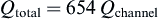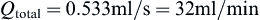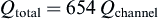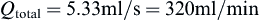Chapter II.6.6
Bioreactors for Tissue Engineering
Introduction
This chapter is a review of the principles of bioreactor design for tissue engineering. We describe the design and operation of tissue engineering bioreactors developed to direct the differentiation and functional assembly of cells cultured on three-dimensional biomaterial scaffolds. We first discuss the general design requirements for tissue engineering bioreactors, with a focus on mass transport considerations associated with environmental control, and biophysical signals necessary to modulate cell differentiation and the formation of engineered tissues. Next, we discuss the specifics of bioreactor design and operation using six examples of distinctly different tissue engineering systems: (1) cartilage tissue engineering with mechanical loading; (2) tissue engineering of anatomically shaped human bone; (3) cardiac tissue engineering with mechanical stretch; (4) cardiac tissue engineering with electrical stimulation and medium perfusion; (5) tissue engineering of heart valves with mechanical stimulation and perfusion; and (6) tissue engineering of blood vessels with pulsatile medium flow. Lastly, we address challenges in bioreactor design and operation in biological research, and translation into animal models and clinical treatment modalities.
Bioreactor Design Considerations
Cells are the key architects of tissues and organs. The cells both respond to and remodel their microenvironment by taking specific differentiation paths, interacting with the neighboring cells, extracellular matrix, molecular and physical signals, and assembling functional tissue units (Kaplan et al., 2005). Both in vivo (during development/regeneration) and in vitro (in tissue engineering settings), the cues with which cells are presented are the principal determinants of the phenotypic nature of the resultant tissues. Notably, the same factors that determine cell fate and function in vivo also determine the progression of cell differentiation in vitro. Hence, for tissue engineering applications, major efforts have been invested into characterizing the native tissue environments, and describing these environments by parameters that may be recapitulated experimentally (Kirouac and Zandstra, 2008).
Cell function and the progression of tissue assembly depend on the entire context of cell environment: the availability of a scaffold for cell attachment and tissue formation; the maintenance of physiological conditions in the cell–tissue environment; the supply of nutrients, oxygen, metabolites, and growth factors; and the presence of physical regulatory factors. The regulatory factors of cell differentiation and tissue assembly can be utilized in vitro to engineer functional tissues by an integrated use of cells, scaffolds, and bioreactors, as depicted in Figure II.6.6.1. Bioreactors are the primary tools for mimicking the native environments, and providing tissue engineered constructs with physiologically relevant stimuli that can orchestrate the conversion of a “collection of cells” into a specific tissue (Freed and Vunjak-Novakovic, 1998; Bilodeau and Mantovani, 2006; Freed et al., 2006; Kretlow and Mikos, 2008).
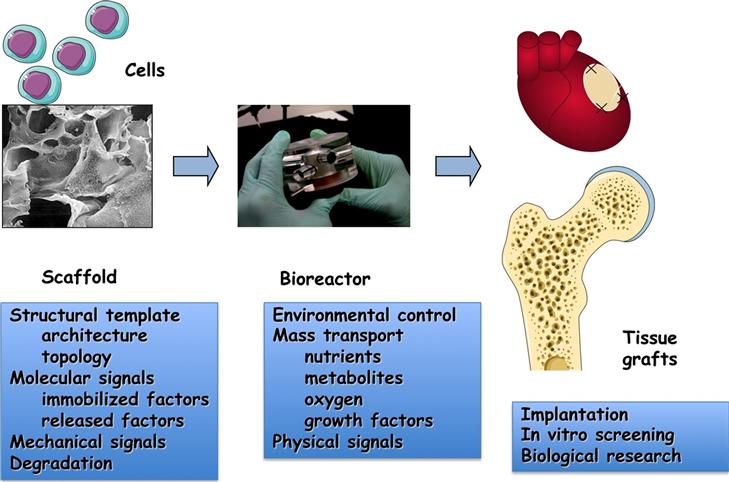
FIGURE ii.6.6.1 The tissue engineering paradigm. The living cells (either differentiated or progenitor/stem cells) are seeded onto a biomaterial scaffold, and cultured in a bioreactor to carry out the process of tissue formation. The scaffold provides a template for cell attachment and tissue formation through its structural properties (overall architecture, surface properties), molecular factors (immobilized and released molecules and cell-receptor ligands), mechanical properties (stiffness and elasticity), and it degrades in parallel with the deposition of new tissue matrix. The bioreactor provides the entire milieu of environmental conditions necessary for regulating cell differentiation and functional assembly, through the control of cell environment (temperature, pH, medium composition) and application of physical stimuli (hydrodynamic shear, mechanical stretch, compression, electrical stimulation).
Key Components
Bioreactors are composed of several building blocks: the culture chamber; medium reservoir; gas and medium exchange system; and instrumentation for monitoring and control (in many cases operated by computer software). These building blocks are either modular (so that they can be used within several different tissue engineering setups) or integrated into a single device. In most cases, the bioreactor design is customized to meet the need of a specific application, although some of the components (as for example the gas exchange system) can be used within various bioreactor systems.
Technical Requirements
As described in the following sections, there is a great diversity in bioreactor designs that mirrors the range of environmental and regulatory signals that need to be provided to the cells to direct their differentiation into various lineages and assembly into engineered tissues. The environments needed to engineer bone or muscle or blood vessels are quite different from each other, in many respects. However, there are some universal requirements that need to be met by all tissue engineering bioreactors.
An essential prerequisite for all bioreactor components that come in contact with the cells and culture medium is their biocompatibility. In contrast to biomaterials used for clinical applications, which are typically designed to be bioactive in some way, all materials used for the bioreactor chamber, gas and medium exchange, and in-line sensors need to be as inert and neutral as possible, so that the cells and molecular factors in culture medium are not affected.
The bioreactor components need to be sterilizable (ideally by autoclaving, ethylene oxide or UV irradiation), and the sterility and sterile containment must be maintained during the entire culture period. This requirement extends to sampling of tissue constructs and culture medium (during which time the sterile envelope may be broken), imaging of the growing tissues or application of external stimuli (such as mechanical loading). The case studies discussed below show some examples of how this requirement is addressed for specific bioreactor designs.
Biomaterial Scaffold
An engineered tissue is, in most cases, formed by cell cultured on biomaterial scaffolds. In general, tissue engineering scaffolds are biodegradable. To this end, it is important that the scaffold degrades at a rate comparable to that of the tissue formation. Fast degradation not only disrupts the initial structure of the cell-biomaterial construct, but can also result in environmental changes (e.g., a decrease in pH associated with hydrolysis of polyesther scaffolds such as polyglycolic acid (PGA) and polylactic acid (PLA)), while slow degradation leaves too much scaffold material in the way of new tissue formation.
A scaffold serves as a structural template for cell attachment and tissue formation, and it is designed to define the shape, size, and structural properties of the forming tissues. The scaffold is also an informational template, by virtue of its mechanical properties, immobilized and released bioactive factors and cell receptor ligands, and overall architecture (including surface properties, pore size and connectiveness, orientation, and alignment). To mimic the anisotropy of native tissues (such as bone or heart), scaffolds are also made of materials that have different structural and mechanical properties in different directions (Peppas and Langer, 1994; Hubbel 1995; Langer and Tirrell, 2004). In all cases, the cells receive important regulatory signals – molecular, topological, and mechanical – from the scaffold. Hence, the bioreactor designs are largely determined by the specific scaffold properties, and the bioreactor chamber accommodating a flexible tubular scaffold used to engineer a blood vessel is distinctly different from the bioreactor chamber that accommodates an anatomically-shaped engineered bone construct.
Gas Exchange
A gas exchange unit is necessary to ensure the maintenance of physiological levels of gases inside the culture chambers: carbon dioxide to regulate the pH of the culture medium; and oxygen to ensure the correct metabolic activity of cells. In most cases, the bioreactor is placed into an incubator, and this function is provided by mass transport between the incubator gas and the culture medium circulating through a coil of silicone tubing or across a silicone membrane. The efficiency of gas exchange depends on the permeability coefficients of each gas through the materials used, the geometry of the system, temperature, composition of the gas atmosphere, and the flow rate that determines the residence time of the fluids during gas exchange. In general, thin silicone tubing and membranes are selected for gas exchange systems because of their high permeability for oxygen and carbon dioxide, high biocompatibility, and possibility for reuse as these materials can be autoclave-sterilized.
