Chapter 1
Matter and Energy: Exploring the Stuff of Chemistry
In This Chapter
Understanding the states of matter
Differentiating between pure substances and mixtures
Measuring matter with the metric system
Examining the properties of chemical substances
Discovering the different types of energy
Simply put, chemistry is a whole branch of science about matter, which is anything that has mass and occupies space. Chemistry is the study of the composition and properties of matter and the changes it undergoes.
Matter and energy are the two basic components of the universe. Scientists used to believe that these two things were separate and distinct, but now they realize that matter and energy are linked. In an atomic bomb or nuclear reactor, for instance, matter is converted into energy. (Perhaps someday science fiction will become a reality and converting the human body into energy and back in a transporter will be commonplace.)
In this chapter, you examine the different states of matter and what happens when matter goes from one state to another. I show you how to use the SI (metric) system to make matter and energy measurements, and I describe types of energy and how energy is measured.
Knowing the States of Matter and Their Changes
Matter is anything that has mass and occupies space. It can exist in one of three classic states: solid, liquid, and gas. When a substance goes from one state of matter to another, the process is called a change of state, or phase change. Some rather interesting things occur during this process, which I explain in this section.
Solids, liquids, and gases
Particles of matter behave differently depending on whether they’re part of a solid, liquid, or gas. As Figure 2-1 shows, the particles may be organized or clumped, close or spread out. In this section, you look at the solid, liquid, and gaseous states of matter.

Figure 2-1: Solid, liquid, and gaseous states of matter.
Solids
At the macroscopic level, the level at which you directly observe with your senses, a solid has a definite shape and occupies a definite volume. Think of an ice cube in a glass — it’s a solid. You can easily weigh the ice cube and measure its volume.
At the microscopic level (where items are so small that people can’t directly observe them), the particles that make up the solid are very close together and aren’t moving around very much (see Figure 2-1a). That’s because in many solids, the particles are pulled into a rigid, organized structure of repeating patterns called a crystal lattice. The particles in the crystal lattice are still moving but barely — it’s more of a slight vibration. Depending on the particles, this crystal lattice may be of different shapes.
Liquids
Unlike solids, liquids have no definite shape; however, they do have a definite volume, just like solids do. The particles in liquids are much farther apart than the particles in solids, and they’re also moving around much more (see Figure 2-1b).
Even though the particles are farther apart, some particles in liquids may still be near each other, clumped together in small groups. The attractive forces among the particles aren’t as strong as they are in solids, which is why liquids don’t have a definite shape. However, these attractive forces are strong enough to keep the substance confined in one large mass — a liquid — instead of going all over the place.
Gases
A gas has no definite shape and no definite volume. In a gas, particles are much farther apart than they are in solids or liquids (see Figure 2-1c), and they’re moving relatively independent of each other. Because of the distance between the particles and the independent motion of each of them, the gas expands to fill the area that contains it (and thus it has no definite shape).
Condensing and freezing
If you cool a gaseous or liquid substance, you can watch the changes of state, or phase changes, that occur. Here are the phase changes that happen as substances lose energy:
Condensation: When a substance condenses, it goes from a gas to a liquid state. Gas particles have a high amount of energy, but as they’re cooled, that energy decreases. The attractive forces now have a chance to draw the particles closer together, forming a liquid. The particles are now in clumps, as is characteristic of particles in a liquid state.
Freezing: A substance freezes when it goes from a liquid to a solid. As energy is removed by cooling, the particles in a liquid start to align themselves, and a solid forms. The temperature at which this occurs is called the freezing point (fp) of the substance.
H2O(g) → H2O(l) → H2O(s)
Here, the (l) stands for liquid, the (g) stands for gas, and (s) stands for solid.
Melting and boiling
As a substance heats, it can change from a solid to a liquid to a gas. For water, you represent the change like this:
H2O(s) → H2O(l) → H2O(g)
This section explains melting and boiling, the changes of state that occur as a substance gains energy.
From solid to liquid
When a substance melts, it goes from a solid to a liquid state. Here’s what happens: If you start with a solid, such as ice, and take temperature readings while heating it, you find that the temperature of the solid begins to rise as the heat causes the particles to vibrate faster and faster in the crystal lattice.
After a while, some of the particles move so fast that they break free of the lattice, and the crystal lattice (which keeps a solid solid) eventually breaks apart. The solid begins to go from a solid state to a liquid state — a process called melting. The temperature at which melting occurs is called the melting point (mp) of the substance. The melting point for ice is 32°F, or 0°C.
From liquid to gas
The process by which a substance moves from the liquid state to the gaseous state is called boiling.
