Chapter II.5.9
Ophthalmologic Applications: Introduction
Overview of Eye Anatomy
The function of the eye is to provide sight. Briefly, images are seen when reflected or emitted light rays from an object are focused by the cornea and lens, and converted by photochemical receptors in the retina to electrical nerve impulses, which are transmitted to the visual cortex of the brain where complex, multi-layer neuronal processing creates the perception of an image. The nerve impulses from the retina are not pixel representation, but rather a complex set of data including shape, movement, and on-off encoded information that is assembled in the visual cortex.
The eye is approximately a sphere, commonly ranging in diameter from approximately 23 to 26 mm. The axial length of the globe is the length from the apex of the cornea to the retina at the macula. The eye is contained in the orbit or eye socket, within the skull. In the orbit, the eyeball is attached to six muscles for movement and surrounded by a cushion of fat. Tenon’s capsule is a layer of connective tissue that encapsulates the globe and the muscles, extending to the back of the orbit. The optic nerve carries several million nerve fibers from their cell bodies in the retina to the first connection point in the brain, known as the lateral geniculate body. The outer protective tissue of the eye globe is the sclera, the “white,” opaque collagen wall of the eye. The thickness of the sclera varies with the location of the globe and with age, typically ranging from 0.4 mm to 1.3 mm. The sclera is a connective tissue that protects the globe, while maintaining its shape. A schematic diagram of the cross-section of the globe is shown in Figure II.5.9.1, and ocular dimensions are summarized in Table II.5.9.1.
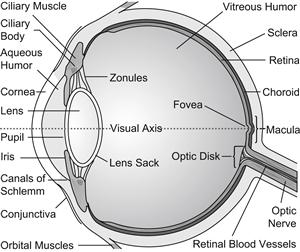
FIGURE II.5.9.1 Schematic representation of the cross-section of the eye (www.99main.com/~charlief/Blindness.htm).
TABLE II.5.9.1 Approximate Dimensions of Globe and Ocular Structures
| Measurement | Length, Thickness or Diameter (mm) |
| Axial length of globe | 23–26 |
| Corneal diameter (white-to-white) | 10.5–12.5 |
| Equatorial diameter | 23–26 |
| Anterior chamber depth (ACD) | 3–4 |
| Anterior chamber diameter | 12.0–12.5 |
| Sulcus diameter | 11.0–11.7 |
| Natural crystalline lens diameter | 9.0–10.2 |
| Natural crystalline lens thickness | 3.5–4.5 |
| Capsular bag diameter after lens extraction | 9.2–10.5 |
| Capsular thickness | 0.004–0.014 |
| Retinal thickness | 0.2–0.25 |
Light enters the eye through the dome shaped cornea that functions both as the window of the eye and the dominant lens. The cornea is the only transparent tissue in nature. The corneal diameter typically ranges from 10.5 to 12.5 mm. The anterior surface of the cornea provides the majority of the refractive component of the eye. The refractive index of the cornea is approximately 1.34. The anterior surface of cornea contributes approximately 48 diopters (D) of positive power towards the convergence of a given image on the retina. (A diopter is a unit of measurement that describes the refractive power of a lens, defined as the reciprocal of the focal length (f) of the lens in meters (D = 1/f)).
The cornea is comprised of five structurally distinct layers. From the anterior surface of the cornea to the posterior surface, the layers are epithelium, Bowman’s layer, stroma, Descemet’s membrane, and endothelium. The cornea is typically 500–600 microns thick, and is non-vascularized. The outermost layer of the cornea, the epithelium, is 5–6 cell layers of about 50 microns thickness. Bowman’s layer is 10–15 microns of acellular collagen, separating the corneal epithelium from the cellular stroma. The stroma comprises approximately 90% of the corneal thickness. It is composed of extracellular collagen fibers, keratocyte cells that produce and repair the collagen, and glycosaminoglycans, complex chemicals interspersed between the collagen fibers. Descemet’s membrane is the basement membrane of the corneal endothelium that lines the inner surface of the cornea. The corneal endothelium is a single layer of cells. In humans, the corneal endothelium does not regenerate. The distance from the posterior surface of the cornea to the anterior surface of the natural crystalline lens is referred to as the anterior chamber depth.
