Chapter 6
Covalent Bonding
In This Chapter
Seeing how one hydrogen atom bonds to another hydrogen atom
Defining covalent bonding
Finding out about the different types of chemical formulas
Taking a look at polar covalent bonding and electronegativity
What holds together sugar, vinegar, and even DNA? Not ionic bonds! In this chapter, I discuss the other major type of bonding: covalent bonding. I explain the basics with an extremely simple covalent compound, hydrogen.
Covalent Bond Basics
Atoms form compounds to achieve a filled valence energy level (see Chapter 2 for more on energy levels). But instead of achieving it by gaining or losing electrons, as in ionic bonding (Chapter 5), the atoms in some compounds share electrons. That’s the basis of a covalent bond.
Sharing electrons: A hydrogen example
Hydrogen is number 1 on the periodic table — upper-left corner. Hydrogen has one valence electron. It’d love to gain another electron to fill its 1s energy level, which would make it isoelectronic with helium (because the two would have the same electronic configuration), the nearest noble gas. Energy level 1 can hold only two electrons in the 1s orbital, so gaining another electron would fill it. That’s the driving force of hydrogen: filling the valence energy level and achieving the same electron arrangement as the nearest noble gas.
Why atoms have to share
Why can’t the simple gain or loss of electrons explain the stability of H2? Imagine one hydrogen atom transferring its single electron to another hydrogen atom. The hydrogen atom receiving the electron fills its valence shell and reaches stability while becoming an anion (H–). However, the other hydrogen atom now has no electrons (H+) and moves further away from stability. This process of electron loss and gain simply won’t happen, because the goal of both atoms is to fill their valence energy levels. So the H2 compound can’t result from the loss or gain of electrons.
What can happen is that the two atoms can share their electrons. At the atomic level, this sharing is represented by the electron orbitals (sometimes called electron clouds) overlapping. The two electrons (one from each hydrogen atom) “belong” to both atoms. Each hydrogen atom feels the effect of the two electrons; each has, in a way, filled its valence energy level. A covalent bond is formed — a chemical bond that comes from the sharing of one or more electron pairs between two atoms.
That’s why the hydrogen found in nature is often not comprised of an individual atom. It’s primarily found as H2, a diatomic (two-atom) compound. Taken one step further, because a molecule is a combination of two or more atoms, H2 is called a diatomic molecule.
Representing covalent bonds
The overlapping of the electron orbitals and the sharing of an electron pair is represented in Figure 6-1a.
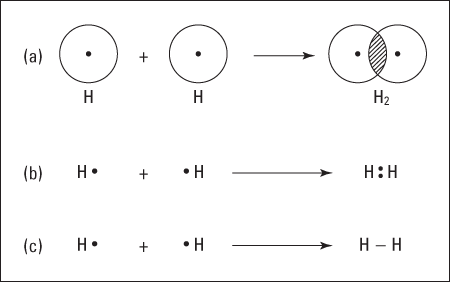
Figure 6-1: The formation of a covalent bond in hydrogen.
Another way to represent this process is through the use of an electron-dot formula. In this type of formula, valence electrons are represented as dots surrounding the atomic symbol, and the shared electrons are shown between the two atoms involved in the covalent bond. Figure 6-1b shows the electron-dot formula representations of H2.
Most of the time, I use a slight modification of the electron-dot formula called the Lewis structural formula; it’s basically the same as the electron-dot formula, but the shared pair of electrons (the covalent bond) is represented by a dash. Figure 6-1c shows the Lewis structural formula.
Comparing covalent bonds with other bonds
The properties of ionic and covalent compounds are different. Table 6-1 shows how the compounds compare. (Note: For the classification between metals and nonmetals, see Chapter 3.)
|
Table 6-1 Properties of Ionic and Covalent Compounds |
||
|
Property |
Ionic Compounds (Salts) |
Covalent Compounds |
|
Bonds occur between |
A metal and a nonmetal |
Two nonmetals |
|
State of the compound at room temperature |
Usually solid |
Can be solid, liquid, or gas |
|
Melting point |
Higher than for covalent compounds |
Lower than for ionic compounds |
|
Electrolytes (they form ions and conduct electricity when dissolved) or nonelectrolytes |
Tend to be electrolytes |
Tend to be nonelectrolytes |
I know just what you’re thinking: If metals react with nonmetals to form ionic bonds, and nonmetals react with other nonmetals to form covalent bonds, do metals react with other metals? The answer is yes and no.
