4
ABSENCE AS A DISEASE

In every street the pipes gushed out where decaying rat carcasses drank everything in.… Bellies in the air, they floated amid apple peels, asparagus stalks and cabbage cores.… it was like a vast infection of tooth decay, like the flatulence of a rotting stomach, like the emanations of a man who has drunk too much, like the dried sweat of rotting animals, like the sour poison of a bedpan.… this avalanche of excretions tumbling down the length of the purulent streets… let off its nocturnal fragrances.
—LE FIGARO
IN THE 1800S the world was seized by spasms of cholera. The first pandemic began in India in 1816 and spread through China, where more than a hundred thousand died. A second pandemic began in 1829 and spread across Europe, and when it was done thirty years later, many hundreds of thousands of people, from Russia to New York, had died. Then, in 1854, cholera started to spread anew, this time globally. In one city after another, whole families died and their dead bodies were loaded into carts together. More than a million people perished in Russia alone. Apartment buildings went from jubilant scenes of the daily circus of work and family to empty shells. In some cities more people died than were born. Ecologists call scenarios in which populations are sustained by immigration alone population sinks, a euphemistic term.1 Cities were sinks down the drains of which human lives poured.
The spread of cholera was blamed on miasma. The miasma theory claimed that diseases, including cholera, were caused by bad-smelling air (the miasma), particularly bad-smelling night air. Miasma is an easy concept to mock, and yet a reasonable sentiment. It encapsulates the idea that bad odors are often associated with illness. Evolutionary biologists argue that an understanding of the relationship between putrid odors and disease is ancient and wired into our subconscious brains.2 Over our long evolutionary history, avoiding odors we perceived as disgusting would have made our ancestors more likely to survive.3 Avoiding the smell of dead bodies would reduce the risk of contagion from pathogens on those dead bodies. Avoiding the smell of feces would reduce the risk of getting sick from pathogens in the feces. In this way, the concept of miasma is perhaps so old as to be almost innate. Unfortunately, as cities evolved, the correlation between the odors of putrefaction and disease was no longer useful. Everything smelled bad; to run from the smell was to get out of town, a solution only the rich could afford.
The quest to understand the true cause of cholera was characterized by decades of false starts and a general inability of scientists and the public to pay enough attention to the data in front of them. But in mid-nineteenth-century London, one man, John Snow, was paying a little more attention than were others. Snow had come to believe that cholera was caused by some sort of “germ” passed not from the air but instead from the feces of one person to the mouth of another person. Although feces smelled, the germs themselves, he reasoned, did not. People didn’t like this idea. It was at odds with the miasma theory. It was also gross. Then, in 1854, building on work of Reverend Henry Whitehead, Snow collected data on where people were getting sick with cholera and where they weren’t in the Soho District of London. Soho had been particularly hard hit by the disease.

Figure 4.1 A re-creation of the map on which Dr. John Snow marked the location of deaths from cholera in Soho, London, in 1854. Each black bar marks a death and each P indicates the location of a water pump. With this map, Snow demonstrated that most of those who died lived near or drank from the Broad Street well. (Figure modified from map re-created by John Mackenzie [2010], based on original map by John Snow [1854].)
What Snow eventually came to see was that deaths in Soho were grouped together in a single large, lumpy patch. He discovered why. All of the people in that patch used water from the same well, a well on Broad Street (now Broadwick Street). Some families not using the Broad Street well also died, but as it turned out, those families had also drunk at least a little water from the Broad Street well when their own well smelled of miasma. Snow then mapped the recent deaths from cholera in Soho with the aim of showing that they emanated from the Broad Street well.
Snow used his map to argue that the contamination of the Broad Street well was making people sick and that if the well handle was removed (hence closing down the well), the disease would stop killing the people of Soho.4 He was right, though it would take years to convince many of his peers of as much. Meanwhile, the cholera epidemic in Soho subsided on its own.5 It was later shown that the well had been contaminated by an old diaper in an abandoned cesspit next to the well. Years later, Robert Koch, the microbiologist who identified Mycobacterium tuberculosis as the cause of tuberculosis, identified the organism that caused cholera, Vibrio cholerae. The pathogen had evolved in India and then spread with trade to London and around the world in the early 1800s.
It took decades to figure out how to reconstruct cities so as to deter such contamination; in London the more immediate answer was to pump water into cities from far enough away that it was less likely to be contaminated. After Snow’s discovery, cities, including London, began to more actively control disposal of human waste. In some, but not all, cases, they also began to treat the incoming drinking water. Hundreds of millions and perhaps even billions of lives were saved.6 Interrupting the movement of pathogens from one person’s feces to another person’s mouth worked.