The endothelium is a physiologically leaky layer that allows water, ions, and glucose to pass from the aqueous humor of the anterior chamber into the stroma. The endothelium has membrane pumps that then eject the water molecules back into the anterior chamber, leaving behind the nutrients necessary for the keratocytes to maintain corneal health. This mechanism is necessary to maintain the physiology of the cornea in the absence of the blood supply that nourishes all other tissues in the body. The transparency of the cornea is due to: (1) the highly organized and tightly packed collagen structure of the stroma; (2) the relative dehydration of the cornea due to the endothelial pump, preventing osmotic swelling of the stroma leading to light scattering; (3) the presence of the glycosaminoglycans, reducing light scattering by occupying spaces between collagen fibers; and (4) the lack of blood vessels.
The iris is a pigmented, vascularized tissue. The iris is responsible for controlling the amount of light that reaches the retina by varying the pupillary opening. The iris separates the anterior chamber from the posterior chamber. The ciliary body is also a pigmented, vascularized tissue. The ciliary body consists of the ciliary muscle and the ciliary processes. The ciliary body has two primary functions: (1) production of aqueous humor; and (2) accommodation, which will be discussed later. The aqueous humor is a clear fluid that fills the anterior chamber. The aqueous humor is produced by the ciliary processes in the posterior chamber of the eye and provides nutrients to the crystalline lens and cornea, as both structures do not have direct blood supplies. The composition of aqueous humor is mostly water and similar to blood plasma. The refractive index is approximately 1.34. The aqueous humor travels around the lens, through the pupil, bathes the back of the cornea, and drains into Schlemm’s canal through the trabecular meshwork. The flow rate of aqueous humor is approximately 2.4 μL/min. In a simplistic view, the intraocular pressure (IOP) of the eye is maintained by the balance of the rate of aqueous humor production by the ciliary processes, and the rate of drainage through the trabecular meshwork. Normal IOPs are usually 10–20 mmHg.
The natural crystalline lens is in the posterior chamber of the eye, and resides behind the iris and pupil. The crystalline lens is connected to the ciliary body by fine suspending ligaments known as zonules. The natural crystalline lens, ciliary body, and zonules are critical structures in accommodation. Accommodation is the ability to change focus from distant to near objects. As a person’s age increases, the ability of the natural crystalline lens to accommodate decreases. Hermann V. Helmholtz described the accommodative mechanism in 1855, and his theory is generally accepted today, although other theories of accommodation persist. Briefly, when the ciliary muscle contracts, the zonular fibers relax their natural state of tension on the periphery of the lens and this allows accommodation. As is well-known from geometry, the shape with the maximal volume for a given external surface area is a sphere. When zonular tension is reduced, the ovoid shape of the lens naturally moves toward a sphere, creating a greater curvature of the anterior and posterior surfaces of the lens. The lens power is therefore increased, allowing for the focus of objects closer to the eye. This will be discussed in greater detail in Chapter II.5.9B.
The natural crystalline lens is encompassed by a thin collagen membrane referred to as the lens capsule (Figure II.5.9.2). The capsule is a thin, elastic basement membrane. The thickness of the capsule varies by location. Beneath the anterior capsule and at the equator of the lens, there is a single layer of lens epithelial cells. In the natural non-cataractous lens, no cells are located on the posterior surface of the lens. The center of the lens is comprised of the nucleus, which is surrounded by lens fiber cells. Lens fiber cells synthesize proteins, crystallins, which are responsible for the refractive index of the lens. The refractive index of lens is approximately 1.42.
The retina covers approximately two-thirds of the inner wall of the posterior surface of the eye (Figure II.5.9.3). The innermost layer of the retina is in contact with the vitreous body. The retinal vasculature provides oxygen and nutrients to the inner layer of the retina. The choroid, the outermost vascular layer behind the retina, supplies oxygen and nutrients to the retinal pigment epithelium and the photoreceptors. The layers of the retina and choroid are shown in Figure II.5.9.3. There are two types of photoreceptors next to the retinal pigment epithelium: rods and cones. Cones are concentrated in the macula, the central region of the retina, and rods dominate in the peripheral region of the retina. Cones are responsible for central vision and daytime (photopic) vision, whereas rods are responsible for peripheral and dim light (scotopic) vision.

