Chapter 2
What’s In an Atom?
In This Chapter
Taking a look at the particles that make up an atom
Understanding elements and atomic mass
Coming to understand electron configurations
Finding out about isotopes and ions
In this chapter, I tell you about atoms, the fundamental building blocks of the universe. I cover the three basic particles of an atom — protons, neutrons, and electrons — and show you where they’re located. And I spend quite a bit of time discussing electrons themselves, because chemical reactions (where a lot of chemistry comes into play) depend on the loss, gain, or sharing of electrons.
Subatomic Particles
The atom is the smallest part of matter that represents a particular element. For quite a while, the atom was thought to be the smallest part of matter that could exist. But in the latter part of the 19th century and early part of the 20th, scientists discovered that atoms are composed of certain subatomic particles and that no matter what the element, the same subatomic particles make up the atom. The number of the various subatomic particles is the only thing that varies.
Scientists now recognize that there are many subatomic particles (this really makes physicists salivate). But to be successful in chemistry, you really only need to be concerned with the three major subatomic particles:
Protons
Neutrons
Electrons
Table 2-1 summarizes the characteristics of these three subatomic particles. The masses of the subatomic particles are listed in two ways: grams and amu, which stands for atomic mass units. Expressing mass in amu is much easier than using the gram equivalent.
|
Table 2-1 The Three Major Subatomic Particles |
|||||
|
Name |
Symbol |
Charge |
Mass (g) |
Mass (amu) |
Location |
|
Proton |
p+ |
+1 |
1.673 × 10–24 |
1 |
In the nucleus |
|
Neutron |
no |
0 |
1.675 × 10–24 |
1 |
In the nucleus |
|
Electron |
e– |
–1 |
9.109 × 10–28 |
0.0005 |
Outside the nucleus |
Atomic mass units are based on something called the carbon-12 scale, a worldwide standard that’s been adopted for atomic weights. By international agreement, a carbon atom that contains six protons and six neutrons has an atomic weight of exactly 12 amu, so 1 amu is defined as 1/12 of this carbon atom. Because the masses in grams of protons and neutrons are almost exactly the same, both protons and neutrons are said to have a mass of 1 amu. Notice that the mass of an electron is much smaller than that of either a proton or neutron. It takes almost 2,000 electrons to equal the mass of a single proton.
Table 2-1 also shows the electrical charge associated with each subatomic particle. Matter can be electrically charged in one of two ways: positive or negative. The proton carries one unit of positive charge, the electron carries one unit of negative charge, and the neutron has no charge — it’s neutral.
The atom itself has no charge. It’s neutral. (Well, actually, certain atoms can gain or lose electrons and acquire a charge, as I explain in the later section “Ions: Varying electrons.” Atoms that gain a charge, either positive or negative, are called ions.) So how can an atom be neutral if it contains positively charged protons and negatively charged electrons? The answer is that there are equal numbers of protons and electrons — equal numbers of positive and negative charges — so they cancel each other out.
The last column in Table 2-1 lists the location of the three subatomic particles. Protons and neutrons are located in the nucleus, a dense central core in the middle of the atom, and the electrons are located outside the nucleus (for details, see “Locating Those Electrons?” later in this chapter).
Centering on the Nucleus
In 1911, Ernest Rutherford discovered that atoms have a nucleus — a center — containing protons. Scientists later discovered that the nucleus also houses the neutron.
The nucleus is very, very small and very, very dense when compared to the rest of the atom. Typically, atoms have diameters that measure around 10–10 meters (that’s small!). Nuclei are around 10–15 meters in diameter (that’s really small!). If the Superdome in New Orleans represented a hydrogen atom, the nucleus would be about the size of a pea.
The protons of an atom are all crammed together inside the nucleus. Now you may be thinking, “Okay, each proton carries a positive charge, and like charges repel each other. So if all the protons are repelling each other, why doesn’t the nucleus simply fly apart?” It’s the Force, Luke. Forces in the nucleus counteract this repulsion and hold the nucleus together. Physicists call these forces nuclear glue. (Note: Sometimes this “glue” isn’t strong enough, and the nucleus does break apart. This process is called radioactivity, and I cover it in Chapter 4.)
Not only is the nucleus very small, but it also contains most of the mass of the atom. In fact, for all practical purposes, the mass of the atom is the sum of the masses of the protons and neutrons. (I ignore the minute mass of the electrons unless I’m doing very, very precise calculations.)
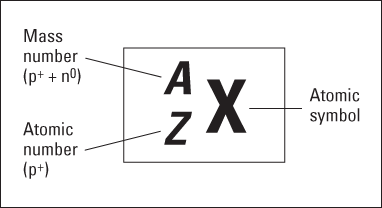
Figure 2-1: Representing a specific element.
