Chapter 5
Chemistry
Chemical reactions happen inside human bodies all the time. That’s how food is broken down inside the stomach in order to digest it, and that’s how the body makes heat to stay alive, among many other processes that constantly take place inside a human body. But chemistry is also everywhere around us. An appetizing brownie can’t be so tasty without a whole lot of chemistry taking place within the ingredients. It’s certain chemicals in foods that make them taste sour, sweet, or salty. Some serious chemistry is also going on inside the batteries that power all the electronic devices society has come to rely on these days.
This chapter will explore some basic ideas in chemistry and give you and your child a peek at how chemistry is such an integral part of your daily lives.
ACTIVITY: How Batteries Work
You use batteries in many things every single day of your life. You charge the battery of your phone every day; your alarm clock may have backup batteries in case the electricity goes out; your rechargeable laptop and tablet are convenient because you can carry them around. The watch on your wrist and even your car require battery power to run.
How does a basic battery work? What happens inside a battery so that it can provide power? What’s necessary to make the battery work? Why does it eventually stop working so that you have to buy a new one? The best way to learn about batteries is by making one at home.
Materials Needed:
- 5 zinc washers, size #10
- 5 pennies
- Freshly squeezed lemon juice
- Thick paper towel
- Scissors
- Plate
- 2 electric wires with alligator clips at their ends
- Piece of aluminum foil (3"–4" in width)
- 1 LED light bulb
Procedure:
- Assist your child in cutting the paper towel into square pieces that are 2" × 2". Tell him to cut up about 10 such pieces.
- Now have your child fold each paper towel piece in half 4 times. In other words, fold the 2" × 2" square in half, making a 2" × 1" rectangle. Then fold that rectangle in half, making a 1" × 1" square. Repeat the last two steps again until the final folded piece would be a thick square with sides that are 1⁄2" × 1⁄2" that are slightly smaller than the penny’s diameter. Have your child fold all the 2" × 2" paper towel pieces in this fashion before moving on to the next step.
- Have your child immerse the folded pieces of paper towel into the freshly squeezed lemon juice, without squeezing out the juice. Ask him to place those pieces on the plate.
- Have your child clip 1 alligator clip wire to 1 arm of the LED bulb, and another wire to the other arm.
- Next, ask your child to place one of the zinc washers on the aluminum foil. On top of the washer, have him place one of the folded paper towels soaked in lemon juice, then a penny on top. Have him place another folded paper towel that’s soaked in lemon juice on the penny, then a zinc washer on top of that. Basically have him alternate between washer and penny (with folded paper towels soaked in lemon juice in between penny and washer) until the last piece on top is the fifth penny. Tell him he doesn’t need a paper towel over the last penny. Now he’s got a battery.
- It’s time for your child to test his battery. Tell him to touch the loose end of one of the alligator clip wires to the aluminum foil. Assist him in touching the other alligator clip wire’s loose end to the top penny. What do you observe? Does the LED bulb light up?
When the LED bulb lights up, tell your child it is totally powered by the pennies, zinc washers, and lemon juice!
STEM Career Choices
Chemical Engineer
Chemical engineers apply principles of chemistry, physics, and biology in solving problems that relate to food, fuel, drugs, and other chemicals. They design the processes for testing chemical compounds, as well as the instruments they use for such testing.
For the most part, chemical engineers work in laboratories. Depending who they work for, they might have to spend time in refineries, industrial plants, processing plants for food, or some other location. This allows them to collect data to analyze later in their office or laboratory. Some of their jobs include improving processing techniques for food, or developing methods to mass-produce foods or drugs in order to make them more affordable, or designing more efficient procedures to refine different petroleum products.
A battery requires three ingredients: a zinc electrode, a copper electrode, and a liquid containing electrolytes, such as the lemon juice. An electrode is a metal used in an electric circuit that comes in contact with some other nonmetal part of the circuit (such as an electrolyte). Copper has a stronger electronegativity than zinc does, which means copper has a stronger ability to attract electrons than zinc does. In this case, zinc gives away electrons that flow toward the copper (via the wires), and the bulb lights up.