Inspired by Snow, maps of the spread of disease became a common feature of the field of epidemiology. Students learn that Snow’s map was the first illustration of the spread of a disease (not really true). They also learn the power of a map to show the likely origin of a disease and to imply a potential cause. Typically, when maps are used in epidemiology, the goal is to describe when and where a particular species, a pathogen, is present, and then to infer why. Maps depict correlations, but they help epidemiologists think about causation, about how and why. But maps can also betray our ignorance, which happened in the 1950s when a new set of diseases emerged.
Crohn’s disease, inflammatory bowel disease, asthma, allergies, and even multiple sclerosis were among this new tribe—the ugly horsemen of malaise and malfunction. All of these diseases were associated with one or another form of chronic inflammation. But what was causing the inflammation?
These diseases were too new to be solely genetic. What was more, just like cholera in London, these diseases had a geography. It was an unusual geography. These diseases, unlike cholera, were more common in regions with good public health systems and infrastructure. The more affluent a region, the more likely it seemed its population was to suffer these diseases. This pattern defied the understanding of “germs” and their geography we’d had since the work of Snow. Yet, in looking at maps of these diseases, in regard to geography or other factors, one might still approach them the way Snow did. He would have used the available maps of the diseases to come up with hypotheses on causes. He would then have looked for natural experiments as a way to test his hypotheses. Finally, once the best hypothesis was tested to his satisfaction, he would have used maps once again to show what he thought to be true. And then, and only then, the details of the biology of the actual agent of disease could begin to be understood. So it was to be with these new diseases. To begin, someone needed to come up with hypotheses and then, on the basis of natural experiments, test them.
The diseases were blamed on new pathogens, on refrigerators, and even on toothpaste. One ecologist, Ilkka Hanski, came to be part of a team that would argue something else entirely was to blame—not exposures to some particular bacterium but instead the absence of exposures. Hanski is an unlikely character in a story about chronic disease and bacteria. He started his career as a world expert on dung beetles. One can read his story, chapter by chapter, in his autobiography. He started to document his life in 2014, writing in a hurry because, as he told his friends in March of 2014, he was dying of cancer. He wanted to get down on paper, for posterity, those things he thought to be most important about the biological world.
In the book, the reader follows Hanski through the stages of his career. Throughout these stages, Hanski was drawn to the study of small patches of island-like habitat. At first those patches were dung piles. For a beetle, a pile of dung is an island that must be discovered and, very rapidly, colonized. Hanski used his own feces or dead fish as bait for the beetles. He attracted and trapped them while hiking up and down Mount Mulu in Borneo to understand the general rules governing when numerous species compete for a pile of shit and when few do. Hanski then moved on to the study of a single butterfly species, the Glanville fritillary (Melitaea cinxia), in the Åland Islands off southern Finland. He used this butterfly species to understand ways rare species wax and wane in small patches of habitat. For decades he followed this butterfly and its parasites and pathogens across more than four thousand patches (they are still being tracked). Through this work, he elaborated mathematical models that quantified just how small and isolated habitats could become before the species in those habitats went extinct. Later, Hanski became interested in why some individuals of a particular butterfly species were able to thrive in the face of habitat fragmentation. He discovered versions of genes that seemed to be associated with the ability of some but not other butterfly individuals to live successfully even in a world of small patches of good habitat. Collectively, Hanski’s insights, based on fieldwork, theories, predictions, and tests won him the Crafoord Prize in Bioscience in 2011, ecology’s version of the Nobel Prize.