As Figure 2-1 shows, chemists use the placeholder X to represent the chemical symbol. You can find an element’s chemical symbol on the periodic table or in a list of elements. The placeholder Z represents the atomic number — the number of protons in the nucleus. And A represents the mass number, the sum of the number of protons plus neutrons. The mass number is listed in amu.
For example, you can represent a uranium atom that has 92 protons and a mass number of 238 as in Figure 2-2.

Figure 2-2: Representing uranium.
But how many electrons does uranium have? Because the atom is neutral (it has no electrical charge), there must be equal numbers of positive and negative charges inside it, or equal numbers of protons and electrons. So there are 92 electrons in each uranium atom.
You can find both the element symbol and its atomic number on the periodic table, but the mass number for a particular element is not shown there. What is shown is the average atomic mass or atomic weight for all forms of that particular element, taking into account the percentages of each found in nature. See the later section “Isotopes: Varying neutrons” for details on other forms of an element.
Locating Those Electrons
Many of the important topics in chemistry, such as chemical bonding, the shape of molecules, and so on, are based on where the electrons in an atom are located. Simply saying that the electrons are located outside the nucleus isn’t good enough; chemists need to have a much better idea of their location, so this section helps you figure out where you can find those pesky electrons.
The quantum mechanical model
Early models of the atom had electrons going around the nucleus in a random fashion. But as scientists discovered more about the atom, they found that this representation probably wasn’t accurate. Today, scientists use the quantum mechanical model, a highly mathematical model, to represent the structure of the atom.
This model is based on quantum theory, which says that matter also has properties associated with waves. According to quantum theory, it’s impossible to know an electron’s exact position and momentum (speed and direction, multiplied by mass) at the same time. This is known as the uncertainty principle. So scientists had to develop the concept of orbitals (sometimes called electron clouds), volumes of space in which an electron is likely present. In other words, certainty was replaced with probability.
The quantum mechanical model of the atom uses complex shapes of orbitals. Without resorting to a lot of math (you’re welcome), this section shows you some aspects of this newest model of the atom.
Scientists introduced four numbers, called quantum numbers, to describe the characteristics of electrons and their orbitals. You’ll notice that they were named by top-rate techno-geeks:
Principal quantum number n
Angular momentum quantum number l
Magnetic quantum number ml
Spin quantum number ms
Table 2-2 summarizes the four quantum numbers. When they’re all put together, theoretical chemists have a pretty good description of the characteristics of a particular electron.
|
Table 2-2 Summary of the Quantum Numbers |
|||
|
Name |
Symbol |
Description |
Allowed Values |
|
Principal |
n |
Orbital energy |
Positive integers (1, 2, 3, and so on) |
|
Angular momentum |
l |
Orbital shape |
Integers from 0 to n – 1 |
|
Magnetic |
ml |
Orientation |
Integers from –l to +l |
|
Spin |
ms |
Electron spin |
+1/2 or –1/2 |
The principal quantum number n
The principal quantum number n describes the average distance of the orbital from the nucleus — and the energy of the electron in an atom. It can have only positive integer (whole-number) values: 1, 2, 3, 4, and so on. The larger the value of n, the higher the energy and the larger the orbital, or electron shell.
The angular momentum quantum number l
The angular momentum quantum number l describes the shape of the orbital, and the shape is limited by the principal quantum number n: The angular momentum quantum number l can have positive integer values from 0 to n – 1. For example, if the n value is 3, three values are allowed for l: 0, 1, and 2.
Orbitals that have the same value of n but different values of l are called subshells. These subshells are given different letters to help chemists distinguish them from each other. Table 2-3 shows the letters corresponding to the different values of l.
|
Table 2-3 Letter Designation of the Subshells |
|
|
Value of l (Subshell) |
Letter |
|
0 |
s |
|
1 |
p |
|
2 |
d |
|
3 |
f |
|
4 |
g |
When chemists describe one particular subshell in an atom, they can use both the n value and the subshell letter — 2p, 3d, and so on. Normally, a subshell value of 4 is the largest needed to describe a particular subshell. If chemists ever need a larger value, they can create subshell numbers and letters.
Figure 2-3 shows the shapes of the s, p, and d orbitals. In Figure 2-3a, there are two s orbitals — one for energy level 1 (1s) and the other for energy level 2 (2s). S orbitals are spherical with the nucleus at the center. Notice that the 2s orbital is larger in diameter than the 1s orbital. In large atoms, the 1s orbital is nestled inside the 2s, just like the 2p is nestled inside the 3p.