Because the zinc gives up electrons to copper, the zinc electrode is the negative terminal of the battery (also known as the anode). And because the copper is eager to receive electrons from the zinc, the copper electrode is the positive terminal of the battery (also known as the cathode).
But what role does the electrolyte play inside the battery? It’s specifically the acid inside the lemon juice that acts as an electrolyte and dissociates into positive and negative ions. A simple example of an electrolyte is table salt. It consists of one sodium atom and one chlorine atom that are combined. When table salt is dissolved in water, the sodium and chlorine atoms dissociate, becoming positive and negative ions. The negative ions from the electrolyte allow the zinc atoms that just lost electrons (and became positive as a result of that) to move away from the zinc electrode. The positive ions in the electrolyte move toward the copper electrode, because they like the newly arriving electrons the copper took from the zinc, and they will snatch those electrons.
An animated picture looks like this: When the zinc gives away electrons, the electrons fly away along the path of the wire toward the copper that’s electron-thirsty. The wire connecting zinc to copper acts like a superhighway for the electrons. The ions inside the electrolyte are heavy, and don’t move around as easily as the lighter electrons can along the superhighway. However, the negative ions can sweep away (neutralize) the zinc that lost its electrons. If the negative ions didn’t do that, then there would be too many zinc atoms that lost electrons congregating around the zinc electrode, and the flow of electrons would stop. The positive ions are bribed by the newly arriving electrons on the copper electrode, and move toward the copper.
STEM Words to Know
electronegativity
Metal atoms, like zinc and copper, have a tendency to either lose some of their electrons or attract other electrons. When a metal atom has a strong tendency to attract electrons from other atoms, it is said to have a strong electronegativity. Copper has a stronger electronegativity than zinc.
So the zinc and copper electrodes on their own can’t make up a battery. Neither does the electrolyte on its own. The lemon juice alone, or the lemon itself, is not a battery. To turn a lemon into a battery, you need the zinc and copper electrodes. In place of a lemon you can use a lime, a potato, or anything that contains acid, such as vinegar.
Eventually all the zinc gets used up. Also, when all the negative and positive ions in the electrolyte find partners on either electrode, the electrolyte no longer exists. Whichever one happens first brings the life of the battery to an end.
STEM Career Choices
Analytical Chemist
Analytical chemists deal with matter. They analyze its structure and determine its composition. They do their work to make sure food, water, and pharmaceutical drugs meet quality standards for consumption, and are compliant with regulations. They decide which samples to isolate and preserve, and then test them.
Analytical chemists can work in government jobs or can be employed by academic institutions or industrial companies. In addition to doing chemical analyses, they invent new methods for measurement, and design the instruments required for them. They can also be found working in law offices and in marketing firms.
ACTIVITY: Alkaline or Acidic?
One of the many ways food can be categorized is by whether it’s alkaline or acidic. Whether a particular food is considered alkaline or acidic depends on a measurement of certain ions in that food.
In order to understand the nature of ions, we must start with a discussion on atoms. An atom is the smallest building block in nature. The smallest atom found in nature is the hydrogen atom. A hydrogen atom (whose symbol is H) is made up of one proton (a positive charge in the center—or nucleus—of the atom) and one electron (a negative charge that moves around the nucleus). One positive charge attracts one negative charge; therefore, if the hydrogen atom has one positive charge and one negative charge, it is electrically neutral. Most atoms can easily share electrons with other atoms; when they do, so that all the combined atoms are electrically neutral, they form what is known as a compound.