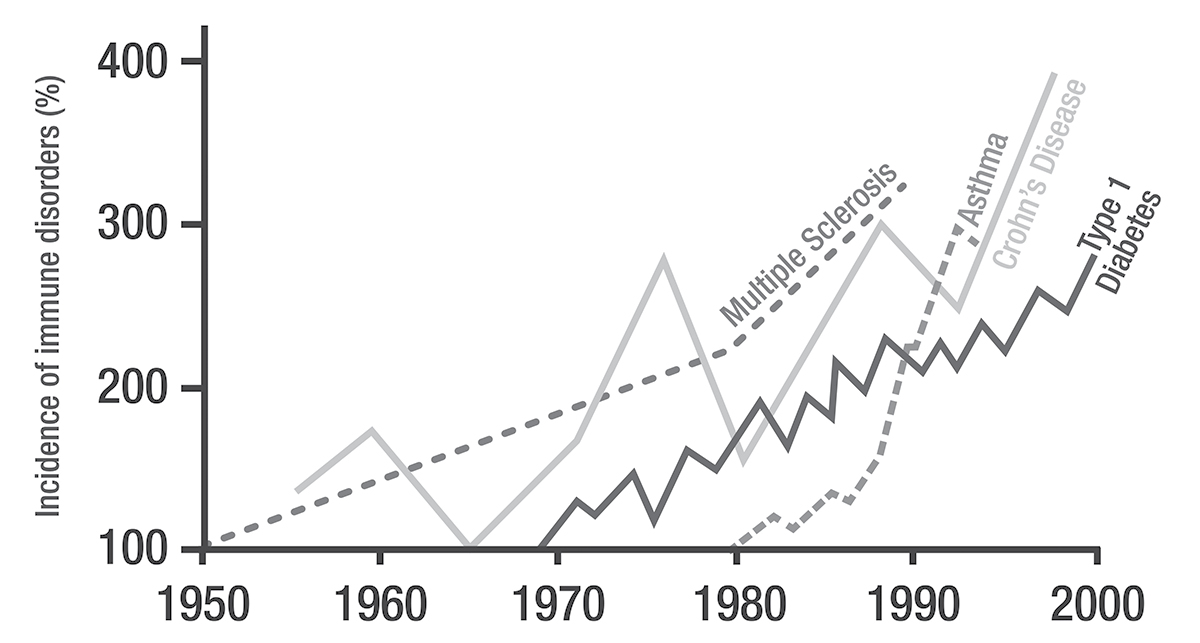
Over decades, Hanski’s work became ever more focused, moving from study of whole dung beetle communities to an individual butterfly species, to a genetic variant of a butterfly species. Then, suddenly, he began to study chronic inflammatory diseases in humans. A chance meeting inspired the change. In 2010 Hanski saw Tari Haahtela, Finland’s preeminent epidemiologist, give a presentation on chronic inflammatory diseases.7 The material Haahtela covered in his talk was different from anything Hanski had ever worked on or even really seen. It was raw and affecting. Haahtela described the rise in the incidence of chronic inflammatory diseases. He showed that these diseases had all become about twice as common every two decades since 1950; more so in wealthier countries. This rise continues. In the last twenty years in the United States, for example, allergies have increased by 50 percent, and asthma has increased by a third. And as poorer countries invested more in urban development, they also saw increases in inflammatory diseases. This global pattern was both striking and worrisome. The rising lines on Haahtela’s graphs could be stock prices, human population sizes, or the cost of butter, but for their labels. Their labels bespoke beasts, terrible chronic diseases, stalking the indoors. Haahtela showed maps of places where these diseases were common and where they were not.
Haahtela argued that the illnesses were not caused by pathogens. It was not about germ theory but instead almost its opposite. Haahtela thought that people were getting sick because of their failure to be exposed to species they needed. He didn’t know which species those might be any more than Snow knew which contaminant in the well caused cholera. In looking at the maps, Hanski had an idea about what might be missing. To Hanski, the maps and trends that Haahtela showed looked like the inverse of maps and trends he would go on to show in his own presentation on the global loss of old-growth forests and their biodiversity of dung beetles, butterflies, birds, and all the rest. As biodiversity declined through time, chronic diseases seemed to become more common. What was more, in the developed regions where the most biodiversity had already been lost (particularly from people’s daily, indoor lives), the diseases were the most common. Hanski thought that perhaps what was missing from people’s lives and what was making them sick was not a species but rather something broader. What was missing was biological diversity itself. What was missing for the first time ever in the history of vertebrates, maybe the history of animals, was wild nature. It was missing from backyards, houses, Manhattan apartments, and the International Space Station (ISS) alike.
At this point, Haahtela had already been thinking about the links between biological diversity and disease, albeit using metaphor as much as hard data. In 2009, he had even written a paper in which he noted that in those places in which the diversity of Finnish butterflies had declined, chronic inflammation was more common. He included pictures in the paper of some of his favorite butterflies—the Elban Heath, De Lesse’s Brassy Ringlet, the Two-Tailed Pasha, the Polar Fritillary, Nogel’s Hairstreak, and a half dozen other species. When the habitat these species needed started to become too fragmented and rare and they started dying out, humans got sick.8 The butterflies were indicators of a deeper connection between the wilderness outside and the wilderness in people’s homes and the consequences of its absence. A disconnection from pathogens such as cholera, one part of nature, benefits humans. But now, humans had gone too far and disconnected themselves not only from the few species that are real and deadly monsters but also from the rest of biodiversity, including beneficial species.