If you heat a liquid, such as a pot of cool water, the temperature of the liquid rises and the particles move faster and faster as they absorb the heat. The temperature rises until the liquid reaches the next change of state — boiling. As the particles heat up and move faster and faster, they begin to break the attractive forces between each other and move freely as a gas, such as steam, the gaseous form of water.
The temperature at which a liquid begins to boil is called the boiling point (bp). The bp depends on atmospheric pressure, but for water at sea level, it’s 212°F, or 100°C. The temperature of a boiling substance remains constant until all of it has been converted to a gas.
Skipping liquids: Sublimation
Most substances go through the logical progression from solid to liquid to gas as they’re heated (or vice versa as they’re cooled). But a few substances go directly from the solid to the gaseous state without ever becoming a liquid. Scientists call this process sublimation. Dry ice — solid carbon dioxide, written as CO2(s) — is the classic example of sublimation. You can see dry ice pieces becoming smaller as the solid begins to turn into a gas, but no liquid forms during this phase change.
The process of sublimation of dry ice is represented as
CO2(s) → CO2(g)
Besides dry ice, mothballs and certain solid air fresheners also go through the process of sublimation. The reverse of sublimation is deposition — going directly from a gaseous state to a solid state.
Pure Substances and Mixtures
One of the basic processes in science is classification. In this section, I explain how all matter can be classified as either a pure substance or a mixture (see Figure 2-2).
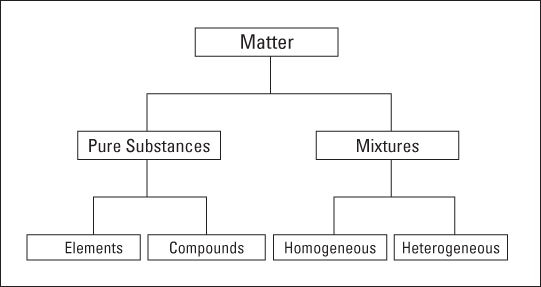
Figure 2-2: Classifying of matter.
Pure substances
Elements
An element is composed of a single kind of atom. An atom is the smallest particle of an element that still has all the properties of the element. For instance, if you slice and slice a chunk of the element gold until only one tiny particle is left that can’t be chopped anymore without losing the properties that make gold gold, then you have an atom. (I discuss properties later in the section “Nice Properties You’ve Got There.”)
The atoms in an element all have the same number of protons. Protons are subatomic particles — particles of an atom. (Chapter 2 covers the three major subatomic particles in great, gory detail.) The important thing to remember right now is that elements are the building blocks of matter. They’re represented in the periodic table, which you explore in Chapter 3.
Compounds
A compound is composed of two or more elements in a specific ratio. For example, water (H2O) is a compound made up of two elements, hydrogen (H) and oxygen (O). These elements are combined in a very specific way — in a ratio of two hydrogen atoms to one oxygen atom (hence, H2O). A lot of compounds contain hydrogen and oxygen, but only one has that special 2-to-1 ratio called water.
Chemists can’t easily separate the components of a compound: They have to resort to some type of chemical reaction.
Throwing mixtures into the mix
Chemists can easily separate the different parts of a mixture by physical means, such as filtration. For example, suppose you have a mixture of salt and sand, and you want to purify the sand by removing the salt. You can do this by adding water, dissolving the salt, and then filtering the mixture. You then end up with pure sand.
Mixtures can be either homogeneous or heterogeneous:
Homogeneous mixtures: Sometimes called solutions, homogeneous mixtures are relatively uniform in composition. Every portion of the mixture is like every other portion. If you dissolve sugar in water and mix it really well, your mixture is basically the same no matter where you sample it. I cover solutions in Chapter 10.
Heterogeneous mixtures: The composition of heterogeneous mixtures varies from position to position within the sample. For instance, if you put some sugar in a jar, add some sand, and then give the jar a couple of shakes, your mixture doesn’t have the same composition throughout the jar. Because the sand is heavier, there’s probably more sand at the bottom of the jar and more sugar at the top.
Measuring Matter
Scientists often make measurements, which may include such things as mass, volume, and temperature. If each nation had its own measurement system, communication among scientists would be tremendously hampered, so scientists adopted a worldwide measurement system to ensure they can speak the same language.
The SI system (from the French Système international) is a worldwide measurement system based on the older metric system. SI is a decimal system with basic units for things like mass, length, and volume and prefixes that modify the basic units. For example, here are some very useful SI prefixes:
kilo- (k) means 1,000
centi- (c) means 0.01
milli- (m) means 0.001
So a kilogram (kg) is 1,000 grams, and a kilometer (km) is 1,000 meters. A milligram (mg) is 0.001 grams — or you can say that there are 1,000 milligrams in a gram.