Environmental Control
One of the key functions of a bioreactor is to control, maintain, and modulate as necessary the environmental parameters such as temperature, pH, oxygen tension, and medium composition. Bioreactors allow the establishment of steady-state conditions thus maintaining, by definition, all parameters of interest at constant levels. On the contrary, standard culture systems such as Petri dishes can be considered batch bioreactors, where the environmental conditions are precisely defined only at time zero, and then continuously vary with time between two medium exchanges, often with diffusion-limited and unpredictable kinetics. It is important that bioreactors can be designed to introduce tightly controlled perturbations into the system (such as an episode of hypoxia), and investigate the dynamics of the resulting biological responses.
Mass Transport
Nutrients, oxygen, and regulatory molecules have to be efficiently transported from the bulk culture medium to the tissue surfaces (external mass transfer), and then through the tissue to the cells (internal mass transfer) to maintain the viability and metabolism of cells within tissue engineered constructs. At the same time, metabolites and CO2 need to be removed from the cells, through the tissue matrix, and into the bulk medium. External mass transfer is determined by the flow conditions at construct surfaces, while the internal mass transport depends on molecular diffusion and convection, and the internal construct structure.
Transport of oxygen to the cells is by far the most critical among all molecules of interest, due to extremely low oxygen solubility in aqueous media. Fully oxygenated culture medium contains only 220 μM of oxygen at physiologic temperature. Oxygen concentration in blood plasma is even lower – only 130 μM. However, the total concentration of oxygen in oxyhemoglobin can reach 8600 μM, through reversible binding of oxygen to hemoglobin. Oxygen transport becomes critically inadequate in large non-perfused constructs (Muschler et al., 2004; Martin and Vermette, 2005), because the diffusional penetration depth of oxygen within native tissues is only 100–200 μm.
Transport phenomena occurring in bioreactors can be subjected to theoretical analysis and precise control once the geometries of the system, the transport distances, and fluid flow rates have been determined. The relative importance of the convective versus the diffusive component to the global mass transport can be calculated and analyzed in conjunction with the kinetics of uptake and release by the cultured cells.
Physical Signals
Tissues and organs in the body are subjected to complex biomechanical environments with dynamic stresses and strains, fluid flow, and electrical signals. Biophysical signals that play a role in cell physiology in vivo can also modulate the activity of cells within engineered tissues cultured in vitro. Innovative bioreactors have been developed to apply one or more regimes of controlled physical and/or electrical stimuli to three-dimensional engineered constructs, in an attempt to improve or accelerate the generation of a functional tissue (Freshney et al., 2007). Shear stress from the tangential forces applied to a surface (typically where cells reside) is important because of the profound effects of flow environment in biological systems (Wang et al., 2005). Mechanical forces play fundamental roles in determining the correct development and function of a multiplicity of organs and systems. The application of mechanical stimulation (compression, tension, torsion) can improve the engineering of tissues that normally provide mechanical function in the body (bone, muscle tissue, cartilage, tendon) (Stolberg and McCloskey, 2009). Likewise, electrical stimulation can improve the physiologic characteristics of tissues that are electrically excitable, such as myocardium (Tandon et al., 2009).
Scale
Tissue engineering bioreactors can have volumes ranging from hundreds of milliliters down to a few milliliters or in some cases only a few microliters. In recent years, micro-bioreactors with volumes of the order of micro- or even nano-liters are helping open new perspectives in studying cell biology and physiology, (Chang et al., 2007; Toner and Irimia, 2005) as they allow experiments conducted at the characteristic time and length scales of biological phenomena (Figallo et al., 2007). While macroscale observations of averaged cell–tissue properties are critical for engineering clinical-size grafts, they cannot be reliable in defining the cell-scale phenomena in biological systems, and should thus be supported by more precise microscale observations (Cimetta et al., 2009). In microscale systems, flow is always laminar (Reynolds numbers Re <1000), and transport is dominated by molecular diffusion or by convective regimes with defined hydrodynamic profiles. Reducing the characteristic dimensions to a microscale level allows working with very short transport distances, which are in turn associated with short time constants. As a result, biological responses are not limited any more by the slow kinetics of physical phenomena. Microscale systems also allow easier decoupling of the effects of mass transport phenomena (such as the generation of specific concentration patterns) from physical phenomena (such as the application of shear forces).
Cartilage Tissue Engineering with Mechanical Loading
The avascular nature of articular cartilage predisposes it to a limited ability to regenerate once injured. Articular cartilage is a loadbearing tissue, and is exposed to cyclical stress, which induces fluid pressurization within the tissue and resultant fluid shear. In a 2000 paper, Hung, Ateshian, and colleagues (Mauck et al., 2000) developed a dynamic loading bioreactor to induce pressurization of the interstitial media within agarose scaffolds seeded with bovine articular chondrocytes. Agarose was selected as a scaffold material because of its open lattice structure that minimizes diffusional distances, while maintaining the three-dimensional structure necessary for the fixation of cells. Agarose is a clear polysaccharide hydrogel that has been used extensively for long-term chondrocyte culture, as it permits the application of deformational loading immediately upon cell encapsulation, and is suitable for the preparation of anatomically correct constructs. The spatial uniformity of cells after seeding (compared to seeded fibrous scaffolds) is considered an additional advantage. Cell encapsulation in agarose, combined with the staged application of growth factors, resulted in engineering of functional articular cartilage with mechanical properties far exceeding those achieved using culture systems without mechanical loading.
Hung, Ateshian, and colleagues developed a custom loading system with a cylindrical loading platen, designed to subject a set of cell-seeded agarose discs that were cultured under sterile conditions in a simple Petri dish covered with a lid to dynamic loading (Figure II.6.6.2). Impermeable platens were used for unconfined compression. The loading platen was connected to an eccentric circular cam driven by a motor. Optimal parameters were determined through empirical testing, using biomechanical properties of tissue constructs as the main readout. Following tare load and tare equilibrium, cyclical displacements were applied to agarose discs, with a saw tooth wave to determine lift-off limits, and recovery of the samples after each loading cycle. Dynamic axial compression was applied as a sinusoidal wave with 10% peak-to-peak compressive strain amplitude, at 1 Hz frequency, to mimic physiologic joint loading.

FIGURE ii.6.6.2 Bioreactor with mechanical loading for tissue engineering of cartilage. Custom designed bioreactor demonstrating how scaffolds experience loading under sterile conditions. The culture well is fitted with a custom designed loading lid imposed by the cam follower system.
(Reproduced by permission from Mauck et al., 2000.)
After four weeks of culture, with loading applied five days per week, with three “one-hour-on-one-hour-off” cycles per day, significant differences in mechanical properties of the engineered articular cartilage were observed as compared to free swelling controls. Specifically, the equilibrium aggregate modulus in the loaded group increased 21-fold over the time of cultivation, and reached a value that was 6-fold higher compared to the free swelling controls. As expected, the improved biomechanical properties correlated with increased amounts of cartilage proteoglycans (Mauck et al., 2000).
The increased nutrient supply interacted synergistically with the mechanical signals to advance tissue growth, as shown by combining dynamic deformational loading and the supplementation of TGF-β1 to the chondrocyte-laden agarose constructs. A particularly interesting result is that dynamic deformational loading applied in sequence with TGF-β (two weeks of growth factor supplementation followed by mechanical loading) yielded significantly increased overall mechanical properties. The equilibrium modulus reached 1306 ± 79 kPa and glycosaminoglycan levels reached 8.7 ± 1.6 % w.w. during the eight-week period of cultivation, and both of these values are similar to host cartilage (994 ± 280 kPa, 6.3 ± 0.9 % w.w.) (Lima et al., 2007).
