Chapter 5
Ionic Bonding
In This Chapter
Finding out why and how ions are formed
Understanding how ions create chemical bonds
Deciphering the formulas of ionic compounds
Naming ionic compounds
Connecting conductivity and ionic bonds
In this chapter, I introduce you to ionic bonding, the type of bonding that holds salts together. I discuss simple ions and polyatomic ions: how they form and how they combine. I also show you how to predict the formulas of ionic compounds and how chemists detect ionic bonds.
Forming Ions: Making Satisfying Electron Trades
In nature, achieving a filled (complete) valence energy level is a driving force of chemical reactions, because when that energy level is full, elements become stable, or “satisfied” — stable elements don’t lose, gain, or share electrons.
The noble gases — the VIIIA elements on the periodic table — are extremely nonreactive because their valence energy level (outermost energy level) is filled. However, the other elements in the A families on the periodic table do gain, lose, or share valence electrons to fill their valence energy level and become satisfied.
In this section, I explain how atoms gain or lose electrons to form ions and achieve stability. I also explain how ions can consist of single atoms or a group of atoms. (For info on achieving stability by sharing electrons, flip to Chapter 6.)
Gaining and losing electrons
Losing an electron to become a cation: Sodium
Consider sodium, an alkali metal and a member of the IA family on the periodic table. Sodium has 1 valence electron and 11 total electrons, because its atomic number is 11. It has an electron configuration of 1s22s22p63s1. (See Chapter 2 for a review of electron configurations.)
By the octet rule, sodium becomes stable when it has eight valence electrons. Two possibilities exist for sodium to become stable: It can gain seven more electrons to fill energy level 3, or it can lose the one 3s electron so that energy level 2 (which is already filled at eight electrons) becomes the valence energy level.
So to gain stability, sodium loses its 3s electron. At this point, it has 11 protons (11 positive charges) and 10 electrons (10 negative charges). The once-neutral sodium atom now has a single positive charge [11 (+) plus 10 (–) equals 1+]. It’s now an ion, an atom that has a charge due to the loss or gain of electrons. You can write an electron configuration for the sodium cation:
Na+: 1s22s22p6
Atoms that have matching electron configurations are isoelectronic with each other. The positively charged sodium ion (cation) has the same electron configuration as neon, so it’s isoelectronic with neon. So does sodium become neon by losing an electron? No. Sodium still has 11 protons, and the number of protons determines the identity of the element.
There’s a difference between the neutral sodium atom and the sodium cation: one electron. As a result, their chemical reactivities are different and their sizes are different. Because sodium loses an entire energy level to change from a neutral atom to a cation, the cation is smaller.
Gaining an electron to become an anion: Chlorine
Chlorine, a member of the halogen family — the VIIA family on the periodic table — often forms anions. It has seven valence electrons and a total of 17 electrons, and its electron configuration is 1s22s22p63s23p5. So to obtain its full octet, chlorine must lose the seven electrons in energy level 3 or gain one at that level.
Because elements don’t gain or lose more than three electrons, chlorine must gain a single electron to fill energy level 3. At this point, chlorine has 17 protons (17 positive charges) and 18 electrons (18 negative charges). So chlorine becomes an ion with a single negative charge (Cl–). The neutral chlorine atom becomes the chloride ion. The electronic configuration for the chloride anion is
Cl–: 1s22s22p63s23p6
The chloride anion is isoelectronic with argon. The chloride anion is also slightly larger than the neutral chlorine atom. To complete the octet, the one electron gained went into energy level 3. But now there are 17 protons attracting 18 electrons, so the electrons can move outward a bit.
Looking at charges on single-atom ions
IA family (alkali metals): Each element has one valence electron, so it loses a single electron to form a cation with a 1+ charge.
IIA family (alkaline earth metals): Each element has two valence electrons, so it loses two electrons to form a 2+ cation.
IIIA family: Each element has three valence electrons, so it loses three electrons to form a 3+ cation.
VA family: Each element has five valence electrons, so it gains three electrons to form an anion with a 3– charge.
VIA family: Each element has six valence electrons, so it gains two electrons to form an anion with a 2– charge.
VIIA family (halogens): Each element has seven valence electrons, so it gains a single electron to form an anion with a 1– charge.
Determining the number of electrons that members of the transition metals (the B families) lose is more difficult. In fact, many of these elements lose a varying number of electrons so that they form two or more cations with different charges.