Here are some basic SI units and how they compare to the English units common in the U.S.:
Length: The basic unit of length in the SI system is the meter (m). A meter is a little longer than a yard; 1.094 yards are in a meter. The most useful SI/English conversion for length is 2.54 centimeters = 1 inch
Mass: The basic unit of mass in the SI system for chemists is the gram (g). And the most useful conversion for mass is 454 grams = 1 pound
Volume: The basic unit for volume in the SI system is the liter (L). The most useful conversion is 0.946 liter = 1 quart
Suppose you want to find the weight of a 5.0-lb. bag of potatoes in kilograms. The setup would look that this:
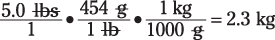
Nice Properties You’ve Got There
When chemists study chemical substances, they examine two types of properties:
Chemical properties: These properties enable a substance to change into a brand-new substance, and they describe how a substance reacts with other substances. Does a substance change into something completely new when water is added — like sodium metal changes to sodium hydroxide? Does the substance burn in air?
Physical properties: These properties describe the physical characteristics of a substance. The mass, volume, and color of a substance are physical properties, and so is its ability to conduct electricity. Physical properties can be extensive or intensive:
• Extensive properties, such as mass and volume, depend on the amount of matter present.
• Intensive properties, such as color and density, don’t depend on the amount of matter present. A large chunk of gold, for example, is the same color as a small chunk of gold.
Intensive properties are especially useful to chemists because intensive properties can be used to identify a substance. For example, knowing the differences between the density of quartz and diamond allows a jeweler to check out that engagement ring quickly and easily.
d = m/v
Usually, mass is described in grams (g) and volume is described in milliliters (mL), so density is g/mL. Because the volumes of liquids vary somewhat with temperature, chemists usually specify the temperature at which they made a density measurement. Most reference books report densities at 20°C, because it’s close to room temperature and easy to measure without a lot of heating or cooling. The density of water at 20°C, for example, is 1 g/mL.
Calculating density is pretty straightforward. You measure the mass of an object by using a balance or scale, determine the object’s volume, and then divide the mass by the volume.
Energy Types
Matter is one of two components of the universe. Energy is the other. Energy is the ability to do work.
Energy can take several forms, such as heat energy, light energy, electrical energy, and mechanical energy. But two general categories of energy are especially important to chemists: kinetic energy and potential energy.
Kinetic energy
Kinetic energy is energy of motion. A baseball flying through the air toward a batter has a large amount of kinetic energy — just ask anyone who’s ever been hit with a baseball.
Chemists sometimes study moving particles, especially gases, because the kinetic energy of these particles helps determine whether a particular reaction may take place. As particles collide, kinetic energy may be transferred from one particle to another, causing chemical reactions.
Kinetic energy can be converted into other types of energy. In a hydroelectric dam, the kinetic energy of the falling water is converted into electrical energy. In fact, a scientific law — the law of conservation of energy — states that in ordinary chemical reactions (or physical processes), energy is neither created nor destroyed, but it can be converted from one form to another.
Potential energy
Potential energy is stored energy. Objects may have potential energy stored in terms of their position. A ball up in a tree has potential energy due to its height. If that ball were to fall, that potential energy would be converted to kinetic energy.
Potential energy due to position isn’t the only type of potential energy. Chemists are far more interested in the energy stored (potential energy) in chemical bonds, which are the forces that hold atoms together in compounds.
Human bodies store energy in chemical bonds. When you need that energy, your body can break those bonds and release it. The same is true of the fuels people commonly use to heat their homes and run their automobiles. Energy is stored in these fuels — gasoline, for example — and is released when chemical reactions take place.
Temperature and Heat
When you measure, say, the air temperature in your backyard, you’re really measuring the average kinetic energy (the energy of motion) of the gas particles in your backyard. The faster those particles are moving, the higher the temperature is.
If you’re in the U.S., you probably use the Fahrenheit scale to measure temperatures, but most scientists use either the Celsius (°C) or Kelvin (K) temperature scale. (Remember: There’s no degree symbol associated with K.) Water boils at 100°C (373 K) and freezes at 0°C (273 K).
Here’s how to do some temperature conversions:
Fahrenheit to Celsius: °C = 5/9(°F – 32)
Celsius to Fahrenheit: °F = 9/5(°C) + 32
Celsius to Kelvin: K = °C + 273
The unit of heat in the SI system is the joule (J). Most people still use the metric unit of heat, the calorie (cal). Here’s the relationship between the two:
1 calorie = 4.184 joules
The calorie is a fairly small amount of heat: the amount it takes to raise the temperature of 1 gram of water 1°C. I often use the kilocalorie (kcal), which is 1,000 calories, as a convenient unit of heat. If you burn a large kitchen match completely, it produces about 1 kcal.

