries from other doctors started coming in, and Methadone Maintenance Treatment (MMT) was touted as the answer to the heroin epidemic.
It was the Seige cycle all over again—wild enthusiasm followed by deep misgivings. Dole remembered the years from 1965 to 1970 as the honeymoon period. Physicians were clamoring to try MMT. Every big city wanted it. Not even the Bureau of Narcotics—which “carped, infiltrated, and attempted to discredit the program,” Dole said—could stop the momentum.
Then MMT became a victim of its own popularity. In the early 1970s, methadone treatment spread so far so fast that it got out of control. It got picked up by overeager centers and sometimes unqualified practitioners to the point where, as Dole put it, “Things became disorderly.” Too many programs were treating too many patients with too little oversight or discipline. In that atmosphere it quickly became clear that MMT was not a perfect answer. An anti-methadone reaction set in, not only from hardcore antidrug types, but from the addicts themselves. They didn’t like the nausea. They didn’t like the state control. Addicts even made up legends centering on the fact that methadone had been developed in Germany during the Nazi years; they nicknamed it “adolphine” and made up conspiracy theories about it. A lot of junkies refused to take methadone, and a lot of those who did relapsed, ending up back on heroin.
Then the sixties were over, and it was time to get tough on drugs again. Methadone treatment was put under increasing government oversight. Paperwork increased. Funding decreased. The emphasis shifted from indefinite maintenance to short-term control using methadone as a stepping-stone, a way to get addicts off their drug of choice and into other, perhaps curative, therapies: psychotherapy, behavioral therapy, twelve-step programs, prayer. The new goal was stopping drugs entirely, not doling them out for life. By the 1980s MMT was out of fashion. But more recently it’s made a comeback. Worries about AIDS transmission led to an appreciation of its role in reducing the use of dirty needles. Funding loosened up again. A National Institutes of Health consensus report in 1997 outlined the proven benefits: less overall drug use; less criminal activity; fewer needle-associated diseases; and an increase in gainful employment. The NIH panel recommended that all opiate-dependent persons under legal jurisdiction should have access to MMT, and the treatment is now FDA approved and growing in use. As one expert notes, “Today the safety, effectiveness, and value of properly applied MMT is no more controversial than is the assertion that the Earth is round.”
But nobody’s arguing that it’s perfect. Many addicts and their families still go into methadone treatment with the idea that they’re going to be “cured,” but more than half of methadone program graduates end up using opiates again after discharge, or go back into treatment to get more methadone—which, remember, is itself a synthetic opioid. Permanent success rates (if you define success as never taking an opioid again) hover around 10 percent or less.
And that is the hard reality for all of opium’s children. Once addiction has started, it is punishingly difficult to stop. It’s certainly true for heroin. And it’s proving true for synthetic opioids as well.
Demerol and methadone were just the start. In the 1950s, one of the great drug discoverers of all time set his mind to creating an even better painkiller. His name was Paul Janssen. And he succeeded so completely that his work is still rocking our society.
He was the son of a Belgian physician and had followed in his father’s footsteps, graduating from medical school at the University of Ghent and planning to teach medicine. But he had a passion for chemistry and new ideas about drug development. So he gave up his teaching, borrowed money from his father, and started a small drug company.
Janssen, the man his friends called “Dr. Paul,” was a rare talent. He had the heart of an old alchemist; his goal was always to strip molecules down to their smallest active component, get to the spirit of the molecule, then build something around this purified essence, adding to it in order to create ever-better variations. Janssen was a deep thinker, able to concentrate intensely, to focus his mind on a given problem without giving up until it was solved. But he was more than a lab rat. He was also a tough-minded businessman, a builder of companies, a man who linked the creativity of an artist/chemist with the money-minded care of an executive.
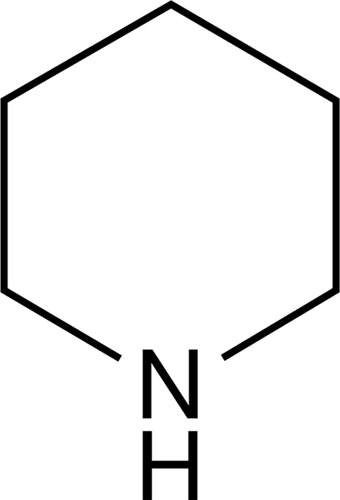
He noticed, for instance, that when you compared the molecular structure of natural opiates like morphine with the newer synthetics like pethidine, there was one bit that they shared, one structure within their structures that the two had in common. It was a six-sided ring of atoms called piperidine. Given the similarity of action between these two families of painkillers, he thought it was likely that this relatively simple structure—this “enchanted ring,” as it came to be called—was the spirit of the opium-like drugs.