Tissue Engineering of Anatomically-Shaped Human Bone
Success of bone reconstructions is uniquely dependent on the viability, mechanical function, and precise geometry of the bone grafts. Traditionally, autologous or allogenic tissues are harvested, reshaped, and implanted, leading to suboptimal geometries, tissue quality, and morbidity. Tissue engineering offers the possibility of generating personalized tissues, tailored to the unique geometrical requirements of each patient and the defect being repaired, by using autologous or allogeneic cells, customized biomaterial scaffolds, and customized bioreactors. Critical to bone development are sufficient access to nutrients, regulated by mass transport, and exposure to shear that results from fluid flow and dynamic mechanical compression. Tissue engineering of bone requires the cultivation of spatially uniform seeded cells on mechanically strong scaffolds, with interstitial flow of culture medium to provide mass transport and hydrodynamic shear at levels necessary for proper bone formation.
The combined anatomical, biological, and functional requirements are probably most stringent for bones of the head and face. Craniofacial bone grafts with clinical utility require mechanical functionality, viability, capacity for integration with blood supply, and precise geometry, furthering the need for new methods to engineer anatomically correct yet functional tissues. Grayson et al. (2009) developed a bioreactor capable of meeting some of the above requirements. The group demonstrated the feasibility of engineering “designer bone grafts,” in their report of engineered anatomically accurate temporomandibular joint (TMJ) condular bone (Figure II.6.6.3A–C), one of the most complex joints in the human body.
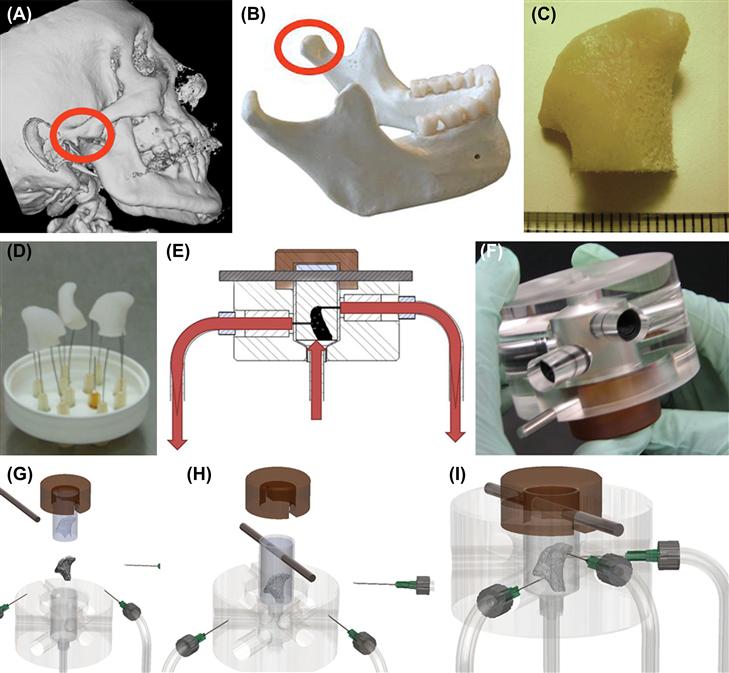
FIGURE ii.6.6.3 Bioreactor for tissue engineering of anatomically shaped bone grafts. (A–C) Scaffold preparation. (A, B) Clinical micro-computed tomography (μCT) images were used to obtain high-resolution digital data for the reconstruction of exact geometry of human TMJ condyles. (C) These data were incorporated into MasterCAM software to machine TMJ-shaped scaffolds from fully decellularized trabecular bone. (D) A photograph illustrating the complex geometry of the final scaffolds that appear markedly different in each projection. (E) The scaffolds were seeded in stirred suspension of human mesenchymal stem cells, to three million cells per scaffold (~1 cm3 volume), precultured statically for one week to allow cell attachment, and then the perfusion was applied for an additional four weeks. (F) A photograph of a perfusion bioreactor used to cultivate-anatomically shaped grafts in vitro.
(G, H, I) Key steps in bioreactor assembly. See text for details. (Reproduced by permission from Grayson et al., 2009.)
The group selected fully decellularized bone in the exact geometry of the TMJ (Figure II.6.6.3D) as the scaffold material. This way, the cells were cultured in a scaffold that had the structural, biochemical, and mechanical properties of native bone (through the use of fully decellularized bone), and the actual geometry of the final graft (through image-guided fabrication). The void volume of decellularized bone was >80%, as determined by microcomputer tomography (μCT), and the pore sizes were approximately 1 mm, as determined by scanning electron microscopy (SEM) and histological analysis.
The exact shape of the patient’s TMJ was recreated from μCT images, and the resulting three-dimensional images were used to shape the scaffold using a computer-driven machine. Human mesenchymal stem cells (hMSCs) were seeded into the scaffold at a physiological density, and cultured in an “anatomical bioreactor” in which bone differentiation and bone formation were induced. The bioreactor itself provided controllable interstitial flow through the scaffold, by adjusting the input and output flow rates throughout the entire scaffold, based on the measurements and mathematical modeling of medium flow.
The bioreactor consisted of an external chamber machined from plastics (acrylic and polyetherimide) (Figure II.6.6.3F). The outer chamber housed a polydimethylsiloxane (PDMS) mold, which in turn held the scaffold. The PDMS mold was created in two steps: digital computer tomography images of a patient’s TMJ condyle were used to generate a three-dimensional copolymer in the exact shape of the TMJ; and PDMS was poured over the model and cured to generate the mold. Once placed inside the mold, the scaffold was further compressed by the acrylic external chamber, which served to ensure perfusion through the scaffold rather than peripheral flow around the scaffold (Figure II.6.6.3G, H). Six ports were built into the acrylic chamber – three inlets and three outlets. A peristaltic pump was used to drive the medium through a common inlet, which interfaced to the three inlet ports (Figure II.6.6.3I). The exiting medium was recirculated through the scaffold via a medium reservoir (Figure II.6.6.3E), which also served as a bubble trap.
Modeling of interstitial flow through the bioreactor revealed flow patterns through the cell-seeded scaffold. The model incorporated physical properties of the scaffold, including its porosity. The fluid was assumed as incompressible and passing through a porous medium, with no-slip boundary conditions at scaffold boundaries. The inlet flow was assumed as fully developed, to achieve laminar flow with minimal shear at construct surfaces. The experimental flow results from the bioreactor substantiated the model. After seeding with hMSCs, tissue constructs were cultured in osteogenic medium for a total of five weeks, at a medium flow rate of 1.8 mL/min. Rapid, spatially uniform proliferation of hMSCs was facilitated by the high porosity and large pore sizes in decellularized bone scaffolds. Enhanced bone formation and mineralization were achieved, resulting in tissue constructs with physiological cell density, and bone-like architecture. The resulting bone grafts were centimeters in size, fully viable, with physiological cell density and the formation of mineralized bone matrix.
The use of this “anatomical” bioreactor system showed, for the first time, the feasibility of engineering anatomically correct pieces of three-dimensional human bone, with the ultimate aim of generating patient-specific bone grafts. Notably, the bioreactor system is modular, and can be readily modified to accommodate the cultivation of bone grafts with different shapes, by simply changing the PDMS mold and adjusting inlet port positions to ensure fluid will perfuse the entire scaffold. Overall, the system has significant potential for bone tissue engineering, in a domain where graft geometry, viability, and function define the success of bone repair.
Cardiac Tissue Engineering with Mechanical Stretch
Motivated by the positive results obtained using neonatal rat hearts to reconstitute tissue-like structures within an elastic scaffold, it was hypothesized that the application of passive and cyclic mechanical stretch within bioreactors would improve cell alignment and differentiation of cardiac cells (Zimmermann et al., 2002, 2006). Over the course of ten years, the Eschenhagen–Zimmermann group has established an effective approach to cardiac tissue engineering by cultivation of neonatal rat heart cells in collagen gel with the application of mechanical stretch. The addition of Matrigel (extracellular matrix from Engelbrecht Swarm tumors) has been essential for the differentiation and functional assembly of cardiac myocyte. This effect is most likely due to the high growth factor content in Matrigel, and was never reproduced using other scaffold materials. Interestingly, mixed populations of heart cells (myocytes, endothelial cells, and fibroblasts) could be cultured in type I collagen gels, but the contractile force developed by the engineered tissue and its responsiveness to calcium and isoprenaline of engineered cardiac constructs were markedly better if Matrigel was included.