Metals don’t really react with other metals to form compounds. Instead, the metals combine to form alloys, solutions of one metal in another. But there is such a situation as metallic bonding, and it’s present in both alloys and pure metals. In metallic bonding, the valence electrons of each metal atom are donated to an electron pool, commonly called a sea of electrons, and are shared by all the atoms in the metal. These valence electrons are free to move throughout the sample instead of being tightly bound to an individual metal nucleus. The ability of the valence electrons to flow throughout the entire metal sample is why metals tend to be conductors of electricity and heat.
Dealing with multiple bonds
I define covalent bonding as the sharing of one or more electron pairs. In hydrogen and most other diatomic molecules, only one electron pair is shared. But in many covalent bonding situations, the atoms share more than one electron pair. For instance, nitrogen (N2) is a diatomic molecule in which the atoms share more than one electron pair.
The nitrogen atom is in the VA family on the periodic table, meaning that it has five valence electrons (see Chapter 3 for details on the periodic table). So nitrogen needs three more valence electrons to complete its octet. A nitrogen atom can fill its octet by sharing three electrons with another nitrogen atom, forming three covalent bonds, a so-called triple bond. Figure 6-2 shows the triple bond formation of nitrogen.

Figure 6-2: Triple bond formation in N2.
A triple bond isn’t quite three times as strong as a single bond, but it’s a very strong bond. In fact, the triple bond in nitrogen is one of the strongest bonds known. This strong bond is what makes nitrogen very stable and resistant to reaction with other chemicals. It’s also why many explosive compounds (such as TNT and ammonium nitrate) contain nitrogen: When these compounds break apart in a chemical reaction, nitrogen gas (N2) is formed, and a large amount of energy is released.
Carbon dioxide (CO2) is another example of a compound containing a multiple bond. Carbon can react with oxygen to form carbon dioxide. Carbon has four valence electrons, and oxygen has six. Carbon can share two of its valence electrons with each of the two oxygen atoms, forming two double bonds. Figure 6-3 shows these double bonds.

Figure 6-3: Formation of carbon dioxide.
Naming Covalent Compounds Made of Two Elements
Binary compounds are compounds made up of only two elements, such as carbon dioxide (CO2). Chemists use prefixes in the names of binary compounds to indicate the number of atoms of each nonmetal present. Table 6-2 lists the most common prefixes for binary covalent compounds.
|
Table 6-2 Prefixes for Binary Covalent Compounds |
|||
|
Number of Atoms |
Prefix |
Number of Atoms |
Prefix |
|
1 |
mono- |
6 |
hexa- |
|
2 |
di- |
7 |
hepta- |
|
3 |
tri- |
8 |
octa- |
|
4 |
tetra- |
9 |
nona- |
|
5 |
penta- |
10 |
deca- |
In general, the prefix mono- is rarely used. Carbon monoxide is one of the few compounds that uses it.
Take a look at the following examples to see how to use the prefixes when naming binary covalent compounds (I’ve italicized the prefixes):
CO2: Carbon dioxide
P4O10: Tetraphosphorus decoxide (chemists try to avoid putting an a and an o together with the oxide name, as in decaoxide, so they normally drop the a off the prefix)
SO3: Sulfur trioxide
N2O4: Dinitrogen tetroxide
This naming system is used only with binary, nonmetal compounds, with one exception: MnO2 is commonly called manganese dioxide.
Writing Covalent Compound Formulas
You can predict the formula of an ionic compound, based on the loss and gain of electrons to reach a noble gas configuration, as I show you in Chapter 5. (For example, if you react Ca with Cl, you can predict the formula of the resulting salt: CaCl2.) However, you really can’t make that type of prediction for covalent compounds, because they can combine in many ways, and many different possible covalent compounds may result.
Most of the time, you have to know the formula of the molecule you’re studying. But you may have several different types of formulas, and each gives a slightly different amount of information. Oh, joy!
Empirical formulas
Molecular or true formulas
The molecular formula, or true formula, tells you the kinds of atoms in the compound and the actual number of each atom.
You may determine, for example, that the empirical formula C2H6O is actually the molecular formula, too, meaning that there are actually two carbon atoms, six hydrogen atoms, and one oxygen atom in the compound. However, you may instead find that the molecular formula is C4H12O2, C6H18O3, C8H24O4, or another multiple of 2:6:1.
Structural formulas: Dots and dashes
For ionic compounds, the molecular formula is enough to fully identify a compound, but it’s not enough to identify covalent compounds. Look at Figure 6-4. Both compounds have the molecular formula of C2H6O. That is, both compounds have two carbon atoms, six hydrogen atoms, and one oxygen atom.
