Chapter I.1.2
The Nature of Matter and Materials
Introduction
Biomaterials are materials.
Biomaterials are materials. What are materials and how are they structured? This is the subject of this chapter, a lead into subsequent chapters with discussions of the bulk properties of materials, mechanics of materials, surface properties, a discussion of water (since all biomaterials function in an aqueous environment, and that environment can alter both the nature of the material and the interaction that occurs with the material), and finally discussions of specific classes of materials relevant to biomaterials (polymers, metals, ceramics, etc.).
Atoms and Molecules
The key to understanding matter is to understand attractive and interactive forces between atoms.
This “Nature of Matter” section aims to communicate an understanding of the basic structure of materials that will drive their properties – both the mechanical properties important for specific applications (strong, elastic, ductile, permeable, etc.), and the surface properties that will mediate reactions with the external biological environment.
The key to understanding matter is to understand attractive and interactive forces between atoms. Argon is a gas at room temperature – it must be cooled to extremely low temperatures to transition it into liquid form. An argon atom interacts (attracts) very, very weakly with another argon atom – so at room temperature, thermal fluctuations that randomly propel the atoms exceed attractive forces that might result in the coalescence to a solid material. A titanium atom strongly interacts with another titanium atom. Extremely high temperatures are required to vaporize titanium and liberate those atoms from each other. The understanding of matter is an appreciation of interactive forces between atoms.
What holds those atoms and molecules together to make a strong nylon fiber or a cell membrane, or a hard, brittle hydroxyapatite ceramic, or a sheet of gold, or a drop of water? Even in the early 18th century, Isaac Newton was pondering this issue: “There are therefore Agents in Nature able to make the Particles of Bodies stick together by very strong Attractions.”
Entropy consideration would say these molecules and atoms should “fly apart” to increase randomness. However, there is an energy term contributing to the stability of the ensemble leading to a negative Gibbs free energy, which, according to the second law of thermodynamics, should make such solids energetically favorable (of course, we intuitively know this). Thus, we must examine this energy term. We know of just four attractive forces in this universe:
Gravity holds us to the surface of the planet Earth (a massive body), but the gravitational potential energy of two argon atoms is only about 10−52 J, 30 orders of magnitude weaker than is observed for intermolecular forces. The weak nuclear force and the strong nuclear force are only significant over 10–4 nm – but molecular dimensions are 5 × 10−1 nm. So these forces do not explain what holds atoms together. This leaves, by default, electromagnetic forces (positive charge attracts negative charge). Electromagnetic forces have appropriate magnitudes and distance dependencies to justify why atoms interact. Interactions can be weak, leading to liquids, or stronger, leading to solids.
Electromagnetic forces manifest themselves in a number of ways. The types of interactions usually observed between atoms (all explained by electrostatics) are summarized in Table I.1.2.1. We consider here van der Waals forces (also called induction or dispersion forces), ionic forces, hydrogen bonding, metallic forces, and covalent interactions.
TABLE I.1.2.1 Forces that Hold Atoms Together
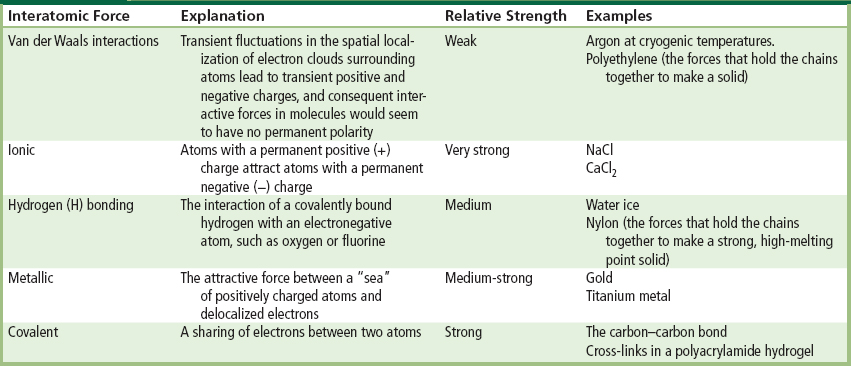
Van der Waals or dispersion forces rationalize the interaction of two atoms or molecules, each without a dipole (no plus or minus faces to the molecules). For example, argon atoms can be liquefied at low temperature. Why should this happen? Why should argon atoms want to interact with each other enough to form a liquid? Figure I.1.2.1 explains the origin of dispersive forces. Such forces are important, as they dictate the properties of many materials (for example, some polymers such as polyethylene which has no obvious dipole), but also they explain why the lipids in cell membranes assemble into the bilayer structure described later in this textbook. A typical van der Waals interactive force (for example, CH4 … CH4) is about 9 kJ/mol. The Ar … Ar interactive force is approximately 1 kJ/mol.