In the first version of this approach, neonatal rat ventricular myocytes were suspended in a gel consisting of collagen type I and Matrigel that was cast into rectangular wells (11 × 17 × 4 mm) holding Velcro-coated silicone tubes (7 mm length, 3 mm outer, 2 mm inner diameter) kept at a fixed distance with a metal wire spacer (Figure II.6.6.4A1) (Fink et al., 2000). The mixture was allowed to gel before culture medium was added to the dish, and the resulting gel–cell constructs were cultured for four days before being exposed to unidirectional stretch, using a motorized device for six days (Figure II.6.6.4A2). In this device, tissues were connected at both ends to stretching bars, which pulled the matrix apart with a constant frequency of 1.5 Hz and +20% of original length. The entire arrangement was held in a CO2 incubator at 37°C, and the culture medium was changed every other day. These early studies showed that the stretch improved organization of cardiac myocytes, increased the mitochondrial density, the length of myofilaments, and the force of contraction generated by the tissue construct.

FIGURE ii.6.6.4 Bioreactors for cardiac tissue engineering with mechanical stimulation. (A) Bioreactor for initial studies for stretching of rectangular engineered cardiac tissues. (1) Culture dish of six tissues before stretching. (2) Bioreactor incorporating motorized phasic stretching (1.5 Hz, +20% of original (spacer) length) (Reproduced by permission from Fink et al. 2000.) (B) Experimental set-up for circular cardiac tissue preparation and culture. (1) Casting mold assembly in which silicone tubing (T) was glued to the surface of glass culture dishes. Either Teflon™ disks (D) or cylinders (C) can be placed over silicone tubing to function as removable spacers during casting mold preparation and tissue culture, respectively. (2) Tissue condensation around the central Teflon™ cylinder in casting molds between culture days 1 to 4. (3) Tissues placed in bioreactor fitted with stretch apparatus to continue culture under unidirectional and cyclic stretch (10%, 2 Hz). Bars = 10 mm. (Reproduced by permission from Zimmermann et al., 2002.) (C) Tissues shown: (1) before; (2) during; and (3) after stacking. Scale bar = 10 mm. (4) implantation of cardiac tissues into rat model.
(Reproduced by permission from Zimmermann et al., 2006.)
In the next version of the approach, they strove to improve upon certain shortcomings, including the simplification of complicated casting techniques and improvement of inhomogeneous cell distribution. In the next generation set-up, neonatal rat cardiac cells were suspended in the collagen–Matrigel mix, and cast around cylindrical molds (Zimmermann et al., 2002). After seven days of culture, the rings of cardiac tissue were placed around two rods of a mechanical stretcher, and subjected to unidirectional cyclic stretch at 10% strain and 2 Hz (Figure II.6.6.4B1–3). With this approach, the level of structural and functional organization of the dissociated cells in hydrogel that was achieved within a relatively short time period was quite remarkable. Cells reconstituted cardiac-like tissue with interconnected, oriented cells displaying sarcomeres, adherens junctions, gap junctions, and desmosomes. Ultrastructurally, cardiac myocytes displayed a predominant orientation of sarcomeres along the longitudinal axis of the cell, and were composed of Z, I, A, and H bands, with the occasional presence of M bands. Importantly, capillary structures positive for CD31 were also noted. Functionally, the constructs exhibited contractile properties with a high ratio of twitch to resting tension, strong β-adrenegenic response, and action potentials characteristic of rat ventricular myocytes.
The size, homogeneity, and relative strength of the engineered tissues consisting of cardiac cells in hydrogel were further improved by fusing engineered cardiac tissue together (Zimmermann et al., 2006). A new bioreactor could accommodate five loop-shaped constructs as they fused to form a single synchronously contracting multi-loop construct ~15 mm in diameter and 1–4 mm thick (Figure II.6.6.4C 1–3). When these constructs were investigated for their ability to support or improve the contractile function of infarcted hearts in an immunosupressed rat model of cardiac ischemia (Figure II.6.6.4C4), grafted hearts were able to develop a greater active force than ungrafted, indicative of the engineered constructs playing a significant role in the regeneration of contractile activity in the infarcted hearts. Although the implants did not reverse the problems associated with infarction, they did significantly improve the diastolic and systolic heart function.
This elegant and effective approach has shown, in a rigorous and convincing way, that immature cardiac cell populations have a remarkable ability to assemble into cardiac constructs, if subjected to mechanical signals during cultivation. The loop-shaped constructs, assembled into larger structures, could not only slow down the progression of heart disease in an animal model of chronic infarction, but could also yield some functional improvements. The use of a hydrogel scaffold that contained growth factors necessary for cell differentiation, enabled encapsulation of the cells, and the application of mechanical stretch was critical for cell assembly and development of mechanical force.
Cardiac Tissue Engineering with Electrical Stimulation and Medium Perfusion
In native heart, mechanical stretch is induced by electrical signals, and the orderly coupling between electrical pacing signals and mechanical contractions is crucial for the development and function of native myocardium. In an attempt to direct the differentiation and functional assembly of cardiac cells by factors guiding native development and function of the heart, the Vunjak-Novakovic group (Radisic et al., (2008); Radisic et al., (2004); Tandon (2009); Maidhof et al., (2010); Maidhof et al., (2011)) developed a “biomimetic” approach to cardiac tissue engineering. They engineered compact, synchronously contracting cardiac tissue constructs, by providing an environment combining two critical functions: (1) convective–diffusive oxygen transport (critical for cell survival and function) via perfusion bioreactors; and (2) excitation–contraction coupling (critical for cell differentiation and assembly) by using bioreactors with perfusion (interstitial flow) of culture medium and electrical stimulation.
Oxygen Supply by Perfusion
In native heart muscle, a capillary bed provides efficient exchange of oxygen, nutrients, and metabolites between the blood and tissue cells, while shielding cardiomyocytes from direct exposure to hydrodynamic shear. Therefore, the design of cardiac tissue engineering scaffolds and bioreactors should be based on the provision of efficient mass transport of nutrients (and most critically oxygen), along with the control of hydrodynamic shear. Both the transport rates and hydrodynamic shear increase with the increase in flow rate of culture medium; while low values of shear stress may induce phenotypic changes in cardiac cells, including elongation, higher values (e.g., >2.4 dyne cm2) cause cell dedifferentiation, apoptosis, and death.
The group developed scaffolds and bioreactors that allow: (1) rapid cell inoculation into scaffolds using forward–reverse flow (Radisic et al., 2008); and (2) interstitial flow of culture medium through inoculated scaffolds. Cardiac cell populations isolated from neonatal rat heart ventricles and enriched for cardiac myocytes were suspended in Matrigel at concentrations of approximately 108 cells/mL (corresponding to physiological cell density in native myocardium), and loaded into a porous collagen scaffold (Ultrafoam™). By thermal gelation, cells were locked in place inside collagen scaffolds, and the perfusion of culture medium could be established. In these hybrid scaffolds, the gel phase provided cell encapsulation and growth factor supply, and the collagen scaffold provided the porous structure and biomechanical properties that supported the formation of contractile cardiac grafts.
The first-generation bioreactor consisted of perfusion loops comprised of tubing and cartridges (outfitted with silicone gaskets made from silicone tubing to hold scaffolds in place) that were maintained inside a cell culture incubator and connected to a peristaltic pump (Figure II.6.6.5A). During cell inoculation, forward–reverse flow was applied to increase the spatial uniformity of cell seeding, critical for engineering thick constructs with high and spatially uniform densities of viable cells. After cell seeding, the volume of culture medium was increased as required for longer-term cell culture, and the pump was switched to unidirectional flow.