Piperidine, the “enchanted ring”
Janssen decided to improve it. He knew that older painkillers worked more slowly than they needed to and lost some effectiveness because they had trouble getting into the central nervous system. They were slowed because they couldn’t easily get across cell membranes, which are mostly built of fat. So Janssen set out to make a fat-soluble opioid.
With that goal in mind, his lab began cranking out experimental drugs with the enchanted ring at the center, surrounded by side structures designed to be fat-soluble. They quickly found dozens of new drugs. In 1957, just after his thirtieth birthday, his fast-growing drug firm found a new opioid that was twenty-five times stronger than morphine and fifty times more powerful than Demerol, which worked more quickly and was cleared from the body faster. Phenoperidine, as his company named it, is still in use today as a general anesthetic.
And that was just the start. In 1960, Janssen’s group synthesized another drug that was more than one hundred times as potent as morphine. It was, at the time of its discovery, the most powerful opioid in the world. This they named fentanyl, and they began working it into a whole family of new painkillers.
Janssen Pharmaceuticals discovered many other drugs as well—a groundbreaking new antipsychotic, anesthesia drugs, a diarrhea medicine used by astronauts in the Apollo program, antifungals, allergy medicines—finding more than eighty successful new drugs in all, four of which are on the World Health Organization’s list of essential medicines. By the time Dr. Paul died in 2003, his company employed more than sixteen thousand workers around the world, and he had earned a reputation, as one of his colleagues wrote, as “the most prolific drug inventor of all time.”
Janssen’s company put fentanyl and its brother drugs into a variety of pills, skin patches, even lollipops for uses in controlling different amounts of pain in different patients. They remain standard medical tools for controlling pain. And they are all highly addictive, legally controlled drugs. As doctors and law enforcement have tightened legal access in recent years, fentanyl has gone underground, made in foreign countries and shipped into the United States. It’s getting more and more common on the streets, in forms that can be snorted, swallowed, put onto blotter paper, used to spike heroin. Because it’s so strong, overdoses keep going up along with increased use.
The spread of ever-more-powerful synthetics gave physicians better and better ways to control pain for their surgical patients, their cancer patients, and others with severe, intractable pain. And they also widened the door to more addiction in more people.
If science wasn’t going to solve the problem, then law enforcement would have to.
In 1971 President Richard Nixon announced his War on Drugs, including a large-scale offensive against opium products and traffickers. There was a mix of forces at play here: a backlash against the in-your-face drug use of the sixties; concern over the heroin addiction that veterans were bringing back from Vietnam; the rising appeal of law-and-order politics; and the growing realization that programs like methadone were having only limited success. His “Silent Majority” voting base, alarmed by what their children were getting into, by drug-related crime on the streets, and by drugs in the schools, wanted illegal drugs stamped out. There was a shift away from treating drug addiction like a disease. Increasingly, the public was liable to agree with writer Philip K. Dick, who wrote, “Drug misuse is not a disease, it is a decision, like the decision to step in front of a moving car. You would call that not a disease, but an error of judgment.”
A decision, not a disease. From that perspective, Nixon’s get-tough War on Drugs made sense.
It even gave the president a chance to show how “hip” he was by bringing celebrities like Elvis Presley into the White House to promote his move. Ironically, Elvis was taking a lot of drugs at the time. Nixon was gone soon afterward, but the Republican Party knew a winning political strategy when they saw it and made the War on Drugs a plank in their platform. “Just Say No,” championed by Nancy Reagan, became the antidrug mantra of its time.
At the same time, a scientific breakthrough allowed scientists to finally figure out how opium works in the body. And with that knowledge came new hope for breaking addiction.
By the early 1970s, it was becoming clear that many processes in the body communicated with other processes, and that communication was done by molecules released by one cell and sensed by another. To deliver the message, specific molecules had to fit into specific receptors on the surface of cells. The old way of thinking was to imagine a key fitting into a lock. It’s not quite like that in the body; it’s maybe more like trying to fit different-shaped wooden pegs into different-shaped holes. You might not be able to put a big square peg into a round hole, but you could loosely fit a small square peg. Or you might be able to carve down a peg that’s too big. In the body, the receptor system can be a little loose like that, recognizing and binding not just one perfect molecule, but also others that are similar. When the molecule binds to the receptor, it kicks off a reaction in the cell.