Haahtela approached Hanski. The two men talked. They had met before. Many years earlier, Haahtela, who photographs butterflies as a hobby, pointed Hanski to the Glanville Fritillary as a species on which to focus his studies. As they reconnected, the two men were reminded that they enjoyed each other’s company. Both men loved butterflies, and now they were also joined by a common set of megatrends: the loss of biodiversity, the increasing rates of chronic inflammatory diseases, and the societal shift to indoor living, where biodiversity tended to be even more reduced than it had become outside.9 If they were right that these trends were related, things were only going to get worse. The threat to biodiversity is growing, and our shift indoors, away from biodiversity, is ever more complete. Haahtela invited Hanski to attend one of his lab meetings, where Hanski also met Leena von Hertzen, a microbiologist and a key partner in what would come next. In the meeting, the excitement was the sort that makes arm hair stand on end. Hanski felt, he would later write in his autobiography, as if he had joined one of the most exciting collaborations of his life. Some major component in the world was, it seemed, about to be worked out.
When Snow proposed that feces in water was spreading something to people that was causing cholera, he didn’t specifically understand what was being spread. In the same way, Hanski, Haahtela, and von Hertzen didn’t understand what aspect of biodiversity loss was making people sick, but they had some ideas about how this loss was making people sick. The possibility of a link between exposure to biodiversity and well-being has been tossed around for decades, both in the context of immune health and more generally. E. O. Wilson argued, in his biophilia hypothesis, that we humans have an innate fondness for biodiversity and our emotional well-being diminishes in its dearth.10 Roger Ulrich has argued that nature reduces stress; Stephen Kaplan, that exposure to biodiversity increases attention spans.11 The nature deficit disorder extends these hypotheses to consider the ways in which biodiversity, and nature more generally, can promote learning and the psychological well-being of children.12 The loss of biodiversity, these theories suggest, causes us to ache emotionally, psychologically, and intellectually. Hanksi and Haahtela were influenced by all of this work but thought there was more going on. They thought the loss of biodiversity was also making our immune systems “ache” and malfunction. In their thinking, the antecedent on which they were most directly building was a hypothesis and series of studies arguing that chronic autoimmune diseases were associated with lives that were too clean, too hygienic. This “hygiene hypothesis” was first proposed by David Strachan, an epidemiologist at Saint George’s University of London, in 1989. Strachan argued that our modern cleanliness had removed from our lives necessary exposures.13 Hanski and Haatehla thought that the kind of exposure that was missing was an exposure to biodiversity, the rest of life.
Like small governments, human immune systems are composed of many working parts, organized into chains of command and consequence, and governed by rules that are followed most but not all of the time. In chronic inflammatory diseases, two pathways are relevant. The pathway we have understood for a while is that when a substance (an antigen), be it a dust mite protein or a deadly pathogen, is detected by immune cells on the skin, in the gut, or in the lungs, it triggers a chain of signals that decide whether the immune system attacks the antigen both in that instance, by relying on white blood cells such as eosinophils, and in the future. When the attack response is triggered, a cascade of signals is sent from one kind of cell to another until those signals trigger the recruitment of a circus of different kinds of white blood cells and (in some but not all cases) initiate the production of specific immunoglobulin E (IgE) antibodies. The IgE antibodies remember the antigen and bind to it whenever it turns up again. The key here is to know that this pathway detects antigens, decides whether to attack them, and decides whether to make future attacks easy. If it does this right, it enables the immune system to be able to respond to pathogens quickly. If it does it wrong, the immune system attacks the wrong things, and allergies, asthma, and other inflammatory disorders develop. A second pathway works to balance the immune response by both preventing the buildup of white blood cells such as eosinophils and preventing IgE antibodies from responding to whichever antigen has been detected. This separate pathway (which includes its own specific receptors, a range of regulatory compounds, and signaling molecules) keeps the peace when it is needed, which is most of the time. Most antigens aren’t dangerous, especially those that occur frequently and that are associated with ordinary environmental exposures or the species that live on the skin, in the lungs, or in the gut; it is the job of this peacekeeper pathway to remind the body of as much. Strachan, and others, suggested that this peacekeeper pathway, the immune system’s soothing voice of reason, wasn’t being sufficiently stimulated by ordinary daily exposures. What they could not explain was just what was missing in urban childhoods or childhoods that were too “clean,” just what kind of absence triggered this lack of regulation. Hanski, Haahtela, and von Hertzen thought that exposure to biodiversity in the environment, in homes, and on the body somehow helped keep the immune system’s peacekeeper pathway functioning normally. In the absence of exposure to biodiversity, the immune system was developing IgE antibodies and inflammation to many antigens that weren’t actually dangerous, such as bits of dust mites, German cockroaches, fungi, or even the body’s own cells. If children weren’t exposed to enough wild species, the regulatory pathway didn’t do its job. Allergies and asthma developed, and a host of other problems also ensued. Or so they speculated. These ideas, however exciting they might be, needed to be tested.