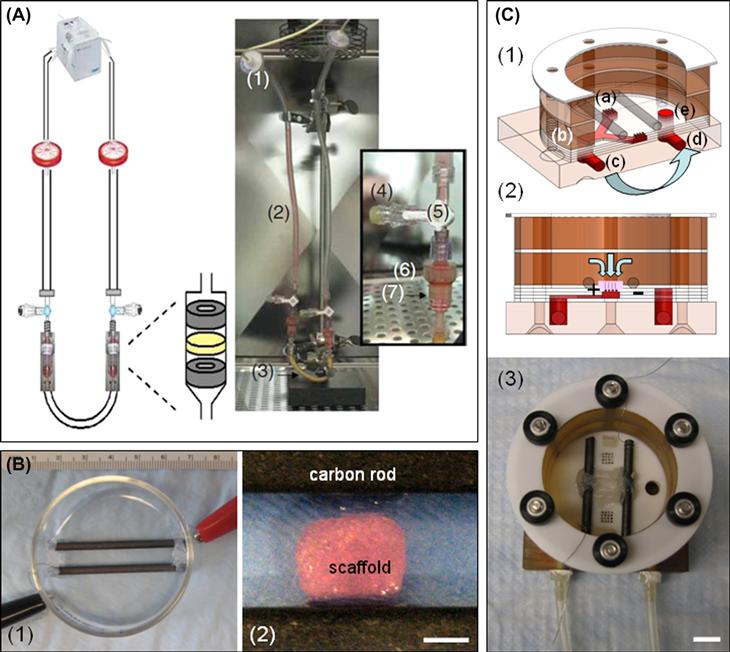
FIGURE ii.6.6.5 Bioreactors for cardiac tissue engineering with perfusion and electrical stimulation. (A) Perfusion loop for cell seeding consisting of a multichannel peristaltic pump: (1) filters; (2,3) U-shaped tubing; (4) injection sites; (5) three-way stopcocks; (6) Apollo perfusion chambers with tissue construct. In the perfusion chamber, the construct was squeezed between (7) two silicone gaskets to force the flow through the 5-mm diameter scaffold core. (Reproduced by permission from Radisic et al., 2008.) (B) (1) photograph of an assembled electrical stimulation bioreactor; and (2) close-up view of scaffold positioned between electrodes. Scale bar = 3 mm. (Reproduced by permission from Tandon et al. 2009.) (C) Perfusion-stimulation bioreactor: (1) Alternating layers of custom machined Teflon™, silicone, and plastic are stacked to a “perfused dish” culture chamber where medium flows from the medium bath (a); into the “Y” shaped channel (b); out of the bioreactor (c); through a peristaltic pump, where it is pumped back to the bioreactor inlet (d); and back into the medium bath (e). Light blue arrows denote medium flow direction. (2) Cross-sectional view of the bioreactor showing flow path through the construct and carbon rod electrodes that provide electrical stimulation. (3) Final assembly of the bioreactor with spacers to keep construct loosely in place and platinum wires to connect electrodes to a cardiac stimulator. Scale bar = 1 cm.
(Reproduced by permission from Maidhof et al., 2011.)
Notably, the final cell viability in perfused constructs cultured for eight days was indistinguishable from the viability of the freshly isolated cells, and markedly higher than the cell viability in dish-grown constructs. Cells expressing cardiac markers (sarcomeric α-actin, sarcomeric tropomyosin, cardiac troponin I) were present throughout the perfused constructs. In response to electrical stimulation, perfused constructs contracted synchronously, had lower excitation thresholds, and readily recovered their baseline function levels after treatment with a gap junction blocker. However, most cells in perfused constructs were round and mononucleated, a situation that was likely due to the exposure of cardiac myocytes to hydrodynamic shear, in contrast to the native heart muscle where blood is confined within the capillary bed and is not in direct contact with cardiac myocytes.
Convective–Diffusive Oxygen Supply in Perfused Channeled Scaffolds
The observed effects of hydrodynamic shear on cardiomyocytes motivated the design of scaffolds with an array of channels that provide a separate compartment for medium flow. In order to reduce the exposure of cardiac myocytes to hydrodynamic shear, the Vunjak-Novakovic group has designed porous elastomer scaffolds of poly(glycerol sebacate) with an array of parallel channels providing a separate compartment for medium flow (Maidhof et al., 2010, 2011). The developed a technique for spatial uniform seeding of these channel’s scaffolds by stacking scaffolds two at a time, with channels not aligned to each other, so as to block medium flow through channels during seeding, and force the flow of cell suspension through the bulk phase of the scaffold. Following seeding, the scaffolds were separated (to reveal channels) and cultured individually. When cultured under these conditions, constructs were observed to contract synchronously in response to electrical stimulation after only three days of culture, while channels remained open. Improved construct properties were correlated with the enhanced supply of oxygen to the cells.
Electrical Stimulation of Contractile Constructs
In order to deliver electrical signals that can induce synchronous contractions of cultured constructs, the Vunjak-Novakovic group designed a custom bioreactor delivering signals mimicking those in native heart (Tandon et al., 2009). This bioreactor has several unique features, including the maintenance of a constant position of the scaffolds with respect to direction of the electrical field gradient, while neither restricting the contractions of cells, nor the ability to observe the constructs under a microscope. The bioreactor is fitted with a pair of parallel carbon rod electrodes that were held in place by silicone adhesive (Figure II.6.6.5B). The spacing between the electrodes accommodates the width of the constructs between them.
When neonatal rat cardiomyocytes suspended in Matrigel onto collagen sponges were cultured for three days subjected to trains of monophasic electrical pulses (5V/cm, 1 Hz, 2 ms duration) for an additional five days, they progressively developed into conductive and contractile cardiac constructs (Radisic et al., 2004). On a molecular level, electrical stimulation increased the expression of myosin heavy chain, Cx-43, creatine kinase-MM, and cardiac troponin-I. Morphologically, cells in stimulated constructs were more aligned and elongated, and contained abundant mitochondria, in addition to cells with clearly visible M and Z lines, and H, I, and A bands closely resembling those in native myocardium.
The effects of electrical stimulation depended strongly on the time of its initiation, indicating that cells required some time to reassemble their excitation–contraction coupling machinery before electrical stimulation could provide any reinforcement of its positive effects on cellular differentiation. Functionally, electrical field stimulation also induced cell alignment and coupling, increased the amplitude of synchronous contractions, and resulted in a remarkable level of ultrastructural organization of engineered myocardium, over only eight days of cultivation.
Bioreactors with Perfusion and Electrical Stimulation
Most recently, the group developed a bioreactor that simultaneously applies two conditioning stimuli – medium perfusion and electrical stimulation (Figure II.6.6.5C1–3) to scaffolds seeded using the perfusion loops (Figure II.6.6.5A) (Maidhof et al., 2011). The perfusion-stimulation bioreactor was machined from alternating layers of Teflon™, silicone, and plastic (polyetherimide), forming a “Y” shaped channel that routes culture medium through two constructs and into a single perfusion outlet. Constructs may freely contract, and are only loosely kept in place between silicone spacers and carbon rods (which also served as electrodes for stimulation) over an array of perforated holes through which culture medium was drawn. The upper bioreactor pieces form a medium bath above the constructs that serves for both gas exchange and as a trap for any bubbles generated during perfusion flow. A silicone tubing loop connects the bioreactor inlet and outlet and a peristaltic pump is used to flow culture medium through the system at a rate of 18 μL/minute.
The bioreactor was characterized by mathematical modeling, and in dye flow studies to show that the electrical field stimulus was linear, and that the flow was restricted to cultured tissues. Cardiac constructs were formed by seeding neonatal rat heart myocytes into highly porous poly(glycerol sebacate) scaffolds with an array of parallel channels (250 μm diameter, 500 μm wall-to-wall spacing) and subject to simultaneous perfusion and electrical stimulation (3 V/cm, 3 Hz, 2 ms duration monophasic square waves) for a period of eight days (three days of pre-culture and five days of stimulation).