The great German physician/researcher Paul Ehrlich had theorized that communication happened this way in the body back in the late 1800s. But he and the next two generations of researchers had problems proving the point, because in the body many of the molecules that turn on receptors are made in very small quantities and are quickly broken down after they are made, disappearing to make room for the next set of reactions. This made them very difficult to study until the 1950s and 1960s, when far more sophisticated and sensitive lab equipment—X-ray and electron diffraction methods to study the structure of crystals; electron microscopes to study the architecture of cells; ultracentrifuges, electrophoresis setups, and chromatography equipment to separate molecules from one another; techniques for tagging molecules with radioactivity—made more sophisticated studies possible.
Including studies on opiates and other drugs. Many (but not all) drugs, it was found, did their work by activating receptors on the surface of cells. This was why certain drugs could have a specific effect on some cells, but not others. If a cell didn’t have a receptor for the drug, nothing happened. If it did, reactions were triggered. Drugs could be used to find receptors and study them. They could also be tweaked, their structures changed slightly, to see what that did, allowing scientists to learn more about how drugs fit into receptors.
It was only logical to think that there must be receptors for morphine and other opium alkaloids. But it wasn’t until 1973 that Solomon Snyder and a graduate student, Candace Pert, found them. Snyder was an MD with a strong interest in clinical psychology; he cut his teeth doing studies on LSD and other hallucinogens in the mid-1960s, trying, like everybody else, to figure out how such vanishingly small quantities of these drugs could produce such profound effects on the mind. He became an expert in doing experiments with molecules tagged with radioactive atoms. By following the radioactivity, he could follow the molecules in the body. He found that LSD, for instance, concentrated in certain parts of the brain after it was taken. Why did it go some places in the brain more than others? Turns out it was because that’s where the receptors for LSD were. Snyder’s lab at Johns Hopkins became a national leader in drug receptor studies.
Pert was a dynamic and determined young woman. Just before entering Johns Hopkins, she broke her back in a horseback-riding accident; her subsequent hospitalization gave her personal experience with the wonders of morphine. How did the drug do what it did? She maintained that interest when she started working in Snyder’s lab as a grad student. As sometimes happens in science labs, friction developed between professor and student: Pert claimed that Snyder wanted her to work on insulin receptors and forbade her to work on morphine; she remembered being so fascinated with the substance that she worked on morphine receptors on her own initiative, even sneaking her five-year-old child into the lab so she could keep an eye on him while she worked at night. Snyder saw her as another grad student who should be doing whatever needed doing in his lab. In his memory, that included opioid studies. However, it worked—between them they found the receptor in the brain that fit opioids. And then they and other researchers found another. And another. The more they looked, the more opioid receptors there seemed to be—three major types have been found so far, plus several more variations (whether the total is three or nine is still being debated). Which raised a question: Why on earth have we evolved so many receptors in our brains for molecules that come from poppy plants? As Pert put it, “God presumably did not put an opiate receptor in our brains so that we could ultimately discover how to get high with opium.”
It turns out that we didn’t. In 1975 a pair of Scottish researchers found out that the brain itself made a natural chemical that those receptors were made to fit. It was called enkephalin, and it was only the first of a growing family of related molecules, all made in our own bodies, that we now call endorphins (for endogenous morphine). You can think of them as our body’s own opiates. They play a vital role in helping us to control pain, calm down, and feel happy. They’re the treat our body gives us when we do something nice for it: the molecules that make us feel good when we get a massage or have sex or experience a runner’s high. They’re even released when we laugh. We make a bunch of them—different stimuli make them flow in different amounts at different times, and they react in varying ways with those different receptors. The result is a variety of effects that make it possible for our bodies to experience an exquisite array of natural pleasures.
Poppy alkaloids and the opiates we’ve made from them, plus the synthetics, all happen to trigger those same receptors. It’s no wonder they’re such bewitching drugs.