Figure 2-3b shows the shapes of the p orbitals, and Figure 2-3c shows the shapes of the d orbitals. Notice that the shapes get progressively more complex.

Figure 2-3: Shapes of the s, p, and d orbitals.
The magnetic quantum number ml
The magnetic quantum number ml describes how the various orbitals are oriented in space. The value of ml depends on the value of l. The values allowed are integers from –l to 0 to +l. For example, if the value of l = 1 (p orbital — see Table 3-4), you can write three values for ml: –1, 0, and +1. This means that there are three different p subshells for a particular orbital. The subshells have the same energy but different orientations in space.
Figure 2-3b shows how the p orbitals are oriented in space. Notice that the three p orbitals correspond to ml values of –1, 0, and +1, oriented along the x, y, and z axes.
The spin quantum number ms
The fourth and final quantum number is the spin quantum number ms. This one describes the direction the electron is spinning in a magnetic field — either clockwise or counterclockwise. Only two values are allowed for ms: +1/2 or –1/2. For each subshell, there can be only two electrons, one with a spin of +1/2 and another with a spin of –1/2.
Putting the quantum numbers together
Table 2-4 summarizes the quantum numbers available for the first two energy levels.
|
Table 2-4 Quantum Numbers for the First Two Energy Levels |
||||
|
n |
l |
Subshell Notation |
ml |
ms |
|
1 |
0 |
1s |
0 |
+1/2, –1/2 |
|
2 |
0 |
2s |
0 |
+1/2, –1/2 |
|
1 |
2p |
–1 0 +1 |
+1/2, –1/2 +1/2, –1/2 +1/2, –1/2 |
|
Table 2-4 shows that in energy level 1 (n = 1), there’s only an s orbital. There’s no p orbital because an l value of 1 (p orbital) is not allowed. And notice that there can be only two electrons in that 1s orbital (ms of +1/2 and –1/2). In fact, there can be only two electrons in any s orbital, whether it’s 1s or 5s.
Each time you move higher in a major energy level, you add another orbital type. So when you move from energy level 1 to energy level 2 (n = 2), there can be both s and p orbitals. If you write out the quantum numbers for energy level 3, you see s, p, and d orbitals.
Notice also that there are three subshells (ml) for the 2p orbital (see Figure 2-3b) and that each holds a maximum of two electrons. The three 2p subshells can hold a maximum of six electrons.
There’s an energy difference in the major energy levels (energy level 2 is higher in energy than energy level 1), but there’s also a difference in the energies of the different orbitals within an energy level. At energy level 2, both s and p orbitals are present. But the 2s is lower in energy than the 2p. The three subshells of the 2p orbital have the same energy. Likewise, the five subshells of the d orbitals (see Figure 2-3c) have the same energy.
Energy level diagrams
Chemists find quantum numbers useful when they’re looking at chemical reactions and bonding (and those are things many chemists like to study). But they find two other representations for electrons — energy level diagrams and electron configurations — more useful and easier to work with.
Chemists use both of these things to represent which energy level, subshell, and orbital are occupied by electrons in any particular atom. Chemists use this information to predict what type of bonding will occur with a particular element and to show exactly which electrons are being used. These representations are also useful in showing why certain elements behave in similar ways.
In this section, I show you how to use an energy level diagram and write electron configurations. I also discuss valence electrons, which are key in chemical reactions.
The dreaded energy level diagram
Figure 2-4 is a blank energy level diagram you can use to depict electrons for any particular atom. It doesn’t show all the known orbitals and subshells, but with this diagram, you should be able to do most anything you need to.
I represent orbitals with dashes in which you can place a maximum of two electrons. The 1s orbital is closest to the nucleus, and it has the lowest energy. It’s also the only orbital in energy level 1 (refer to Table 2-4). At energy level 2, there are both s and p orbitals, with the 2s having lower energy than the 2p. The three 2p subshells are represented by three dashes of the same energy. The figure also shows energy levels 3, 4, and 5.
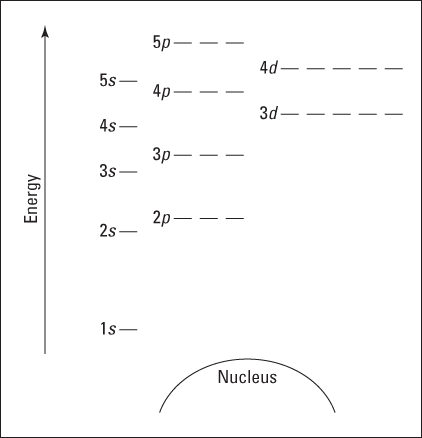
Figure 2-4: An energy level diagram.