FIGURE II.5.9.3 Histological section of retina and choroid (Colthurst et al., 2000) demonstrating the 10 layers from outermost layer to inner layer: (A) retinal pigment epithelium; (B) outer and (C) inner segments of photoreceptors; (D) outer nuclear layer; (E) outer plexiform layer; (F) inner nuclear layer; (G) inner plexiform layer; (H) ganglion cell layer; (I) nerve fiber layer; and (J) internal limiting membrane.
Eye-Related Conditions and Statistics
In the United States, “legal” blindness is defined as the best-corrected visual acuity of 6/60 or 20/200 or worse in the better-seeing eye, and vision impairment (low vision) is defined as the best-corrected visual acuity less than 6/12 or <20/40 in the better-seeing eye (The Eye Diseases Prevalence Research Group, 2004).
In the US, Prevent Blindness America and the National Eye Institute estimate that approximately 2.4 million Americans over the age of 40 are visually impaired, and approximately 1 million are blind (Table II.5.9.2). The World Health Organization (WHO) estimates that approximately 269 million people live with low vision, and 45 million people live with blindness worldwide.
TABLE II.5.9.2 US Statistics on Ocular Conditions
| Ocular Disease | Number of Americans Affected |
| Vision impairment/low vision | 2.4 million (over age 40) |
| Blindness | 1 million (over age 40) |
| Refractive error: | |
| Myopia (> 1D) Hyperopia (> 3 D) Astigmatism Presbyopia |
30.5 million (over age 40) 12.0 million (over age 40) Affects 30% of population Affects almost all over age 50 |
| Cataracts | 21 million (over age 40) |
| Primary open-angle glaucoma | 2.2 million (over age 40) |
| Age-related macular degeneration (AMD) | 1.6 million (over age 50) (late AMD) |
| Diabetic retinopathy | 5.3 milllion (over age 18) |
Source: Prevent Blindness America, 2002.
In the US, the leading causes of vision impairment and blindness are primarily age-related diseases, such as cataract, glaucoma, age-related macular degeneration, and diabetic retinopathy. In the US, the annual cost of the ocular problems in adults age 40 or older is approximately $51 billion (Prevent Blindness America, 2002). The most common ocular disorders are refractive errors: myopia; hyperopia; astigmatism; and presbyopia. In a normal eye (emmetrope), light is focused directly on the retina to produce an image. If the light is focused in front or behind the retina, then either an object at distance or a near object will be blurry or out-of-focus. An individual with 20/20 vision can see near and distance objects clearly. Most refractive errors can be corrected with spectacles or contact lenses. Contact lenses will be discussed in detail in Chapter II.5.9.A.
Myopia, commonly referred to as nearsightedness, is an ocular disorder in which one can focus well on near objects, but cannot focus on objects in the distance. An object in the distance will appear out-of-focus or blurry. In myopic eyes, the image is focused in front of the retina, as opposed to directly on the retina. The axial length of a myopic eye is generally longer than that of an emmetrope. Hyperopia is referred to as farsightedness, as one cannot focus on near objects, but can focus on objects in the distance. That is, in hyperopic eyes, the image is formed behind the retina. A schematic of image formation in myopic and hyperopic eyes is shown in Figure II.5.9.4. Astigmatism is an ocular disorder that describes a change in the curvature of the cornea, such that the cornea is no longer spherical. Astigmatism affects approximately 30% of Americans. Presbyopia occurs as a result of a loss of accommodation. Presybyopic individuals with good distance vision, whether naturally or with glasses, contact lenses or refractive surgery, have difficulty in focusing on near objects. Presbyopia typically becomes symptomatic at age 40–45.
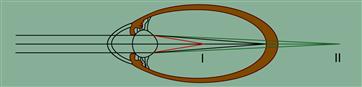
FIGURE II.5.9.4 Schematic of formation of images in myopes and hyperopes as compared to emmotropes. (I) The image is formed in front of the retina in myopia. (II) The image in formed behind the retina in hyperopia. (Kohnen et al., 2008.)