Seeing some common one-atom ions
Table 5-1 shows the family, element, ion name, and ion symbol for some common monoatomic (one-atom) cations.
|
Table 5-1 Common Monoatomic Cations |
|||
|
Family |
Element |
Ion Name |
Ion Symbol |
|
IA |
Lithium |
Lithium cation |
Li+ |
|
Sodium |
Sodium cation |
Na+ |
|
|
Potassium |
Potassium cation |
K+ |
|
|
IIA |
Beryllium |
Beryllium cation |
Be2+ |
|
Magnesium |
Magnesium cation |
Mg2+ |
|
|
Calcium |
Calcium cation |
Ca2+ |
|
|
Strontium |
Strontium cation |
Sr2+ |
|
|
Barium |
Barium cation |
Ba2+ |
|
|
IB |
Silver |
Silver cation |
Ag+ |
|
IIB |
Zinc |
Zinc cation |
Zn2+ |
|
IIIA |
Aluminum |
Aluminum cation |
Al3+ |
Table 5-2 gives the same information for some common monoatomic anions.
|
Table 5-2 Common Monoatomic Anions |
|||
|
Family |
Element |
Ion Name |
Ion Symbol |
|
VA |
Nitrogen |
Nitride anion |
N3– |
|
Phosphorus |
Phosphide anion |
P3– |
|
|
VIA |
Oxygen |
Oxide anion |
O2– |
|
Sulfur |
Sulfide anion |
S2– |
|
|
VIIA |
Fluorine |
Fluoride anion |
F– |
|
Chlorine |
Chloride anion |
Cl– |
|
|
Bromine |
Bromide anion |
Br– |
|
|
Iodine |
Iodide anion |
I– |
|
Possible charges: Naming ions with multiple oxidation states
The electrical charge that an atom achieves is sometimes called its oxidation state. Many of the transition metal ions (the B families) have varying oxidation states because these elements can vary in how many electrons they lose. Table 5-3 shows some common transition metals that have more than one oxidation state.
|
Table 5-3 Common Metals with More than One Oxidation State |
|||
|
Family |
Element |
Ion Name |
Ion Symbol |
|
VIB |
Chromium |
Chromium (II) or chromous |
Cr2+ |
|
Chromium (III) or chromic |
Cr3+ |
||
|
VIIB |
Manganese |
Manganese (II) or manganous |
Mn2+ |
|
Manganese (III) or manganic |
Mn3+ |
||
|
VIIIB |
Iron |
Iron (II) or ferrous |
Fe2+ |
|
Iron (III) or ferric |
Fe3+ |
||
|
Cobalt |
Cobalt (II) or cobaltous |
Co2+ |
|
|
Cobalt (III) or cobaltic |
Co3+ |
||
|
IB |
Copper |
Copper (I) or cuprous |
Cu+ |
|
Copper (II) or cupric |
Cu2+ |
||
|
IIB |
Mercury |
Mercury (I) or mercurous |
Hg22+ |
|
Mercury (II) or mercuric |
Hg2+ |
||
|
IVA |
Tin |
Tin (II) or stannous |
Sn2+ |
|
Tin (IV) or stannic |
Sn4+ |
||
|
Lead |
Lead (II) or plumbous |
Pb2+ |
|
|
Lead (IV) or plumbic |
Pb4+ |
||
Current method: Use the metal name, such as chromium, followed by the ionic charge written as a roman numeral in parentheses, such as (II). For example, Cr2+ is chromium (II) and Cr3+ is chromium (III).
Traditional method: An older way of naming ions uses -ous and -ic endings. When an element has more than one ion, do the following:
• Give the ion with the lower oxidation state (lower numerical charge, ignoring the + or –) an -ous ending.
• Give the ion with the higher oxidation state (higher numerical charge) an -ic ending.
So for chromium, the Cr2+ ion is named chromous and the Cr3+ ion is named chromic.
Grouping atoms to form polyatomic ions
Ions can be polyatomic, composed of a group of atoms. For example, take a look at Table 5-3 in the preceding section. Notice anything about the mercury (I) ion? Its ion symbol, Hg22+, shows that two mercury atoms are bonded together. This group has a 2+ charge, with each mercury cation having a 1+ charge. The mercurous ion is classified as a polyatomic ion.