However, these are two entirely different compounds with two entirely different sets of properties. The difference is in the way the atoms are bonded, or what’s bonded to what. The compound on the left, dimethyl ether, is used in some refrigeration units and is highly flammable. The one on the right, ethyl alcohol, is the drinking variety of alcohol. Simply knowing the molecular formula isn’t enough to distinguish between the two compounds. Can you imagine going into a restaurant, ordering a shot of C2H6O, and getting dimethyl ether instead of tequila?
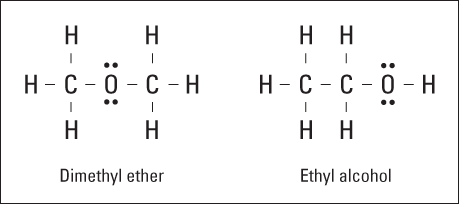
Figure 6-4: Two possible compounds of C2H6O.
The structural formula shows the elements in the compound, the exact number of each atom in the compound, and the bonding pattern for the compound. The electron-dot formula and Lewis formula, which I cover in this section, are common structural formulas.
Basic bonds: Writing the electron-dot and Lewis formulas
The following steps explain how to write the electron-dot formula for a simple molecule — water — and provide some general guidelines to follow:
1. Write a skeletal structure showing a reasonable bonding pattern using just the element symbols.
 Often, most atoms are bonded to a single atom. This atom is called the central atom. Hydrogen and the halogens are very rarely, if ever, central atoms. Carbon, silicon, nitrogen, phosphorus, oxygen, and sulfur are always good candidates, because they form more than one covalent bond in filling their valence energy level. In the case of water, H2O, oxygen is the central element and the hydrogen atoms are both bonded to it. The bonding pattern looks like this:
Often, most atoms are bonded to a single atom. This atom is called the central atom. Hydrogen and the halogens are very rarely, if ever, central atoms. Carbon, silicon, nitrogen, phosphorus, oxygen, and sulfur are always good candidates, because they form more than one covalent bond in filling their valence energy level. In the case of water, H2O, oxygen is the central element and the hydrogen atoms are both bonded to it. The bonding pattern looks like this:

It doesn’t matter where you put the hydrogen atoms around the oxygen. I put the two hydrogen atoms at a 90° angle to each other.
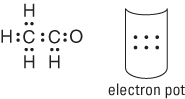
2. Take all the valence electrons from all the atoms and throw them into an electron pot.
Each hydrogen atom has one electron, and the oxygen atom has six valence electrons (VIA family), so you have eight electrons in your electron pot. Those are the electrons you use when making your bonds and completing each atom’s octet.
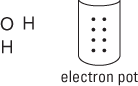
3. Use the N – A = S equation to figure the number of covalent bonds in this molecule.
In this equation,
• N equals the sum of the number of valence electrons needed by each atom. N has only two possible values — 2 or 8. If the atom is hydrogen, it’s 2; if it’s anything else, it’s 8.
• A equals the sum of the number of valence electrons available for each atom. A is the number of valence electrons in your electron pot. (If you’re doing the structure of an ion, you add one electron for every unit of negative charge or subtract one electron for every unit of positive charge.)
• S equals the number of electrons shared in the molecule. And if you divide S by 2, you have the number of covalent bonds in the molecule.
So in the case of water,
• N = 8 + 2(2) = 12 (eight valence electrons for the oxygen atom, plus two each for the two hydrogen atoms)
• A = 6 + 2(1) = 8 (six valence electrons for the oxygen atom, plus one for each of the two hydrogen atoms)
• S = 12 – 8 = 4 (four electrons shared in water), and S ÷ 2 = 4 ÷ 2 = 2 bonds
You now know that there are two bonds (two shared pairs of electrons) in water.
4. Distribute the electrons from your electron pot to account for the bonds.
You use four electrons from the eight in the pot, which leaves you with four electrons to distribute later. There has to be at least one bond from your central atom to the atoms surrounding it.
5. Distribute the rest of the electrons (normally in pairs) so that each atom achieves its full octet of electrons.
Remember that hydrogen needs only two electrons to fill its valence energy level. In this case, each hydrogen atom has two electrons, but the oxygen atom has only four electrons, so you place the remaining four electrons around the oxygen. This empties your electron pot. Figure 6-5 shows the completed electron-dot formula for water.

Figure 6-5: Electron-dot formula of H2O.
Notice that this structural formula shows two types of electrons: bonding electrons, the electrons that are shared between two atoms, and nonbonding electrons, the electrons that aren’t being shared. The last four electrons (two electron pairs) that you put around oxygen aren’t being shared, so they’re nonbonding electrons.