Conversations about how and where to test these ideas almost invariably came back to one place, modern Finland. In modern Finland a kind of natural experiment has been under way since the end of World War II. Among Finns, the incidence of chronic inflammatory disorders had increased everywhere except in one place: the Russian half of Karelia, a region that had once been part of Finland but that was no longer. Before World War II, the Karelia region, which stood on the border of Finland and Russia, was united under Finnish rule. After the war, the new Finnish-Russian border bisected the region, yielding Russian Karelians and Finnish Karelians, people with a common heritage but distinct futures.
Today, in Russian Karelia, life expectancies are relatively short owing to traffic accidents, alcoholism, smoking, and every possible combination of each of these problems. In Finnish Karelia, all of these causes of death are less common. For the most part, folks in Russian Karelia were on the bad side of the fence. But the Finnish Karelians are prone to illnesses that Russians are not: chronic inflammatory disorders. Asthma, hay fever, eczema, and rhinitis were (and have remained) three to ten times more common in Finland than in Russia. Hay fever and peanut allergies are nonexistent in Russian Karelia.14 Finnish Karelia, on the other hand, is a microcosm of the parts of the world where chronic inflammatory diseases have become ever more common. Since the war, each generation in Finnish Karelia has been more likely than preceding generations to suffer from inflammatory disorders. Not their relatives across the border in Russian Karelia.
Haahtela and von Hertzen had spent the better part of a decade comparing the lives of Karelians on both sides of the border in the aptly named “Karelia Project.” On the basis of intensive surveys and blood tests for the IgE antibodies associated with particular allergies, they were able to show that the differences in the prevalence in allergies between the two populations were real. More significantly, they had come to believe that the diseases in Finnish Karelia were due to the absence of exposure to environmental microbes.
Russian Karelians live lives very much like those their ancestors lived fifty or a hundred years ago. They live in small, rural houses lacking central air-conditioning and heating systems, have daily exposure to domestic animals, including cattle, and grow most of their own produce in small vegetable gardens. Their drinking water comes from wells drilled into the groundwater below their homes, or it comes from the surface water of nearby Lake Ladoga. The region is still largely forested and biologically diverse. The Finnish Karelians live in a very different environment. They live in much more developed towns and cities, with much less biological diversity. Compared to Russian Karelians, the Finnish Karelians spend much more time inside, in houses that are sealed more tightly from the outdoors. The life they are exposed to is ever more like that of the ISS and ever less like that of a trail through an old and wild woods.
Haahtela and von Hertzen along with their students had shown that some plant-associated microbes seemed to be missing from the daily lives of children growing up in Finnish Karelia, but they hadn’t connected all the pieces. Now, with Hanski involved, they started to develop their full argument, which was that the loss of outdoor biological diversity (be it butterflies, plants, or anything else) led to the loss of indoor biological diversity, which then led to immune systems in which eosinophils were too abundant and, as a result, chronic inflammatory diseases. In a paper led by Leena von Hertzen, the scientists called this idea the “biodiversity hypothesis,”15 which together they would proceed to test.
The ideal project would have been to experimentally alter the biodiversity to which children were exposed in their homes and backyards and then follow those same children over the next few decades. In theory, this might have been possible, but the process would have also been very expensive and prolonged. Another approach would have been to compare the lives and exposures of the Karelians in Russia and in Finland, but this proved untenable at the time. So Hanski, Haahtela, and von Hertzen decided to take a third approach. They would study a single region within Finland, one Haahtela and von Hertzen had been working in since 2003. In this region, they would test whether teens (fourteen- to eighteen-year-olds) living in houses with less biodiversity were more likely to have immune systems prone to allergy and asthma.
The chosen region was a square, 100 kilometers by 100 kilometers. It included a small town, villages of different sizes, and isolated houses. Within the region, Haahtela and von Hertzen randomly selected homes. Nearly all of the families in the randomly selected homes had not moved in many years, hence their teens had grown up in the same house in which they now lived (an impossibility in many regions). One might criticize the scientists for not choosing a more diverse region or working in many regions. One could criticize many things. But as the ecologist Dan Janzen has often noted,16 the Wright brothers did not take off in a thunderstorm. Hanski, Haahtela, and von Hertzen chose to begin their work where they could control as many extraneous factors as possible and build on the data already in hand.
The team tested each of the teens for allergies. They then also measured the biodiversity in their backyards and the biodiversity on their skin. They predicted that teens with less biodiversity in their backyards would have less biodiversity on their skin and would, in turn, be more likely to have allergies. They measured biodiversity by counting the number of kinds of nonnative plants, native plants, and rare native plants present in backyards. Each plant tends to have its own associated bacteria and fungi and even its own associated insects, so measuring plants was a way to capture a simple proxy of the rest of the life the teens might encounter. Plants are also easier to measure than other organisms because they are visible (in contrast to microbes) and don’t move (in contrast to, say, butterflies or birds).17 Skin bacterial biodiversity was measured on the middle of the forearm of teens’ writing hands. Bacterial biodiversity was tallied in much the same way we tallied it in homes in Raleigh. Finally, allergies were measured as a function of the IgE antibodies in the blood of the teens. In general, more IgE equals more allergies. In those teens with high IgE levels, the team also tested for allergies to specific antigens such as cats, dogs, or mugwort.