Cataract is the progressive clouding of the natural lens with age. Cataracts can also be due to trauma or injury, congenital defect, or can result from other underlying pathologies or medication, such as long-term steroid use. Cataracts are the leading cause of low vision in the US, and are highly treatable. Cataracts affect approximately 20.5 million people in the US over the age of 40 years. In the US, approximately 2 million cataract surgeries are performed each year. Cataract surgery is one of the most successful surgical procedures, as the success rate is above 90%. In cataract surgery, the natural crystalline lens of the eye is replaced by an artificial intraocular lens (IOL), referred to as a “pseudophakic IOL.”
In cataract surgery, the natural crystalline lens of the eye is usually extracted with a procedure known as phacoemulsification. Ultrasound energy is delivered to the cataractous lens to break it up into small pieces that can be aspirated out. There are many types of IOLs: monofocal, multifocal; toric; and accommodating. Certain IOLs can also be placed in the eye without removal of the lens; these are referred to as phakic IOLs. Phakic IOLs correct refractive errors for myopes or hyperopes, while leaving the natural lens intact in individuals with a clear lens. Intraocular lenses are discussed in greater detail in Chapter II.5.9B.
Primary open angle glaucoma is the most common form of glaucoma. Other forms of glaucoma include angle-closure, secondary, and congenital. Glaucoma is typically characterized by elevated intraocular pressure (IOP), changes in the optic nerve head (increased cupping), and/or visual field loss. Vision loss is due to the death of nerve cells. The vision lost from glaucoma cannot be restored. The clinical strategy to treat glaucoma usually begins with a topical pharmaceutical treatment to lower IOP. If IOP is not managed with medication, either laser treatment or surgery to implant a glaucoma shunt or stent is performed. Two types of laser treatment are argon laser trabeculoplasty (ALT) and selective laser trabeculoplasty (SLT). Both laser treatments use a laser to create space in the trabecular meshwork to increase aqueous humor outflow. A glaucoma or aqueous shunt is comprised of a plate and a tube with or without a valve. The tube is placed in the anterior chamber, and the plate resides in the sub-conjunctival space, where the aqueous humor flows out through a sieve into the tissues where it is absorbed. The shunt also aids in aqueous humor outflow by creating another outflow path. Glaucoma treatments are discussed in Chapter II.5.9.D.
There are two types of age-related macular degeneration (AMD): dry AMD and wet AMD. Dry AMD accounts for approximately 90% of all cases and is the milder form of AMD. It develops gradually over time and usually causes mild to moderate loss of vision. Often small yellow deposits, called drusen, accumulate in the retinal pigmented epithelium (RPE) cells adjacent to the macula. The dry form may eventually develop into the wet form of AMD.
While wet age-related macular degeneration accounts for approximately 10% of all cases, it is responsible for approximately 90% of cases of severe vision loss associated with AMD. Wet AMD involves the growth of abnormal blood vessels, known as choroidal neovascularization (CNV). These abnormal blood vessels invade from the choroid under the macula, hemorrhage, and lead to major cellular damage. This causes very sudden loss of central vision. Bleeding is followed by the formation of scar tissue that leads to vision loss.
Diabetic retinopathy (DR) also affects the retina and is associated with both diabetes type 1 (juvenile onset diabetes) or type 2 (adult onset diabetes). Diabetic retinopathy affects approximately 5.3 million Americans. There are two types of diabetic retinopathy: non-proliferative or proliferative. Non-proliferative DR is less severe. Blurred vision is a result of blood vessels leaking edema fluid into the retina. Proliferative DR is usually more severe. Neovascularization (formation of new blood vessels) occurs on the surface of the retina, and sometimes reaches up into the vitreous. The new vessels are delicate and ultimately hemorrhage, resulting in profound vision loss due to blurring as a result of the blood cells. The blood in the vitreous often stimulates a fibrotic scar reaction in the vitreous which pulls on the retina, ultimately leading to traction retinal detachment. The current approved treatments for AMD and DR are pharmaceutical injections into the vitreous, and laser treatments of the hypoxic retina and/or the abnormal vessels. New implants and drug delivery systems are in development, as discussed in Chapter II.5.9.E.