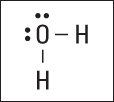
Figure 6-6: The Lewis formula for H2O.
Double bonds: Writing structural formulas for C2H4O
Drawing the structural formula for a molecule that contains a double or triple bond can be a bit tricky (see the earlier section “Dealing with multiple bonds”). In those cases, your equations may tell you that you have more covalent bonds that you know what to do with.
For example, here’s an example of a structural formula that’s a little more complicated — C2H4O. The compound has the following framework:

Notice that it has not one but two central atoms — the two carbon atoms. You can put 18 valence electrons into the electron pot: four for each carbon atom, one for each hydrogen atom, and six for the oxygen atom.
Now apply the N – A = S equation:
N = 2(8) + 4(2) + 8 = 32 (two carbon atoms with eight valence electrons each, plus four hydrogen atoms with two valence electrons each, plus an oxygen atom with eight valence electrons)
A = 2(4) + 4(1) + 6 = 18 (four electrons for each of the two carbon atoms, plus one electron for each of the four hydrogen atoms, plus six electrons for the oxygen atom)
S = 32 – 18 = 14, and S ÷ 2 = 14 ÷ 2 = 7 covalent bonds
Put single bonds between the carbon atoms and the hydrogen atoms, between the two carbon atoms, and between the carbon atom and oxygen atom. That’s six of your seven bonds.
There’s only one place that the seventh bond can go, and that’s between the carbon atom and the oxygen atom. It can’t be between a carbon atom and a hydrogen atom, because that would overfill hydrogen’s valence energy level. And it can’t be between the two carbon atoms, because that would give the carbon on the left ten electrons instead of eight. So there must be a double bond between the carbon atom and the oxygen atom. The four remaining electrons in the pot must be distributed around the oxygen atom, because all the other atoms have reached their octet. Figure 6-7 shows the electron-dot formula.

Figure 6-7: Electron-dot formula of C2H4O.
If you convert the bonding pairs to dashes, you have the Lewis formula of C2H4O, as in Figure 6-8.

Figure 6-8: The Lewis formula for C2H4O.
Grouping atoms with the condensed structural formula
I like the Lewis formula because it enables you to show a lot of information without having to write all those little dots. But it, too, is rather bulky. Sometimes chemists (who are, in general, a lazy lot) use condensed structural formulas to show bonding patterns. They may condense the Lewis formula by omitting the nonbonding electrons (dots) and grouping atoms together and/or by omitting certain dashes (covalent bonds). For instance, condensed formulas often group all the hydrogens bonded to a particular carbon atom.
Figure 6-9 shows a couple of condensed formulas for C2H4O.
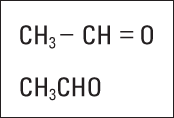
Figure 6-9: Condensed structural formulas for C2H4O.
Electronegativities: Which Atoms Have More Pull?
Atoms may share electrons through covalent bonds, but that doesn’t mean they share equally. When the two atoms involved in a bond aren’t the same, the two positively charged nuclei have different attractive forces; they “pull” on the electron pair to different degrees. The end result is that the electron pair is shifted toward one atom. But the question is, “Which atom does the electron pair shift toward?” Electronegativities provide the answer.
Figure 6-10 shows the electronegativity values of the various elements below each element symbol on the periodic table. Notice that with a few exceptions, the electronegativities increase from left to right in a period and decrease from top to bottom in a family.
Predicting the type of bond
Electronegativities are useful because they give information about what will happen to the bonding pair of electrons when two atoms bond.
A bond in which the electron pair is equally shared is called a nonpolar covalent bond. You have a nonpolar covalent bond anytime the two atoms involved in the bond are the same or anytime the difference in the electronegativities of the atoms involved in the bond is very small. For example, consider the Cl2 molecule. The table in Figure 6-10 shows that chlorine has an electronegativity value of 3.0. Each chlorine atom attracts the bonding electrons with a force of 3.0. Because there’s an equal attraction, the bonding electron pair is shared equally between the two chlorine atoms and is located halfway between the two atoms.