Snyder and Pert’s early studies blossomed into entire fields of research. We now have far more refined tools for studying the receptors on our cells and the ways they can be stimulated or blocked. Much of modern drug-making is built around these studies. Existing drugs are often used to find the receptors; once found, the receptors can be studied to see what turns them on and off; the result is both new drugs and a better understanding of how the body works. It’s a sort of virtuous cycle, with new drugs spurring a better understanding of the body, then that new understanding spurring a next round of better drugs. This is expensive, painstaking, and very important work. And it has led to hundreds of new medicines.
The discovery of opioid receptors and the molecules they work with also opened another door to pain control. Just as, seventy years earlier, organic chemists dreamed that some manipulation of the morphine structure might yield a nonaddictive substitute, now molecular biologists dreamed of another new path, one that led through the newly discovered opiate receptors. Receptors are turned on by molecules called “agonists”—morphine, heroin, oxycodone, and fentanyl are all agonists—but can also be turned off by “antagonists,” molecules that attach to and block receptors without switching them on. When an antagonist blocks a receptor, it can’t be turned on by anything else. Researchers found a way to do this with opioid receptors, developing antagonists like naloxone (marketed as Narcan). Naloxone attaches to opioid receptors but doesn’t switch them on. One website compares taking Narcan to putting a piece of tape over the fingerprint scanner on your phone; you can put your finger on the scanner all you want, but the tape keeps the phone from getting the message.
Narcan attaches so strongly to opioid receptors that it can actually sort of muscle the real drugs off, taking their place, sticking tight, and preventing any more from switching on the receptor. That’s why a dose of Narcan can save an addict’s life. The opioid is still in excess in the bloodstream, looking for a receptor to land on, but can’t find one. The resulting crash can be both horrible for drug users and near-miraculous for caregivers trying to save their lives; Narcan not only can wipe away all the euphoria from the opioid, throwing addicts into a form of instant withdrawal, but it can also stop an overdose in its tracks, pulling the victim back from the brink of death.
Researchers kept coming up with more and more new drugs that could modulate opiate receptors, new agonists and antagonists and partial agonists and agonist-antagonists (which have some properties of both), molecules specific for certain receptors and not others, molecules that acted different ways at different dosages, molecules that worked faster or slower, molecules that were quickly flushed out of the body and others that lasted a long time, a trove of new medicines that could turn receptors on and off selectively without using opiates.
In the 1970s and 1980s, the hope once again was that this fast-growing science might solve the whole heroin/opioid addiction problem.
But, no.
A respected expert gives a speech at a medical gathering, reporting that America is the world’s center of a growing drug crisis. America consumes fifteen times as many opiates as Austria, Germany, and Italy combined; only 20 percent of these drugs are taken for legitimate medical reasons. There’s evidence that almost a quarter of medical professionals themselves have some sort of personal opiate habit.
That’s from a newspaper story that ran in 1913. Since then we’ve had more than a century of scientific research, social programs, and government pronouncements. And the problem has only gotten worse.
Today the United States, with less than 5 percent of the world’s population, consumes 80 percent of the world’s opioids. The number of prescriptions written for opioid drugs—both synthetic and non-synthetic—more than doubled between 1992 and 2015; the number of overdose deaths in the country went up almost fivefold over the same period. Today, opioid overdoses kill more Americans than car accidents and gun homicides put together.
How has this happened? Science plays a role. Drug companies keep looking for that magic combination of addiction-free painkilling, and they keep coming up short. As they explored, they found other things—more powerful, more targeted opioids—so the total number of available opioids and related drugs keeps going up year after year: specialized formulations that work fast or slow, pills that are time-released and pills that are coated to prevent abuse, pills that are tailored for all levels of pain. Trailing behind them are all the drugs that are not opioids but are designed to help treat opioid addiction (like methadone and buprenorphine); to reverse the action of opioids (like naloxone and others); to treat opioid-linked constipation; to energize opioid patients so they can get out of bed; to take the edge off the energized patients so they can get some sleep; and the list goes on.
Another major factor fueling the opioid epidemic is money. Prescription opioids are a $10 billion per year business; pain drugs in general in 2017 were second only to cancer drugs in terms of sales, with more than three hundred million prescriptions filled per year. That’s not to mention the ancillary drug income, the illegal money from street drugs, the government program dollars, and money flowing through the burgeoning rehab, detox, and treatment businesses.