Some Special Consideration for Ophthalmic Biomaterials and Commonly Used Materials
Many considerations for ophthalmic biomaterials are similar to the considerations in other disciplines, such as biocompatibility, mechanical properties and stability, material degradation, manufacturability, and implant design optimization. Some common ophthalmic biomaterials are listed in Table II.5.9.3.
TABLE II.5.9.3 Biomaterials Commonly Used in Ophthalmology
| Ophthalmic Implants | Materials Commonly Used |
| Contact lenses | Poly(methyl methacrylate) (PMMA), 2-hydroxyethyl methacrylate (HEMA) copolymers, silicone hydrogels |
| Inlays or onlays | Hydrogels, collagen, permeable membranes |
| Intraocular lenses | Optic: PMMA, hydrophobic acrylic, silicone, hydrophilic acrylic Haptic: polypropylene, PMMA, polyimide, polyvinylidene fluoride (PVDF) |
| Ophthalmic viscosurgical device (OVD) | Chrondroitin sulfate, sodium hyaluronate, hyaluronic acid, hydroxypropyl methylcellulose (HPMS), polyacrylamide, collagen, or combinations of these materials |
| Glaucoma shunts | Plates: silicone (impregnated with barium), polypropylene Tubing: silicone |
| Vitreous replacements | Silicone oil, gases |
Unique to ophthalmology, the functionality of ocular biomaterials is often based on the optical properties of the material. The optical properties are critical for contact lenses, artificial corneas, corneal inlays or onlays, as well as intraocular lenses. The materials for these devices must be transparent and have a refractive index equal to or greater than that of the tissue it is replacing. These materials often incorporate an ultraviolet (UV) blocker to protect the retina from UV light. Additionally, the stability of the material in the eye, lack of toxicity, and long-term functionality of the product or device over time is critical. Optical devices need to be mechanically stable, because any tilt or decentration of these optical medical devices will lead to unfavorable visual performance. As the ophthalmic industry is moving to smaller surgical incisions, the materials for ophthalmic implants must be able to recover their shape after folding and unfolding, with no affect on the optical properties. This is clearly demonstrated with intraocular lenses.
Any material used for a corneal application, such as contact lenses or corneal inlays, must have acceptable oxygen and nutrient permeability. As the cornea is avascular tissue, the metabolic needs of the cornea depend on permeability to oxygen. For contact lenses, the surface wettability is important for a tear film for patient comfort. As contact lenses are implants exposed to proteins and lipid deposits in the tear film, the material must allow removal and/or be resistant to these deposits.
When designing a new ocular material or modifying an existing material, special attention should be given to the relevant standards. For example, for intraocular lenses, the biocompatibility of the material must meet the guidelines in ISO 10993-1: 2003, Biological Evaluation of Medical Devices. Part 1: Evaluation and testing, and ISO 11979-5(E): 2006, Ophthalmic Implants: Intraocular Lenses. Part 5: Biocompatibility.
Bibliography
1. Bhat SP. The ocular lens epithelium. Bioscience Reports. 2001;21(4):537–563.
2. Colthurst MJ, Williams RL, Hiscott PS, Grierson I. Biomaterials used in the posterior segment of the eye. Biomaterials. 2000;21:649–665.
3. ISO 10993-1. Biological Evaluation of Medical Devices. Part 1: Evaluation and testing 2003.
4. ISO 11979-5(E). Ophthalmic Implants: Intraocular lenses Part 5: Biocompatibility. 2006.
5. Kohnen T, Strenger A, Klaproth OK. Basic knowledge of refractive surgery: Correction of refractive errors using modern surgical procedures. Dtsch Arztebl Int. 2008;105(9):163–172.
6. Prevent Blindness America and National Eye Institute. Vision Problems in the U.S.: Prevalence of Adult Vision Impairment and Age-Related Eye Disease in America. 2002.
7. Prevent Blindness America. The Economic Impact of Vision Problems. 2007.
8. The Eye Diseases Prevalence Research Group. Causes and prevalence of visual impairment among adults in the United States. Arch Ophthalmol. 2004;122:477–485.
9. World Health Organization. Visual Impairment and Blindnesss (Fact Sheet N 282). 2009; (http://www.who.int/mediacentre/factsheets/fs282/en/; 2009;).