The study was straightforward, and each investigator had a specific role. Haahtela was in charge of blood samples to test for allergies, von Hertzen was in charge of skin samples to measure bacterial communities, and Hanski was in charge of sampling and studying the diversity of plants. Everyone worked together on the analyses. It was exciting, a potentially big step forward, though also, in some ways, far-fetched.
WHEN HANSKI AND his colleagues looked at the data, they were excited but also anxious. Would the diversity of plants in the homes of the teens really matter? Although the scientists had controlled for as many factors as they could, predicting differences in the health of humans is notoriously difficult. For Hanski, in particular, this was trying. Humans, he was quickly learning, were far more difficult to study than were dung beetles and butterflies. He would have liked to have done an experiment. He worried that if they found no pattern, the study wouldn’t mean anything. Perhaps they would just need to study more teens, or more countries, or across more years.
But what Hanski, Haahtela, and von Hertzen saw was, to them, remarkably clear. Teens living in houses with a higher diversity of rare native plants in their backyards had different bacterial species on their skin. They tended to have a higher diversity of bacteria on their skin, particularly those kinds of bacteria associated with soil. Presumably, those bacteria were landing on teens in their backyards or maybe coming into their homes through open windows and doors and landing on them while they went about their days or even slept. In addition, teens with a higher number of rare native plant species in their backyards and more diverse skin bacteria were also at a reduced risk of allergies. Any allergies.18 The scientists hadn’t done an experiment; they had merely observed a correlation, but the correlation they observed was entirely in line with their hypothesis.
In particular, one group of bacteria, the Gammaproteobacteria, seemed to be more diverse when plant diversity was high and more common on teens with fewer allergies. More than forty years earlier, species of this same group of bacteria had been shown to vary in abundance on human skin with the seasons.19 In the samples Megan Thoemmes took from chimpanzee nests, the abundance of Gammaproteobacteria also varied from season to season. Hanski, Haahtela, and von Hertzen found that the Gammaproteobacteria also varied in space. Again, it didn’t matter whether they considered allergies to cats, dogs, horses, birch pollen, timothy grass, or mugwort. In each case, individuals with more kinds of Gammaproteobacteria, particularly more kinds of one genus, Acinetobacter, on their bodies were less likely to have allergies. In a subsequent study, Hanski and Haahtela, along with another group of researchers, were able to show that individuals (again in Finland) with more of a kind of Acinetobacter on their skin tended to have immune systems that produced more of a compound associated with immunological peacekeeping.20 This same peacekeeping compound was also produced by mice in the lab when they were experimentally dosed with Acinetobacter.21
An additional test of the idea that bacterial diversity, and presence of Acinetobacter in particular, was helping to keep allergies in check was to compare the bacteria on the skin of teens in the Russian and Finnish parts of Karelia. Haahtela led a separate study to find out. The backyard biodiversity should be higher in Russian Karelia than in Finnish Karelia. It was. The skin biodiversity should be higher in Russian Karelia than in Finnish Karelia. It was. Finally, the abundance of Acinetobacter bacteria should be higher on the skin of teens in Russian Karelia than in Finnish Karelia. It was, too.22
WE CAN SEE in Hanski, Haahtela, and von Hertzen’s results a direct relationship between exposure to native plant diversity and the effect of native plant diversity on Gammaproteobacteria on the skin (and other bacteria with similar effects in the lungs and gut), which in turn triggers the peacekeeping pathway of the immune system and keeps inflammation in check.23 We achieved such exposures for tens of millions of years without even having to try. Gammaproteobacteria are diverse in wild plants but also in our food plants. They live as mutualists of seeds, fruits, and stems. We breathed them, we ate them, we walked through them. Then we moved indoors, where the Gammaproteobacteria disappear. They seem to be rare in food plants kept very cold. They disappear when our food plants are processed. They were entirely absent from the ISS and are rare in most urban apartments we have studied. Maybe the diversity of Gammaproteobacteria could be useful not only in the garden but also in potted plants indoors and in fresh fruits and vegetables.24 To test the specific role of Gammaproteobacteria, scientists would need to alter the diversity of plants in backyards, bring a diversity of plants into homes, feed families fresh fruit and vegetables that have (or have not) been sterilized, and then study whether those changes alter, over years, immune health. It would be analogous to when Snow took off the well handle, just something of the opposite, letting biodiversity flow back in. One could do this. No one has.25 One study, though, has come close, building on insights from the study of Amish children, Hutterite children, and mice.