Similarly, the symbol for the sulfate ion, SO42–, indicates that one sulfur atom and four oxygen atoms are bonded together and that the whole polyatomic ion has two extra electrons: a 2– charge.
Polyatomic ions are treated the same as monoatomic ions (see “Naming ionic compounds,” later in this chapter). Table 5-4 lists some important polyatomic ions.
|
Table 5-4 Some Important Polyatomic Ions |
|
|
Ion Name |
Ion Symbol |
|
Sulfite |
SO32– |
|
Sulfate |
SO42– |
|
Thiosulfate |
S2O32– |
|
Bisulfate (or hydrogen sulfate) |
HSO4– |
|
Nitrite |
NO2– |
|
Nitrate |
NO3– |
|
Hypochlorite |
ClO– |
|
Chlorite |
ClO2– |
|
Chlorate |
ClO3– |
|
Perchlorate |
ClO4– |
|
Chromate |
CrO42– |
|
Dichromate |
Cr2O72– |
|
Arsenite |
AsO33– |
|
Arsenate |
AsO43– |
|
Phosphate |
PO43– |
|
Hydrogen phosphate |
HPO42– |
|
Dihydrogen phosphate |
H2PO4– |
|
Carbonate |
CO32– |
|
Bicarbonate (or hydrogen carbonate) |
HCO3– |
|
Cyanide |
CN– |
|
Cyanate |
OCN– |
|
Thiocyanate |
SCN– |
|
Peroxide |
O22– |
|
Hydroxide |
OH– |
|
Acetate |
C2H3O2– |
|
Oxalate |
C2O42– |
|
Permanganate |
MnO4– |
|
Ammonium |
NH4+ |
|
Mercury (I) |
Hg22+ |
Creating Ionic Compounds
Ionic bonding, the bonding that holds the cations and anions together, is one of the two major types of bonding in chemistry. (I describe the other type, covalent bonding, in Chapter 6.)
In this section, you look at how ionic bonding works, and you see how to write formulas for and name ionic compounds.
Making the bond: Sodium metal + chlorine gas = sodium chloride
For instance, sodium, a metal, can fill its octet and achieve stability by losing an electron. Chlorine, a nonmetal, can fill its octet by gaining an electron. (See the earlier section “Gaining and losing electrons” for details on the octet rule.) If the two are in the same container, then the electron that sodium loses can be the same electron that chlorine gains. The Na+ cation attracts the Cl– anion and forms the compound NaCl, sodium chloride.
Compounds that have ionic bonds are commonly called salts. In sodium chloride — table salt — a crystal is formed in which each sodium cation is surrounded by six different chloride anions and each chloride anion is surrounded by six different sodium cations.
Different types of salts have different crystal structures. Cations and anions can have more than one unit of positive or negative charge if they lose or gain more than one electron. In this fashion, many different kinds of salts are possible.
Figuring out the formulas of ionic compounds
When an ionic compound is formed, the cation and anion attract each other, resulting in a salt. This section shows you how to write the formula of that salt.
Balancing charges: Magnesium and bromine
Suppose you want to know the formula, or composition, of a compound that results from reacting a metal and a nonmetal. You start by putting the two atoms side by side, with the metal on the left. Then you add their charges.
Figure 5-1 shows this process for magnesium and bromine. (Forget about the crisscrossing lines for now. I explain them in the upcoming section “Using the crisscross rule.”)
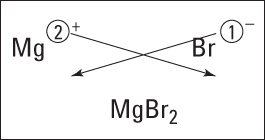
Figure 5-1: Figuring the formula of magnesium bromide.
The electron configurations for magnesium and bromine are
Magnesium (Mg): 1s22s22p63s2
Bromine (Br): 1s22s22p63s23p64s23d104p5
Magnesium, an alkaline earth metal, has two valence electrons that it loses to form a cation with a 2+ charge. The electron configuration for the magnesium cation is
Mg2+: 1s22s22p6
Bromine, a halogen, has seven valence electrons, so it gains one electron to complete its octet (eight valence electrons) and form the bromide anion with a 1– charge. The electron configuration for the bromide anion is
Br1–: 1s22s22p63s23p64s23d104p6
The magnesium ion has a 2+, so it requires two bromide anions, each with a single negative charge, to balance the two positive charges of magnesium. So the formula of the compound that results from reacting magnesium with bromine is MgBr2.