Speaking of which, Figure 2-5 shows the Aufbau principle, a method for remembering the order in which orbitals fill the vacant energy levels.
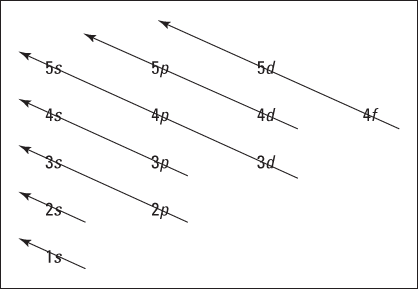
Figure 2-5: The Aufbau filling chart.
Electrons fill the lowest vacant energy levels first.
When there’s more than one subshell at a particular energy level, such as at the 3p or 4d levels (see Figure 2-4), only one electron fills each subshell until each subshell has one electron. Then electrons start pairing up in each subshell. This rule is named Hund’s rule.
Suppose you want to draw the energy level diagram of oxygen. You look on the periodic table and find that oxygen is atomic number 8. This number means that oxygen has eight protons in its nucleus and eight electrons. So you put eight electrons into your energy level diagram. You can represent electrons as arrows, as in Figure 2-6. Note that if two electrons end up in the same orbital, one arrow faces up and the other faces down. This is called spin pairing. It corresponds to the +1/2 and –1/2 of ms (see “The spin quantum number ms” section, earlier in this chapter, for details).
The first electron goes into the 1s orbital, filling the lowest energy level first, and the second one spin-pairs with the first one. Electrons 3 and 4 spin-pair in the next-lowest vacant orbital — the 2s. Electron 5 goes into one of the 2p subshells (no, it doesn’t matter which one — they all have the same energy), and electrons 6 and 7 go into the other two totally vacant 2p orbitals. The last electron spin-pairs with one of the electrons in the 2p subshells (again, it doesn’t matter which one you pair it with). Figure 2-6 shows the completed energy level diagram for oxygen.
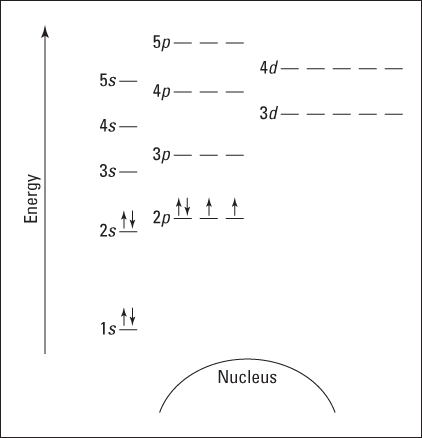
Figure 2-6: Energy level diagram for oxygen.
Electron configurations
Energy level diagrams are useful when you need to figure out chemical reactions and bonding, but they’re very bulky to work with. Wouldn’t it be nice if there were another representation that gives just about the same information but in a much more concise form? Well, there is. It’s called the electron configuration.
The electron configuration for oxygen is 1s22s22p4. Compare that notation with the energy level diagram for oxygen in Figure 2-6. Doesn’t the electron configuration take up a lot less space? You can derive the electron configuration from the energy level diagram. The first two electrons in oxygen fill the 1s orbital, so you show it as 1s2 in the electron configuration. The 1 is the energy level, the s represents the type of orbital, and the superscript 2 represents the number of electrons in that orbital. The next two electrons are in the 2s orbital, so you write 2s2. And finally, you show the four electrons in the 2p orbital as 2p4. Put it all together, and you get 1s22s22p4.
Here are a couple of electron configurations you can use to check your conversions from energy level diagrams:
Chlorine (Cl): 1s22s22p63s23p5
Iron (Fe): 1s22s22p63s23p64s23d6
Valence electrons: Clues about chemical reactions
Knowing the number of electrons that are an atom’s outermost energy level gives you a big clue about how that atom will react.
When chemists study chemical reactions, they study the transfer or sharing of electrons. The electrons more loosely held by the nucleus — the electrons in the energy level farthest away from the nucleus — are the ones that are gained, lost, or shared.
The electrons in the outermost energy level are commonly called valence electrons. Chemists really only consider the electrons in the s and p orbitals in the energy level that’s currently being filled as valence electrons. In the electron configuration for oxygen, 1s22s22p4, energy level 1 is filled, and there are two electrons in the 2s orbital and four electrons in the 2p orbital for a total of six valence electrons. Those valence electrons are the ones lost, gained, or shared.