Electrical Versus Mechanical Stimulation
Because of the high degree of electro-mechanical coupling, it is difficult to decipher whether there are indeed roles for the application of separate mechanical and/or electrical stimulation for engineering cardiac constructs. In constructs prevented from contracting by supplementation of verapamil, electrical stimulation maintained the gap junction protein connexin-43 (Cx-43) at levels comparable with stimulated drug-free controls, suggesting that electrical stimulation, even without contractile activity, aids in the establishment of functional gap junctions (Radisic et al., 2004). Similarly, mechanical stimulation resulted in functional coupling of cardiac cells (Zimmermann et al., 2002). Mechanical stretch and electrical stimulation each appeared to greatly enhance the differentiation of cardiomyocytes, and it may be speculated that a method incorporating the application of both factors could outperform a regime applying just one.
Constructs grown with simultaneous perfusion and electrical stimulation exhibited improved functional properties, including a significant increase in contraction amplitude, greater DNA content and a more uniform cell distribution throughout the scaffold thickness. Simultaneous perfusion and electrical stimulation also enhanced cell morphology, tissue organization, and cardiac protein expression, as shown by immunostaining and Western blot analysis. This novel bioreactor that enables the simultaneous application of culture medium perfusion and electrical conditioning may be appropriate for generating cardiac constructs with clinical size and organization, and may pave the way for further studies incorporating the delivery of combinations of biophysical forces in tissue engineering applications.
Tissue Engineering of Heart Valves with Mechanical Stimulation and Perfusion
To repair heart valve defects, especially in children, it has been proposed to engineer replacement valves consisting of viable cells that could grow in sync with the developing heart. An autologous cell source offers the best chances of graft compatibility, but the method of obtaining differentiated heart valve cells (i.e., smooth muscle and endothelial cells) requires an invasive procedure. Therefore, research has been directed towards obtaining stem cells from less invasive sources (i.e., bone marrow-derived mesenchymal stem cells), and differentiating these cells during culture in vitro.
In the body the biophysical forces the heart valve cells are exposed to include: (1) mechanical stretch as the valves are forced open and closed; and (2) hydrodynamic shear as the blood flows over the valve surface. Each of these forces was independently shown to enhance the differentiation, organization, and mechanical properties of engineered heart tissue valves in in vitro bioreactor cultures. Therefore, it was proposed that a combination of these conditioning regimes applied during culture could yield further improvement of the engineered tissues.
To test this hypothesis, a flex-stretch-flow (FSF) bioreactor was developed to simultaneously apply mechanical stimulation and hydrodynamic shear to engineered heart valves (Figure II.6.6.6) (Engelmayr et al., 2008). The bioreactor consists of two stationary posts to which tissue specimens can be affixed, and cyclic flexure is applied by a third post attached to a linear actuator. Parameters for the amount of flexure of the engineered valves, and the flow rate for hydrodynamic shear, were selected based on measurements of typical valve opening and closing distances in the heart, and estimations of shear stress in the native heart. Culture medium is recirculated within the FSF bioreactor chamber by a magnetically coupled paddle-wheel driven by an immersible magnetic stirrer. The entire FSF bioreactor is constructed from sterilizable parts, and may be operated in an incubator for several weeks of culture.
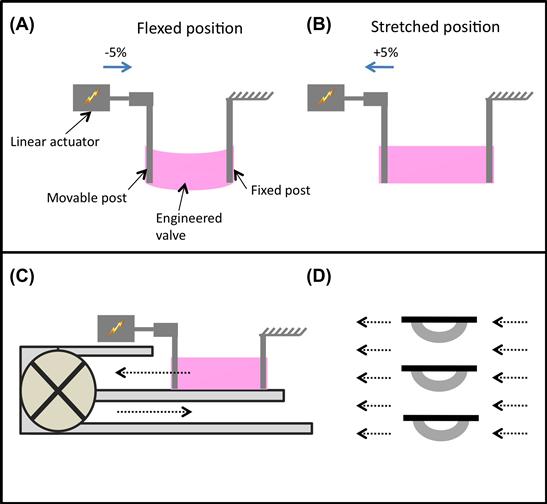
FIGURE ii.6.6.6 Flex-stretch-flow bioreactor for engineering a heart valve. (A) Each scaffold is attached to a movable post coupled to a linear actuator and a stationary post. (Reproduced by permission from Engelmayr et al., 2008.) The scaffolds are then flexed (A) or stretched (B) via activation of the linear actuator along the direction of the blue arrow. Culture medium can be recirculated (C, dashed black arrows) within the bioreactor chamber via a magnetically coupled paddle-wheel to provide laminar flow and associated fluid shear stresses to scaffold specimens. In the no flex groups, scaffold specimens are maintained in the straight, undeformed configuration (solid black bars) with or without flow (D). In the flex groups, scaffold specimens are cycled at 1 Hz between the undeformed and flexed configurations (curved gray bars), with or without medium flow.
Mesenchymal stem cells (MSCs) derived from sheep bone marrow aspirates were cultured on sheets of nonwoven scaffold containing 50% fibers of polyglycolic acid and 50% fibers of poly-L-lactic acid that were assembled into a valved conduit by manual and machine needle punching, and cultured in the bioreactor. The combined application of mechanical stimulation and hydrodynamic shear resulted in increased collagen content and effective stiffness of engineered valves after three weeks of culture. In control groups that excluded either one or both of these conditions, collagen content and mechanical properties were lower. By the end of three weeks the combination of mechanical stimulation and hydrodynamic shear resulted in engineered valves with a modulus comparable to those generated starting from smooth-muscle cells in previous studies. Thus, the FSF bioreactor can be used to provide cyclic flexure and laminar flow, and can synergistically accelerate bone MSC-mediated tissue formation in engineered heart valves.
Tissue Engineering of Blood Vessels with Pulsatile Medium Flow
Atherosclerotic vascular disease is a major cause of mortality in the US. A commonly used treatment modality is bypass grafting with autologous veins or arteries, but adequate tissue for bypass vessels is lacking in many patients. Engineered blood vessel grafts could make up for this tissue shortage; however, issues including insufficient mechanical properties of the engineered vessels, and lack of a differentiated cell population, have limited clinical implementation. To help overcome these shortcomings, the design of a pulsatile perfusion bioreactor was proposed over a decade ago (Niklason et al., 1999).
During vasculogenesis and throughout life, vascular cells (endothelial and smooth muscle cells) are exposed to pulsatile physical forces generated with each heartbeat. Studies have shown that mechanical stretch is an important factor in extracellular protein synthesis and expression; specifically, cyclic stretch upregulates collagen and other extracellular matrix protein production in vascular cells. A biomimetic system to culture engineered vessels should replicate this cyclic stretch, with culture medium serving as the “blood” and a pulsatile pump providing the “heart beat.”
A suspension of smooth muscle cells (SMCs) isolated from the medial layer of bovine aorta and expanded in culture was seeded onto tubular scaffolds made of fibrous polyglycolic acid (PGA). The surface of the PGA scaffolds was chemically modified with sodium hydroxide to cause ester hydrolysis on the surface of the fibers, and enhance hydrophilicity, adsorption of serum proteins, and cell attachment. The seeded scaffolds were fitted with silicone tubing, placed into the vessel lumen, and transferred into bioreactors and cultured with the application of pulsatile flow for eight weeks. At the end of cultivation, the silicone tubing was removed, a suspension of bovine endothelial cells was introduced, and the cells were allowed to adhere for three days to the lumen surfaces.
The bioreactor was designed to support the cultivation of tubular scaffolds seeded with cells and attached to a perfusion loop, so that culture medium was forced to flow through the lumen (Figure II.6.6.7). A pulsatile pump was used to vary the medium pressure inside the vessel over each cycle, causing the vessel to cyclically stretch in the radial direction. The cultivation conditions were designed to mimic those found during fetal development (~165 beats per minute and 5% radial distension of the vessel).

FIGURE ii.6.6.7 Pulsatile flow bioreactor for engineering blood vessels. A pulsatile pump is used to flow culture medium through the lumen of an engineered blood vessel causing cyclic stretch of the tissue during culture. After exiting the vessel the medium is returned to a reservoir that provides oxygenation and allows for medium exchange throughout the culture period. (Niklason et al., 1999.)