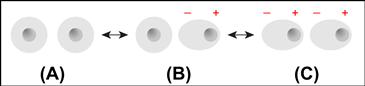
FIGURE I.1.2.1 (A) Consider the electron clouds (charge density in space) of two atoms or molecules, both without permanent dipole moments. (B) Electron clouds are continuously in motion and can shift to one side of the atom or molecule; therefore, at any moment, the atoms or molecules can create a “fluctuating instantaneous dipole.” (C) The “fluctuating instantaneous dipole” in one molecule electrostatically induces such an “instantaneous dipole” in the next molecule.
Ionic forces are probably the easiest of the intermolecular forces to understand. Figure I.1.2.2 illustrates a unit cell of a sodium chloride crystal. The + and − charges are arrayed to achieve the closest interaction of opposite charges, and the furthest separation of similar charges. This unit cell can be repeated over and over in space, and the forces that hold it together are the electrostatic interaction of a permanent + charge and a permanent − charge. Typical ionic bond strengths (for example, NaCl) are about 770 kJ/mol.
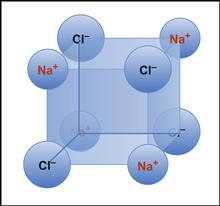
FIGURE I.1.2.2 The unit cell of a sodium chloride crystal illustrating the plus–minus electrostatic interactions.
Hydrogen bonding interactions are also straightforward to appreciate as electrostatic interactions. An electronegative element such as oxygen (it demands electrons) can distort the binding electron cloud from the hydrogen nucleus leaving the hydrogen (just a proton and electron), with less electron and thus more plus-charged proton. This somewhat positive charge will, in turn, then interact with an electronegative oxygen (Figure I.1.2.3). Typical hydrogen bond strengths (for example, O–H … H)are about 20 kJ/mol.
FIGURE I.1.2.3 A hydrogen bond between two water molecules.
Metallic bonding is explained by a delocalized “sea” of electrons with positively charged nuclear cores dispersed within it (Figure I.1.2.4). A single metallic bond is rarely discussed. The total interactive strength is realized through the multiplicity of the plus–minus interactions. The strength of this interaction can be expressed by heats of sublimation. For example, at 25°C, aluminum will have a heat of sublimation of 325 kJ/mol, while titanium will be about 475 kJ/mol.
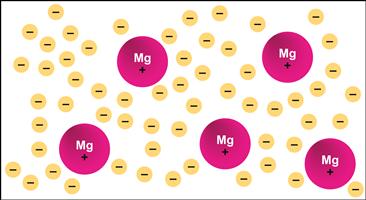
FIGURE I.1.2.4 Metallic bonding in magnesium. The 12 electrons from each Mg atom are shared among positively charged nuclear cores (the single + charge on each magnesium atom in the figure is simply intended to indicate there is some degree of positive charge on each magnesium nuclear core).
Covalent bonds are relatively strong bonds associated with sharing of pairs of electrons between atoms (Figure I.1.2.5). Typical covalent bond strengths (for example, C–C) are about 350 kJ/mol.
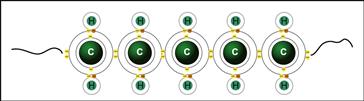
FIGURE I.1.2.5 Covalent bonding along a section of polyethylene chain. Carbons share pairs of electrons with each other, and each hydrogen shares an electron pair with carbon.
Molecular Assemblies
Atoms can combine in defined ratios to form molecules (usually they combine with covalent bonds), or they can form cohesive assemblies of atoms (think of gold and metallic interactive forces, for example). Thus, materials can be made of atoms or of molecules (i.e., covalently joined atoms). The difference between the dense, lubricious plastics used in orthopedics, the soft, elastic materials of catheters, and the hard, strong metals of a hip joint is associated with how those atoms and molecules are organized (due to attractive and interactive forces) in materials. Metals used in biomaterials applications can be strong, rigid and brittle, or flexible and ductile. Again, the difference is largely how the atoms making up the metal are organized, and how strongly their atoms interact.