It’s a huge industry. And most of the players have a vested interest in keeping their business going. So, as has been true for more than a century, drugmakers keep promoting the next anti-addiction tweak, rehab centers keep promising more effective programs, and the government keeps announcing new efforts to wage war on drugs. Most of these efforts seem eerily familiar to anyone who has studied the history of these drugs. President Donald Trump’s recent idea about killing drug dealers, for instance, is the same one that was used by—and to some degree worked for—the Communists in China in the 1950s. Those kinds of programs are far easier to implement in centralized dictatorships than in Western democracies. Whatever the promised benefits of new formulations from drugmakers, newly redesigned drug rehab programs, or newly announced government initiatives, virtually none of these programs have, in any positive sense, worked. And the money keeps flowing.
If that sounds cynical, it is. Many, many people truly want to end this menace, and many organizations are honestly committed to bringing opioids under control and ending the scourge of addiction and overdose. But you can’t get around the simple fact that money motivates many players.
And that includes doctors. Drug companies are masters at promoting their wares, and a lot of their effort goes toward convincing doctors to prescribe their latest and greatest. In the old days drugmakers would have ballyhooed their products, bought the doctor lunch, and offered a cigar. Today they offer the physician some payment as a consultant, or pay for some research; they invite the physician to a winter conference at a tropical resort, where other physicians—experts whose opinions support the drugmaker—highlight the results from supportive scientific studies. These studies might also be financially backed, the results sometimes tailored, and the final papers sometimes written with help from the drug companies. They make sure that the right information gets into the right journals. They might make sure that negative experimental results—the kind that might sink a promising drug—are smoothed over or buried. It’s all very “scientific” and persuasive. And lucrative.
Physicians are also subject to trends in care. In the 1980s and 1990s, for instance, some leading experts in pain management argued that patients taking opioids for legitimate pain were unlikely to become addicted. The message of the day was: Prescribe until the pain is under control, even if the doses are high. Drugmakers obliged by coming up with more and more powerful variations on opioids, boosting the popularity of stronger semisynthetics like Oxycontin and synthetics like fentanyl. They became more and more common in medical practice.
Opioids were perfect for doctors who were increasingly short on time, especially the time needed for chronic pain patients, many of whom have complicated health histories and sometimes hard-to-diagnose reasons for their pain. Patients like that can eat up a lot of time talking about their condition; real answers can be very hard to find. A prescription for an opioid is an easy fix.
But far short of a perfect one. Patients would start on a relatively low dose, get relief, then find that they needed to increase their dose to get the same effect. They became drug tolerant. Their original pain was often replaced or increased by the pain of withdrawal, of simply not getting enough drug. In other words, it was easy for pain patients to become addicted.
But by the time that lesson became clear—and remember, this is the same lesson doctors grappled with in the 1840s with opium and the 1890s with morphine and the 1900s with legal heroin—in the first decade of the twenty-first century, opioid prescriptions were starting to go through the roof, followed by widespread dependence and addiction. The more that oxy and fentanyl were prescribed, the more they ended up on the street, either sold by patients with legitimate prescriptions or by dealers who found illegal ways to get them by the crate. Some addicts are experts at “doctor-shopping,” taking their pain complaints to physician after physician, some of whom will run them out of their office, and some of whom will write them a script. Then the addicts take their duplicate prescriptions to multiple drugstores to get them filled. They take some and sell some. There’s a huge black market for prescription opioids.
By 2010, the media and the public woke up to the fact that we were suffering through yet another opioid crisis. And the brakes were applied. In the past few years, consumption has dropped a bit. Physicians are prescribing less, moving away from the 1980s idea of “pain control no matter what” to a mind-set that more evenly matches risks with benefits. Government controls on the distribution of opioids have helped. Many drugmakers seem eager to cooperate with strategies to fight the epidemic and are seeking ways to curb abuse with better tracking of the flow of drugs from manufacturer to final user, and by continuing to make abuse-deterrent forms of opioids, with waxy coatings and time-release formulations that make it harder to get high.
But addicts, it turns out, are just as innovative as drug engineers. As soon as a new abuse-deterrent model of opioid comes out, somebody figures out how to smash, shave, microwave, snort, chew, or dissolve the drug to get past the deterrent and get their hit.
And that’s the thing: The hit is always there. No matter how protected it is, at the heart of every opioid pain pill is the opioid itself. Taking the pill gets the drug sooner or later to the receptors in the brain. The drug attaches to the receptor, the receptor fires—and there’s relief. The pain eases, the spirits soar, the jones is pushed away for a little while. There will always be some out there, on the street, as long as the poppy is harvested, the labs make synthetic versions, and doctors prescribe the drugs. And doctors will always prescribe the drugs because opioids are still, hands down, the best things we have to control pain.