After eight weeks of culture, the gross appearance of the engineered vessels was similar to that of native arteries. Bioreactor-cultured vessels developed significantly greater mechanical properties (rupture strength, suture retention) than those cultured without pulsatile flow, and matched and correlated with the increased collagen content. Upon implantation into the right saphenous vein of a miniature pig, vessels subjected to cyclic stretch remained open to blood flow for up to four weeks, whereas non-stretched vessels developed thrombosis after only three weeks. Taken together, these results indicate that a bioreactor that subjects engineered vessels to cyclic mechanical stretch can improve the differentiation of engineered vascular grafts prior to implantation.
Challenges in Bioreactor Design
Part of the motivation behind bioreactor development has been to create greater correlation between in vitro studies and in vivo outcomes. The thought has been that advanced tissue culture platforms, recapitulating the in vivo environment in a controllable manner, would aid in the transition from cell studies to animal models, and eventually to human clinical trials and even the clinic. These engineered “biomimetic” environments allow cells to be measured and physically manipulated in unprecedented ways, and they are now replacing the simple but deficient environment of culture dishes. However, several important challenges remain to be resolved to establish conditions that are predictive of cell behavior in vivo, and provide bioreactors beyond the laboratory bench.
Producing Conditions More Predictive of Cell Behavior In Vivo
In terms of providing bioreactors that will better recapitulate the actual cell–tissue environment, and be more predictive of cellular responses in vivo, the main challenge is the difficulty of identifying and optimizing necessary biophysical and molecular cues. Although bioreactors outperform standard culture systems in their ability to provide matrix, molecular, and biophysical factors, current systems still lag behind nature’s ability to deliver the highly coordinated sequences of spatial and temporal gradients of regulatory factors at the level of the cell which are necessary to regulate cell function in a developing and adult organism. This performance gap is likely responsible for at least some of tissue engineering’s limited success in delivering on the promise of a “one stop shop” of engineered tissues for the entire body.
For reasons that are still little understood, certain tissues (such as cardiac and osteochondral) are more conducive to tissue assembly within bioreactors than others (such as pancreatic and hepatic). Perhaps some bioreactor limitations will be ameliorated through future research in developmental biology, which may shed more light on how tissues emerge from coordinated sequences of cell proliferation, differentiation, and functional assembly that are orchestrated by factors originating from the surrounding cells, matrix, and the external environment. Likewise, future research on regeneration of adult tissues may shed more light on how cells respond to the entire milieu of injury or disease, and help incorporate the most critical biophysical and/or molecular cues into the repertoire of current bioreactors. Some known biophysical cues, such as molecular gradients, although relatively facile to apply in microfluidic conditions, are difficult to produce in the larger-scale three-dimensional culture settings of the bioreactor, without applying significant shear stresses or using excessive amounts of expensive culture medium.
Furthermore, the large cell-number and media requirements for most bioreactors still preclude the performance of screening studies, which are key to optimizing culture conditions, let alone transitioning results from animal models to human stem cells. Overcoming this challenge may indeed involve the development of high-throughput microbioreactors that are equipped to provide certain well-known biophysical cues (e.g., transport, mechanical stimulation, electrical stimulation).
Providing Bioreactors Beyond the Laboratory Bench
In the current tissue engineering paradigm, a common approach is to culture cells on a biomaterial scaffold and regulate the environmental conditions via bioreactors toward reconstructing a functional tissue. With this approach, one can attempt to manipulate the cells’ responses in predictable ways, by utilizing the same complex factors known to direct development and remodeling in vivo, with the eventual goal of producing a tissue with enough fidelity for implantation. Several challenges related to current bioreactor fabrication economics and logistics remain, before tissue engineering may deliver on this promise.
A bioreactor should ideally provide a native-like cell environment, and address the specific requirements for the tissue of interest. Therefore, bioreactors are often custom-designed to recapitulate the specific mechanisms of nutrient transfer and specific biophysical signals in each type of tissue. These custom-designed systems require an understanding not only of general cell culture principles, but also of the specific mechanisms of relevance to the tissue at hand. As cells are highly sensitive to changes in their environment, even minute changes in molecular and biophysical cues may have implications on the reproducibility of a bioreactor’s regulation of cell survival, behavior, and differentiation capacity. Therefore, quality control and implementation of protocol transfer are of great importance to the tissue engineering community.
Further challenges to bioreactor design are also related to the complexity of regulatory issues, and the design constraints of surgical techniques and the operating theater. For tissue-engineered constructs to be viable and clinically useful, bioreactor design and protocols should be compatible with the transportation of biologically stable tissue engineered implants. Therefore, the application of engineered tissues in vitro – as experimental models of development and disease – will remain much easier than the cultivation of engineered tissues for implantation.
Interestingly, the best strategy for engineering functional tissue grafts is, in many cases, a simple one – not everything needs to be recapitulated, and one key factor provided within the right context can make the difference. The application of mechanical or electrical stimulation to high-density cultures of cells on scaffolds is an excellent example of this situation. The field has made remarkable strides with the application of limited sets of molecular and biophysical cues, and identified the next set of challenges that call for true interdisciplinary work of biomedical engineers, basic scientists, and clinicians.
Worked Examples
Perfusion bioreactors have been used extensively in tissue engineering in order to provide adequate nutrient supply, as well as mechanical stimuli to the cells inside a scaffold. A round perfusion bioreactor (Figure II.6.6.8) consists of an inlet, an outlet, and a culture chamber, and allows the cultivation of six scaffolds simultaneously. The culture medium coming into the inlet splits into six channels, and flows through the scaffolds into the media chamber. One major function of a bioreactor is to mimic physiologic conditions in vitro. In previous studies, bone cells were exposed to physiologic shear stress ranging from 0.25 dynes/cm2 to 25 dynes/cm2. In an experiment designed to assess the effect of shear stress on bone tissue engineering, the medium flow rates corresponded to shear stresses of 1 dynes/cm2 and 10 dynes/cm2. Cylindrical scaffolds (5 mm in diameter and 5 mm long) can be modeled as 70% porous material consisting of an array of channels 400 μm in diameter (Figure II.6.6.9), with the cells lining the channel lumens. Estimate the flow rate required to provide the low and high shear stress, assuming fully developed laminar flow in the longitudinal direction, steady-state, incompressibility of culture medium, μ = 7.7 × 10−4 Pa⋅s, and ρ = 1 g/ml.
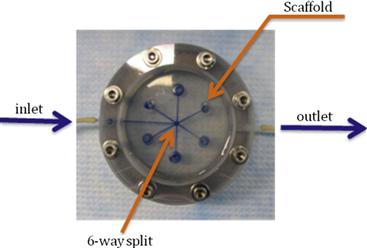
FIGURE ii.6.6.8 Schematic of a simple bioreactor with direct perfusion of culture medium through cultured tissues.

FIGURE ii.6.6.9 Schematic of a single channel within a tissue construct perfused with culture medium.
Solution
The analysis can be done in the single channel within the scaffold.
Flow Rate in the Single Channel
The velocity profile of a fully developed laminar circular pipe flow can be derived from the Navier–Stokes equations:
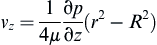
The volumetric flow rate of single channel can be determined by:
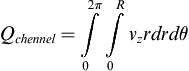
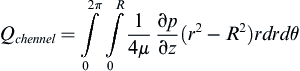
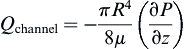
The relationship can be expressed in terms of the pressure drop:
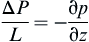
Or:
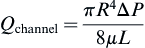
The average velocity is:
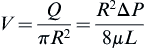
The maximum velocity occurs at the center of the tube:
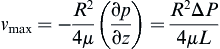
Hence:
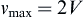
The velocity profile can now be written as:
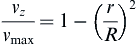
and the shear stress equation becomes:
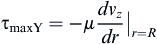
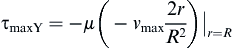
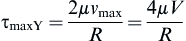
Now we can solve for the flow rate:
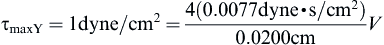

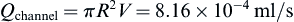
Checking the Reynolds number:
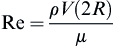
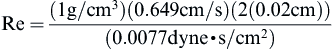
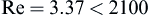
we confirm that the laminar flow assumption was valid.