Using the crisscross rule
To see how to use this rule, look back at Figure 5-1. For magnesium and bromine, you make magnesium’s 2 a subscript of bromine and make bromine’s 1 a subscript of magnesium (but because it’s 1, you don’t show it). You get the formula MgBr2.
So what happens if you react aluminum and oxygen? Figure 5-2 shows the crisscross rule for this reaction. You get Al2O3.
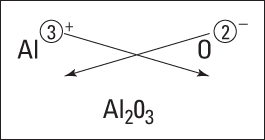
Figure 5-2: Figuring out the formula of aluminum oxide.
Compounds involving polyatomic ions work in exactly the same way. For example, here’s the compound made from the ammonium cation (NH4+) and the sulfide anion (S2–):
(NH4)2S
Notice that because you need two ammonium ions (two positive charges) to neutralize the two negative charges of the sulfide ion, you enclose the ammonium ion in parentheses and add a subscript 2.
For example, suppose that you want to write the compound formed when calcium reacts with oxygen. Calcium, an alkaline earth metal, forms a 2+ cation, and oxygen forms a 2– anion. So you may predict that the formula is
Mg2O2
But you need to divide each subscript by 2 to get the correct formula:
MgO
Naming ionic compounds
Suppose, for example, that you want to name Li2S, the compound that results from the reaction of lithium and sulfur. You first write the name of the metal, lithium, and then write the name of the nonmetal, adding an -ide ending so that sulfur becomes sulfide:
Li2S: Lithium sulfide
Ionic compounds involving polyatomic ions follow the same basic rule: Write the name of the metal first, and then simply add the name of the nonmetal. However, with polyatomic anions, it’s not necessary to add the -ide ending:
(NH4)2CO3: Ammonium carbonate
K3PO4: Potassium phosphate
Dealing with multiple oxidation states
When the metal involved is a transition metal with more than one oxidation state (see Table 5-3, earlier in the chapter), there can be more than one way to correctly name the compound, based on how you name the metal.
For example, suppose that you want to name the compound formed between the Fe3+ cation and the cyanide ion, CN–. The preferred method is to use the metal name followed in parentheses by the ionic charge written as a roman numeral: iron (III). But an older naming method, which is still sometimes used (so it’s a good idea to know it), is to use -ous and -ic endings.
After you write the name of the metal, name the nonmetal. So the compound Fe(CN)3 can be named
Fe(CN)3: iron(III) cyanide, or ferric cyanide
Getting names from formulas and formulas from names
Sometimes figuring out the charge on an ion can be a little challenging (and fun), so take a look at how to name FeNH4(SO4)2. I show you earlier in Table 5-4 that the sulfate ion has a 2– charge, and from the formula you can see that there are two of these ions. Therefore, you have a total of four negative charges. Table 5-4 also indicates that the ammonium ion has a 1+ charge, so you can figure out the charge on the iron cation:
|
Ion |
Charge |
|
Fe |
? |
|
NH4 |
1+ |
|
(SO4)2 |
(2–) × 2 |
Because you have a 4– charge for the sulfates and a 1+ for the ammonium, the iron must be a 3+ to make the compound neutral. So the iron is in the iron (III), or ferric, oxidation state. You can name the compound:
FeNH4(SO4)2: Iron (III) ammonium sulfate, or ferric ammonium sulfate
And finally, if you have the name, you can derive the formula and the charge on the ions. For example, suppose that you’re given the name cuprous oxide. You know that the cuprous ion is Cu+ and the oxide ion is O2–. Applying the crisscross rule (from the earlier section “Using the crisscross rule”), you get the following formula:
Cuprous oxide: Cu2O
Bonding Clues: Electrolytes and Nonelectrolytes
Electrolytes are substances that conduct electricity in the molten state or when dissolved in water. For instance, sodium chloride is an electrolyte because it conducts an electrical current when dissolved in water. If you were to melt pure NaCl (which requires a lot of heat!) and then check the conductivity of the molten salt, you’d find that the molten table salt also conducts electricity. In the molten state, the NaCl ions are free to move and carry electrons, just as they are in the saltwater solution.
Substances that don’t conduct electricity when in these states are called nonelectrolytes. Table sugar, or sucrose, is a good example of a nonelectrolyte. You can dissolve sugar in water or melt it, but it won’t have conductivity. No ions are present to transfer the electrons.