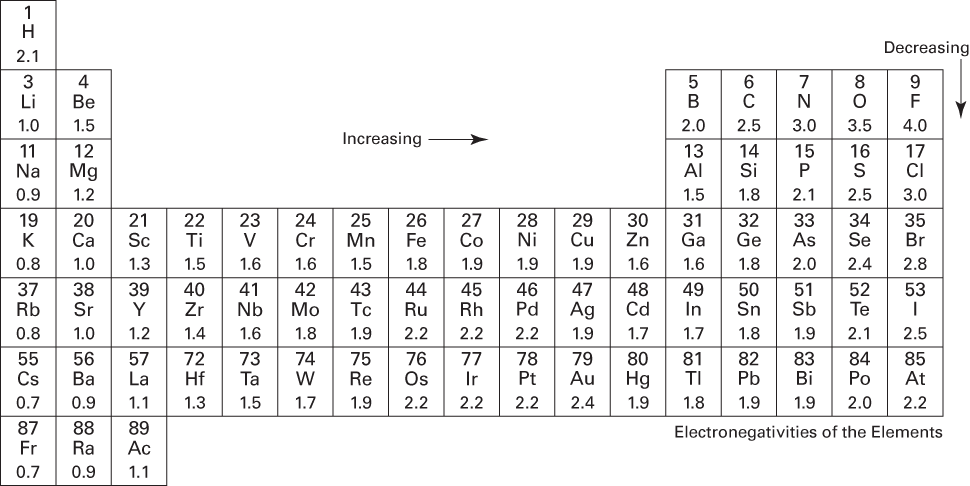
Figure 6-10: Electronegativities of the elements.
If the two atoms have extremely different electronegativities, the atoms will probably form ionic, not covalent bonds. For instance, sodium chloride (NaCl) is ionically bonded. An electron has transferred from sodium to chlorine. Sodium has an electronegativity of 1.0, and chlorine has an electronegativity of 3.0. That’s an electronegativity difference of 2.0 (3.0 – 1.0), making the bond between the two atoms very, very polar.
|
Electronegativity Difference |
Type of Bond Formed |
|
0.0 to 0.2 |
Nonpolar covalent |
|
0.3 to 1.4 |
Polar covalent |
|
> 1.5 |
Ionic |
The presence of a polar covalent bond in a molecule can have some pretty dramatic effects on the properties of a molecule, as you see in the next section.
Polar covalent bonding: Creating partial charges
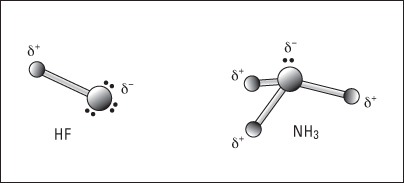
Figure 6-11: Polar covalent bonding in HF and NH3.
In hydrogen fluoride (HF), the bonding electron pair is pulled much closer to the fluorine atom than to the hydrogen atom, so the fluorine end becomes partially negatively charged and the hydrogen end becomes partially positively charged. The same thing takes place in ammonia (NH3): The nitrogen has a greater electronegativity than hydrogen, so the bonding pairs of electrons are more attracted to it than to the hydrogen atoms. The nitrogen atom takes on a partial negative charge, and each hydrogen atom takes on a partial positive charge.
In addition, a polar covalent molecule can act as a weak electrolyte because a polar covalent bond allows the substance to act as a conductor. So if a chemist wants a material to act as a good insulator (a device used to separate conductors), he or she looks for a material with as weak of a polar covalent bond as possible.
Attracting other molecules: Intermolecular forces
A polar molecule is a dipole — with one end having a partial negative charge and the other end having a partial positive charge — so it acts like a magnet. These charged ends can attract other molecules. For instance, the partially negatively charged oxygen atom of one water molecule can attract the partially positively charged hydrogen atom of another water molecule. This attraction between the molecules occurs frequently and is a type of intermolecular force (force between different molecules).
London force (dispersion force): This very weak type of attraction generally occurs between nonpolar covalent molecules, such as nitrogen (N2), hydrogen (H2), or methane (CH4). It results from the ebb and flow of the electron orbitals, giving a very weak and very brief charge separation around the bond.
Dipole-dipole interaction: This intermolecular force occurs when the positive end of one dipole molecule is attracted to the negative end of another dipole molecule. It’s much stronger than a London force, but it’s still pretty weak.
Hydrogen bond: The third type of interaction is really just an extremely strong dipole-dipole interaction that occurs when a hydrogen atom is bonded to one of three extremely electronegative elements: O, N, or F. These three elements have a very strong attraction for the bonding pair of electrons, so the atoms involved in the bond take on a large amount of partial charge. This bond turns out to be highly polar — and the higher the polarity, the more effective the bond.
When the O, N, or F on one molecule attracts the hydrogen of another molecule, the dipole-dipole interaction is very strong. This strong interaction (only about 5 percent of the strength of an ordinary covalent bond but still very strong for an intermolecular force) is called a hydrogen bond. The hydrogen bond is the type of interaction that’s present in water.