In the end, if addicts can’t get oxy or fentanyl or some other pharmaceutical-grade opioids, they can always fall back on heroin. Heroin use is exploding as the black market for prescription opioids is becoming more constrained. Many addicts, finding it harder to get a legal fix from their doctors after the recent round of watchdogging, simply switch to the old favorite. Today heroin is flooding the streets; it’s cheap and available. Street price of a single pill of a strong opioid, Oxycontin or better, can run as high as $30 to $100 today. A bag of heroin, on the other hand, costs around $10, depending on your city. In many places, you can score a hit of heroin for less than a pack of cigarettes. And the heroin can be stronger than ever, boosted with a sprinkling of fentanyl or some other powerful synthetic. When you get it on the street, you never know how strong your fix is going to be. Overdoses have skyrocketed accordingly. The only winner seems to be the pharmaceutical industry. Every few years, drug companies come up with another opioid variation, some brand-new, can’t-fail, anti-abuse version that promises a different result, just like heroin was going to fix the problem of morphine. As drug after drug fails, there’s always another one to help switch addicts off the harder stuff, and untold millions of dollars are spent testing it out and trying to make some marginal difference.
Why America? Why are opioids America’s particular problem, so much more than any other country’s? Experts have been thinking about this question for decades, focusing on a few prime suspects. Part of the answer is rooted in the structure of our medical system, with its emphasis on short appointments for patients, reliance on powerful technologies, and bias toward finding a pill for every problem. Part of it comes from our economic system, with its insistence on increasing sales and profit. We are a wealthy society, and we can afford heavy pharmaceutical use. Part of it comes from our now-ingrained mind-set that drugs are a criminal problem, not a medical one. This funnels a lot of money toward the criminal justice system, police, DEA, and prisons, and dials back funding for medical approaches—clean-needle programs, addiction counseling, legalization of some drugs—that seem to work in other countries. There’s also a bit related to our peculiar national character. We Americans love our freedom to do what we want, when we want, including taking the drugs we want.
And, disquietingly, there’s the underlying fact that we’re drawn to opioids for the same reason the Chinese were nearly two centuries ago: It’s a way to escape. As one opioid expert put it, “We thought the big problem with these drugs is addiction. Now we realize the problem is with patients who take them and basically opt out of life.”
And maybe it’s because we’re wimps. As a physician said during a recent symposium, “Americans think we should never be in pain.” This is the flip side of our risk-taking adventurism. In part because of the quality of our drugs, we seem to have grown unaccustomed to pain and are unwilling to bear it. And not only physical pain. We are also lowering our tolerance for any sort of psychic discomfort, from minor anxiety to minor depression.
Increasingly, when we suffer any sort of discomfort, we pester our physicians for pills, and they prescribe them to us. This is not to say that millions of Americans do not suffer severe, long-term, very real pain, or severe depression, or crippling anxiety, and need opiates or antidepressants or tranquilizers to manage their illness. But in theory, a similar proportion of patients in every other culture or country should fall into the same category. The question is why American usage, both medically and on the street, is often so much higher. Are we in more pain than other nations? Are we suffering from more mental illness? There is little evidence that we are.
These issues are obviously complicated—as complicated as the workings of the human body—and dauntingly hard to tackle. Opioids are the ultimate case, because, as one expert concluded, “Opiate dependence is not a habit, nor is it a simple drive for some emotional craving. It is as fundamental to an addict’s existence as food and water, a physiochemical fact: an addict’s body is chemically reliant upon its drug, for opiates actually alter the body’s chemistry so it cannot function properly without being periodically primed. A hunger for the drug forms when the quantity in the bloodstream falls below a certain level, the addict becoming anxious and irritable. Fail to feed the body and it deteriorates and may die from drug starvation.” Read that again: Denied their fix, addicts are not just uncomfortable. They’re starving.
Despite all the politicians’ programs, the medical studies, the police task forces, and the social workers’ best efforts, addiction rates have gone nowhere but up. The predictions are that Americans, as they age, will continue to take more, and stronger, opioids. Drug companies will continue to profit. And opium’s thousand-year-old story will be rewritten for a new age.