The Amish and the Hutterites both moved to the United States in the eighteenth and nineteenth centuries. Genetically, they have similar backgrounds, particularly with regard to genes known to influence susceptibility to asthma. Culturally, they tend to live relatively similar lives: they eat the same German farm foods, have large families, get vaccinated, drink raw cow’s milk, and otherwise go about life in remarkably similar manners. Neither group watches television or uses any other sort of electricity. Nor does either group believe in keeping animals as pets. All domestic animals in both communities are working animals. In both groups, marriage outside of the group means leaving the group. At a glance, their genes, lives, and experiences are the same. The main difference, biologically, between the Amish and the Hutterites is that the Hutterites have decided to practice industrial agriculture. They drive tractors. They use pesticides. They plant relatively few varieties of crops. In contrast, the Amish farm as they have always farmed, using horses for labor. Amish children are more directly, physically, connected to their fields, animals, and soils than are Hutterite children. Also, the front doors of Amish houses tend to be fifty feet or so away from the barn door, whereas Hutterite houses and Hutterite farms are often separated by great distances. And, as Hanski, Haahtela, and von Hertzen might have predicted, given this difference, asthma is rare among the Amish. The Hutterites, meanwhile, suffer from asthma at rates higher than almost anyone else in the United States. Twenty-three percent of Hutterite children have asthma. And, just like Finnish kids with few wild plant species in their backyards, the Hutterite kids have elevated levels of the IgE antibodies against common allergens in their blood. Nor are these differences in IgE antibodies the extent of the immunological differences.
Recently, a large scientific team, headed by scientists and clinicians at the University of Chicago and the University of Arizona, compared the immune systems of Amish children and Hutterite children. When the team from the University of Chicago studied the blood of a sample of Amish and Hutterite kids in more detail, they found that when challenged with a compound associated with the cell walls of bacteria, the blood of the Amish kids produced fewer of the compounds, cytokines, that signal alarm. In addition, the Amish kids had different kinds and quantities of white blood cells. They had fewer eosinophils, the white blood cells most associated with inflammation. Also, their neutrophils tended to be of a variety that was, to put it plainly, less likely to indiscriminately attack. Finally, the Amish kids had more of a variety of monocyte (yet another kind of white blood cell) associated with suppressing the immune system. In short, the blood of the Hutterite kids was a schoolyard thug and, in comparison, the blood of the Amish kids was peaceful.
The team from Chicago and Arizona decided that one way to isolate the effects of the Amish dust and its microbes on immune systems was to experimentally give individuals with inflammatory diseases doses of the dust. They couldn’t ethically do this experiment on humans, but they could do it with mice. Scientists have bred a variety of mice that suffers from a chronic inflammatory disease akin to allergic asthma. The mice develop asthma symptoms when exposed to egg proteins. Egg proteins are their Kryptonite. The team gave the asthmatic mice three treatments. One group had egg proteins sprayed into their noses every two or three days for a month. One group had egg proteins plus dust from the bedrooms of Hutterite families sprayed into their noses the same number of times. The third group got egg proteins and Amish bedroom dust (dust that it would later be shown tended to have more kinds of bacteria than the Hutterite dust, or more biodiversity). The mice given the egg protein suffered an allergic response akin to asthma. No surprise. The mice given the egg protein and Hutterite dust actually suffered a worse allergic response than did those that got just the egg. But what about the mice given egg protein and Amish dust? The Amish dust nearly completely prevented the allergic response of the mice to the eggs. Not only did the biodiverse Amish dust keep the mice from getting sick, it damn near made them well, even though they were getting dosed every other day with egg protein, their great weakness.26 A Finnish team was able to show a similar effect in mice using dust from barns in rural Finland (but not dust from homes in urban Helsinki).27 This isn’t to say that if you are asthmatic you should go sneaking around Amish bedrooms or Finnish backyards snorting dust (especially not without asking permission), but it may well suggest that you need to be sniffing more biodiversity, more of the wild.