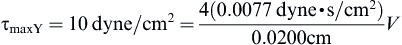
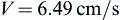

Checking the Reynolds number:

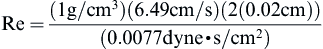
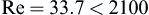
we confirm that the laminar flow assumption was valid.
where vz indicates the velocity in the z–direction (see Figure II.6.6.9), µ indicates the viscosity of the fluid, ρ indicates the pressure within the pipe, r indicates the radial position, and R indicates the radius of the pipe. Qchannel indicates the flow within a channel, L indicates the length of the channel, V is average velocity, vmax is the maximum velocity, τmaxY is the maximum shear in the Y-direction.
Scaffold Geometry
Cross-sectional area of the scaffold is:
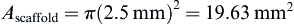
Void area of the scaffold is:

Cross-sectional area of single channel is:

Therefore, each scaffold contains the following number of channels:
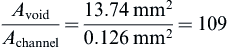
As there are six scaffolds per bioreactor, there are 654 channels in the scaffold.
Acknowledgment
We gratefully acknowledge the NIH support of the work described in this chapter (HL076485, DE016525, HL089913, EB002520, RR026244).
Bibliography
1. Bilodeau K, Mantovani D. Bioreactors for tissue engineering: Focus on mechanical constraints A comparative review. Tissue Engineering. 2006;12(8):2367–2383.
2. Cheng X, Irimia D, Dixon M, Sekine K, Demirci U, et al. A microfluidic device for practical label-free CD4+ T-cell counting of HIV infected subjects. Lab on a Chip. 2007;7:170–178.
3. Cheng X, Gupta A, Chen C, Tompkins RA, Rodriguez W, et al. Enhancing the performance of a point-of-care CD4+ T-cell counting microchip through monocyte depletion for HIV/AIDS diagnostics. Lab on a Chip. 2009;9:1357–1364.
4. Cimetta E, Figallo E, Cannizzaro C, Elvassore N, Vunjak-Novakovic G. Micro-bioreactor arrays for controlling cellular environments: Design principles for human embryonic stem cell applications. Methods. 2009;47:81–89.
5. Engelmayr GC, Soletti L, Vigmostad SC, Budilarto SG, Federspiel WJ, et al. A novel flex-stretch-flow bioreactor for the study of engineered heart valve tissue mechanobiology. Annals of Biomedical Engineering. 2008;36(5):700–712.
6. Figallo E, Cannizzaro C, Gerecht S, Burdick JA, Langer R, et al. Micro-bioreactor array for controlling cellular microenvironments. Lab on a Chip. 2007;7:710–719.
7. Fink C, Ergün S, Kralisch D, Remmers U, Weil J, et al. Chronic stretch of engineered heart tissue induces hypertrophy and functional improvement. The FASEB Journal: Official Publication of the Federation of American Societies for Experimental Biology. 2000;14(5):669–679.
8. Freed LE, Vunjak-Novakovic G. Culture of organized cell communities. Advanced Drug Delivery Reviews. 1998;33:15–30.
9. Freed LE, Guilak F, Guo XE, Gray ML, Tranquillo R, et al. Advanced tools for tissue engineering: Scaffolds, bioreactors, and signaling. Tissue Engineering. 2006;12(12):3285–3305.
10. Freshney I, Obradovic B, et al. Principles of tissue culture and bioreactor design. In: Lanza RP, Langer R, Vacanti J, eds. Principles of Tissue Engineering. San Diego, CA: Academic; 2007;155–184.
11. Grayson WL, Fröhlich M, Yeager K, Bhumiratana S, Chan ME, et al. Regenerative medicine special feature: Engineering anatomically shaped human bone grafts. Proceedings National Academy of Sciences USA. 2009;107(8):3299–3304.
12. Hubbel JA. Biomaterials in tissue engineering. Biotechnology. 1995;13:565–575.
13. Kaplan D, Moon RT, Vunjak-Novakovic G. It takes a village to grow a tissue. Nature Biotechnology. 2005;23(10):1237–1239.
14. Kirouac DC, Zandstra PW. The systematic production of cells for cell therapies. Cell Stem Cell. 2008;3:369–381.
15. Kretlow JD, Mikos AG. From material to tissue: Biomaterial development, scaffold fabrication, and tissue engineering. AIChE Journal. 2008;54(12):3048–3067.
16. Langer R, Tirrell DA. Designing materials for biology and medicine. Nature. 2004;428:487–492.
17. Lima EG, Bian L, Ng KW, Mauck RL, Byers BA, et al. The beneficial effect of delayed compressive loading on tissue-engineered cartilage constructs cultured with TGF-beta3. Osteoarthritis Cartilage. 2007;15(9):1025–1033.
18. Maidhof R, Marsano A, Lee EJ, Vunjak-Novakovic G. Perfusion seeding of channeled elastomeric scaffolds with myocytes and endothelial cells for cardiac tissue engineering. Biotechnology Progress. 2010;26(2):565–572.
19. Maidhof R, Tandon N, Lee EJ, et al. Biomimetic perfusion and electrical stimulation applied in concert improved the assembly of engineered cardiac tissue. Journal of Tissue Engineering and Regenerative Medicine 2011; 10.1002/term.525; 2011; [Epub ahead of print].
20. Martin Y, Vermette P. Bioreactors for tissue mass culture: Design, characterization, and recent advances. Biomaterials. 2005;26(35):7481–7503.
21. Mauck RL, Soltz MA, Wang CC, Wong DD, Chao PH, et al. Functional tissue engineering of articular cartilage through dynamic loading of chondrocyte-seeded agarose gels. Journal of Biomechanical Engineering. 2000;122(3):252–260.
22. Muschler GF, Nakamoto C, Griffith LG. Engineering principles of clinical cell-based tissue engineering Journal of Bone and Joint Surgery. American Volume. 2004;86A(7):1541–1558.
23. Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, et al. Functional arteries grown in vitro. Science. 1999;284(5413):489–493.
24. Peppas NA, Langer R. New challenges in biomaterials. Science. 1994;263(5154):1715–1720.
25. Radisic M, Park H, Shing H, Consi T, Schoen FJ, et al. Functional assembly of engineered myocardium by electrical stimulation of cardiac myocytes cultured on scaffolds. Proceedings National Academy of Sciences USA. 2004;101(52):18129–18134.
26. Radisic M, Marsano A, Maidhof R, Wang Y, Vunjak-Novakovic G. Cardiac tissue engineering using perfusion bioreactor systems. Nature Protocols. 2008;3(4):719–738.
27. Stolberg S, McCloskey KE. Can shear stress direct stem cell fate?. Biotechnology Progress. 2009;25(1):10–19.
28. Tandon N, Cannizzaro C, Chao PH, Maidhof R, Marsano A, et al. Electrical stimulation systems for cardiac tissue engineering. Nature Protocols. 2009;4(2):155–173.
29. Toner M, Irimia D. Blood-on-a-chip. Annu Rev Biomedical Engineering. 2005;7:77–103.
30. Wang H, Riha GM, Yan S, Li M, Chai H, et al. Shear stress induces endothelial differentiation from a murine embryonic mesenchymal progenitor cell line. Arteriosclerosis Thrombosis and Vascular Biology. 2005;25:1817–1823.
31. Zimmermann WH, Schneiderbanger K, Schubert P, Didié M, Münzel F, et al. Tissue engineering of a differentiated cardiac muscle construct. Circulation Research. 2002;90(2):223–230.
32. Zimmermann W, Melnychenko I, Wasmeier G, Didié M, Naito H, et al. Engineered heart tissue grafts improve systolic and diastolic function in infarcted rat hearts. Nature Medicine. 2006;12(4):452–458.