Isotopes and Ions
The number of protons in an atom determines which element you have. But sometimes the number of neutrons or electrons varies, so you see several different versions of the atoms of that element. In this section, I introduce you to two variations: isotopes and ions.
Isotopes: Varying neutrons
Hydrogen is a common element here on Earth. Hydrogen’s atomic number is 1 — its nucleus contains 1 proton. The hydrogen atom also has 1 electron. Because it has the same number of protons as electrons, the hydrogen atom is neutral (the positive and negative charges have canceled each other out).
Most of the hydrogen atoms on Earth contain no neutrons. You can use the symbolization in Figure 2-1 to represent hydrogen atoms that don’t contain neutrons, as shown Figure 2-7a shows.
However, approximately one hydrogen atom out of 6,000 contains a neutron in its nucleus. These atoms are still hydrogen, because they each have one proton; they simply have a neutron as well, which most hydrogen atoms lack. So these atoms are called isotopes. Figure 2-7b shows an isotope of hydrogen, commonly called deuterium. It’s still hydrogen, because it contains only one proton, but it’s different from the hydrogen in Figure 2-7a, because it also has one neutron. Because it contains one proton and one neutron, its mass number is 2 amu.
There’s even an isotope of hydrogen containing two neutrons. This one’s called tritium, and it’s represented in Figure 2-7c. Tritium is extremely rare, but it can easily be created.
Figure 2-7 also shows an alternative way of representing isotopes: Write the element symbol, a dash, and then the mass number.

Figure 2-7: The isotopes of hydrogen.
For hydrogen, you have to take into consideration that there’s a lot more H-1 than H-2 and only a very tiny amount of H-3. That’s why the atomic mass of hydrogen on the periodic table isn’t a whole number: It’s 1.0079 amu. The number shows that there’s a lot more H-1 than H-2 and H-3.
Ions: Varying electrons
Because an atom itself is neutral, I say that the number of protons and electrons in atoms are equal throughout this book. But in some cases, an atom can acquire an electrical charge. For example, in the compound sodium chloride — table salt — the sodium atom has a positive charge and the chlorine atom has a negative charge. Atoms (or groups of atoms) in which there are unequal numbers of protons and electrons are called ions.
The neutral sodium atom has 11 protons and 11 electrons, which means it has 11 positive charges and 11 negative charges. Overall, the sodium atom is neutral, and it’s represented like this: Na. But the sodium ion contains one more positive charge than negative charge, so it’s represented like this: Na+ (the + represents its net positive electrical charge).
Gaining and losing electrons
Atoms become ions by gaining or losing electrons. And ions that have a positive charge are called cations. The progression goes like this: The Na+ ion is formed from the loss of one electron. Because it lost an electron, it has more protons than electrons, or more positive charges than negative charges, which means it’s now called the Na+ cation. Likewise, the Mg2+ cation is formed when the neutral magnesium atom loses two electrons.
Now consider the chlorine atom in sodium chloride. The neutral chlorine atom has acquired a negative charge by gaining an electron. Because it has unequal numbers of protons and electrons, it’s now an ion, represented like this: Cl–. And because ions that have a negative charge are called anions, it’s now called the Cl– anion. (You can get the full scoop on ions, cations, and anions in Chapter 5, if you’re interested. This here’s just a teaser.)
Writing electron configurations
Here are some extra tidbits about ions for your chemistry reading pleasure:
You can write electron configurations and energy level diagrams for ions. The neutral sodium atom (11 protons) has an electron configuration of 1s22s22p63s1. The sodium cation has lost an electron — the valence electron, which is farthest away from the nucleus (the 3s electron, in this case). The electron configuration of Na+ is 1s22s22p6.
The electron configuration of the chloride ion (Cl–) is 1s22s22p63s23p6. This is the same electron configuration as the neutral argon atom. If two chemical species have the same electron configuration, they’re said to be isoelectronic. Figuring out chemistry requires learning a whole new language, eh?
This section has been discussing monoatomic (one atom) ions. But polyatomic (many atom) ions do exist. The ammonium ion, NH4+, is a polyatomic ion, or specifically, a polyatomic cation. The nitrate ion, NO3–, is also a polyatomic ion, or specifically, a polyatomic anion.
Predicting types of bonds
Ions are commonly found in a class of compounds called salts, or ionic solids. Salts, when melted or dissolved in water, yield solutions that conduct electricity. A substance that conducts electricity when melted or dissolved in water is called an electrolyte. Table salt — sodium chloride — is a good example.
On the other hand, when table sugar (sucrose) is dissolved in water, it becomes a solution that doesn’t conduct electricity. So sucrose is a nonelectrolyte.