The special stuff in the Amish house dust may have been Gammaproteobacteria that triggered the peacekeeping pathway in the lungs (rather than on the skin) in keeping with the prediction of Hanski and colleagues. But even if it isn’t, even if what is key in the lungs and gut is some other group of bacteria such as, say, the Firmicutes and Bacteroidetes, or maybe even some special fungi, the researchers’ work offers a broader insight that has as much to do with how they framed their question as it does with the details of what they saw: as our exposure to biological diversity, including the biodiversity of plants but also animals and much else, decreases, the odds that we are exposed to the right bacteria, including Gammaproteobacteria, also decreases. We can consider this as probabilistic. Imagine there is a certain number of kinds of bacteria to which you need to be exposed to stay healthy. If this is the case (and given that we don’t know where to even find most kinds of bacteria), the more plants and animals and soil you interact with, the more likely you will pick up some of those key bacteria. The fewer kinds to which you are exposed, the less likely you get the right ones, the ones that activate your innate immune system in the right way to keep the eosinophils in check. But chance is chance, so you could also be exposed to great biodiversity and fail to get what you need; some Amish kids get allergies just as do some kids who live in the Russian part of Karelia; it is just less likely.
It would, of course, be much more satisfying to figure out exactly which of these bacteria we need, make sure we are exposed to them, and leave it at that. Until we do, we are just one step beyond the miasma stage of understanding chronic inflammatory diseases. Taking another step may take a while. Consider the fecal transplant. The best treatment for people whose gut ecosystems are invaded by the weedy pathogen Clostridium difficile is a fecal transplant. In a fecal transplant, a sick person is given a heavy dose of antibiotics. The feces and fecal microbes of a healthy person are then transplanted into the sick person as an attempt to restore the sick person’s ecosystem. It works. Many lives are saved thanks to fecal transplants, which restore enough of the gut ecosystem to prevent Clostridium difficile from thriving. For practitioners, fecal transplants have been a great relief for the treatment of patients who had few other options. Microbiologists, too, have hailed them as innovative, a sign of the future. But they are also an acknowledgment that we don’t know which species is essential, and in lieu of more knowledge, the best option is to restore everything, to reboot and rewild the gut.
Scientists love making and testing predictions. Among the most predictable features of science is its sociopolitics. I predict that over the next ten years any of a variety of pills and treatments will be offered up as ways to cure yourself of chronic inflammatory problems. Some scientists will continue to suggest that the key missing factor is our exposure to particular kinds of tapeworms, hookworms, and other wrigglers. Others will suggest it is exposure to Gammaproteobacteria. Others, that it is exposure to a single species of bacteria, though different labs will argue for different species, and some will argue that we need those bacteria in our food, others that they are more necessary in our water. In the meantime, someone will find a set of human genes that seems to make some people more susceptible to these diseases than others. It will become apparent that different people need exposure to different microbes based on their genetic backgrounds. But, in those studies, the geneticists will realize (relatively late) that they have mostly sampled white male college students and that when they consider a truly diverse population, the story will become even more complex. Ultimately, it will appear that the microbes, or at least exposures to microbes, people need to stay healthy depend on where people live and even their culture. Maybe a perfectly prescriptive model will come out of this suggesting what each person should do. I wouldn’t bet on it, but we need to continue to try to figure it out. It was useful when Snow figured out the mode of transmission of cholera. It was even better when the cholera bacterium, Vibrio cholerae, was identified and water systems could be tested for its presence to be make sure they were safe to drink.
While we wait for perfect clarity, we can acknowledge the problems with the status quo and choose an alternative approach, one that is not perfect and yet that is undeniably better. The status quo is that we are exposed to far different species than we used to be, far fewer because we have diminished the biological diversity of the world around us and because we spend nearly all of our time indoors, a realm we appear to be making ever less diverse. As a result, Crohn’s disease, asthma, allergy, multiple sclerosis, and their kin have become far more common. What then can we offer our children? We need to offer them the chance to interact with a diversity of microbes that, in doing so, increases the odds that they are exposed to the ones they need. Play the ecological lottery more times and you better your chances of getting it right.
Plant a greater diversity of plants outside of your home and interact with those plants. Tend them. Watch them. Take a nap on them. It may well be that having a greater diversity of plants indoors triggers the same sorts of benefits. Grow a garden and sink your fingers into the soil. Or go full Amish and get a cow that you keep near your backdoor. This may well help and it won’t hurt. Meanwhile, we also need to make sure that whatever species we most need still exist in the future. We need to, as Haahtela put it in 2009, “take care of the butterflies,” which is to say, save the biodiversity of life around in general until we know for sure what we need. Save the butterflies for our own good. Save the butterflies because where butterflies are diverse and wild, so too are the microbes, so too are species that we may need but have yet to study. Save the butterflies to pay homage to Ilkka Hanski. Hanski died on May 10, 2016, still in love with butterflies. He died still fascinated by the workings of the world. He died aware that though the flapping of a butterfly’s wings may not change the weather, the extinction of butterflies, or the plants on which both the butterflies and many bacteria depend, can make us sick. We need biodiversity in order to be well. We need it in our backyards and in our homes; we may even need it, as it turns out, in our showerheads.