Molecules also organize or assemble. The widely varying properties of polymers are due to molecular organization. The assembly of lipid molecules to make a cell membrane is another example of this organization.
A key concept in appreciating the properties of materials is hierarchical structures. The smallest size scale that we need consider here in materials is atoms, typically about 0.2 nm in diameter. Atoms combine to form molecules with dimensions ranging from 1 nm to 100 nm (some large macromolecules). Molecules may assemble or order to form supramolecular structures with dimensions up to 1000 nm or more. These supramolecular structures may themselves organize in bundles, fibers or larger assemblies with dimensions reaching into the range visible to the human eye. This concept of hierarchical structure is illustrated in Figure I.1.2.6, using collagen protein as the example.
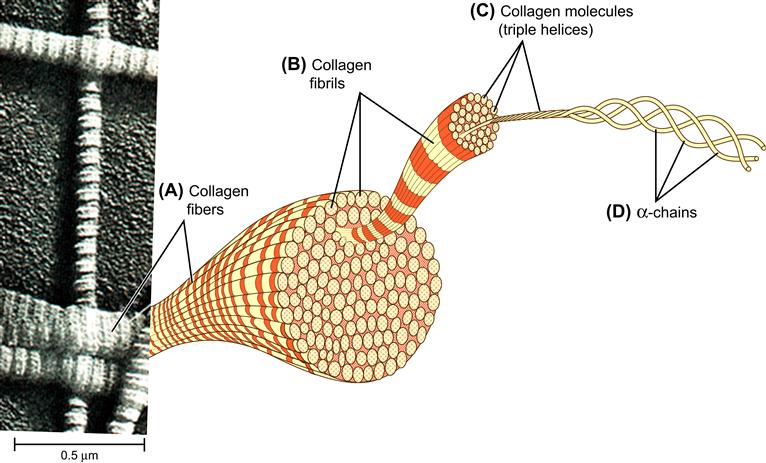
FIGURE I.1.2.6 Collagen fibers make up many structures in the body (tendons, for example). Such anatomical structures as tendons are comprised of collagen fibrils, formed of aligned bundles of collagen triple helices that are themselves made up of single collagen protein chains (α-chains). The α-chains are constructed of joined amino acid units, and the amino acids are molecules of carbon, oxygen, nitrogen, and hydrogen atoms in defined ratios and orders. (Illustration from Becker, W. (2002). The World of the Cell. Reprinted with permission of Pearson Education, Inc.)
The single α-chains comprising the collagen triple helix would break under tension with an application of nanograms of force. On the other hand, the collagen fibers in a hierarchical structure such as a tendon can support many kilograms of force. Such hierarchical structures are noted frequently in both materials science and in biology.
Surfaces
As assemblies of atoms and/or molecules form, within the bulk of the material, each unit is uniformly “bathed” in a field of attractive forces of the types described in Table I.1.2.1. However, those structural units that are at the surface are pulled upon asymmetrically by just the units beneath them. This asymmetric attraction distorts the electron distributions of the surface atoms or molecules, and gives rise to the phenomenon of surface energy, an excess energy associated with this imbalance. For this reason surfaces always have unique reactivities and properties. This idea will be expanded upon in Chapter I.1.5.
Conclusion
In this section we have reviewed the transition from chemistry to matter. Interacting assemblies of atoms and molecules comprise matter. Without matter, we cannot have biomaterials. Matter exists because of electrostatic forces – positive and negative charges, in all cases, hold atoms together. The strength of those interactions, associated with the magnitude of the charge on each atom, and the environment the atoms are in (water, air, etc.) ultimately dictates the properties of matter (a soft gel, a hard metal, etc.). Now that we have a general idea what “matter” is, we can take these concepts from physics and chemistry and bring them to a consideration of the mechanical properties of materials, the surface properties of materials, and then into the specifics of polymers, metals, ceramics, and other types of materials.
Bibliography
1. Barton A. States of Matter, States of Mind. Bristol, UK: Institute of Physics Publishing; 1997.
2. Holden A. The Nature of Solids. New York, NY: Dover Publications Inc; 1992.
3. Becker WM. World of the Cell. In: Kleinsmith LJ,, Hardin J, eds. 5th ed. Pearson Education, Inc. Upper Saddle River, NJ 2003.