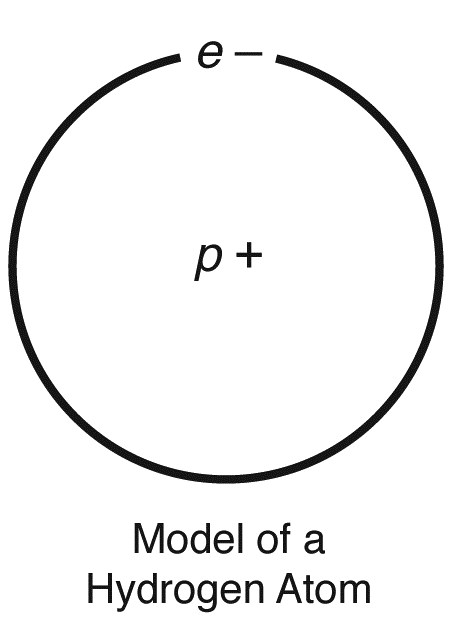
Atoms can lose or gain electrons. When a neutral hydrogen atom, H, loses an electron, it is left with only one positive charge (or proton), but no electrons; it is then called a hydrogen ion (and gets the symbol H+). An ion is not electrically neutral. Remember that atoms can combine together; they do so by sharing electrons. A hydrogen atom, H, can share one electron, while an oxygen atom, O, can share two electrons. If a neutral oxygen atom, O, shares one electron with a neutral hydrogen atom, H, there is still another electron the oxygen atom can share in that union. Such a union between one hydrogen atom and one oxygen atom produces an ion known as a hydroxide ion, OH–.
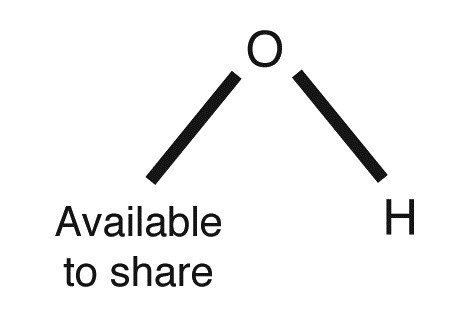
What do ions have to do with a food substance being acidic or alkaline? If the substance has a high concentration of hydrogen ions, H+, it’s considered acidic. An acid is a chemical compound that turns litmus paper red because its pH is low. It usually tastes sour. Acids have the ability to react with some metal compounds. For example, the acetic acid found in vinegar can react with calcium carbonate (found in eggshells), forming bubbles of carbon dioxide gas.
However, if the substance has a high concentration of hydroxide ions, OH–, the substance is considered alkaline. The measurement of acidity or alkalinity is called a pH measurement. To measure the pH of a substance is to measure the concentration of hydrogen ions in it. In fact, the letters pH refer to the “power of hydrogen.”
The numerical measurement of pH ranges from 0 to 14. At pH=0, the substance is strongly acidic, and at pH=14, the substance is strongly alkaline. Pure water has pH=7 (neutral pH). Therefore, any substance that has a pH less than 7 is considered acidic, and if its pH is higher than 7 it is alkaline. For example, white vinegar has a pH range between 2 and 2.4, making it acidic. Milk has a pH of about 6.6, making it closer to neutral. Baking soda has a pH that is slightly higher than 8, making it alkaline.
What about other foods you have in your kitchen? Are they acidic or alkaline? You can easily measure the pH of any liquid food using litmus paper.
Obtain some litmus paper and test the pH of the following foods. (Consult the chart that comes with your litmus paper to establish what color changes indicate acids and alkalines.) It’s best to test these foods in a liquid form, so make sure to juice a fruit or vegetable before you test it. Record your results in the following table so you can categorize your findings. Also, feel free to add more food items to the list of things you would like to test. For example, you can find out what happens to the pH of your milk once you add a sugar-rich cereal to it.
NOTE: Make sure you use freshly squeezed fruits and vegetables.
| Record of Food pH: | ||
|---|---|---|
| Food (in liquid form) | pH value | Acid, Alkaline, or Neutral |
Club soda | ||
Cow’s milk | ||
Coconut milk | ||
White vinegar | ||
Apple cider vinegar | ||
Sugar in spring water | ||
Salt in spring water | ||
Carrot | ||
Cucumber | ||
Tomato | ||
Lemon | ||
Orange | ||
Pineapple | ||
Bell pepper | ||
Sweet potato | ||
Eggplant | ||
Buttermilk | ||
Sugar-sweetened fruit juice | ||
Different foods have an acidifying or an alkalizing effect once in the human body. If you would like to find out how different foods affect the body in terms of pH, read the next activity, The pH of Foods.
STEM Career Choices
Biochemist
Biochemistry combines both chemistry and biology. Biochemists explore chemical principles and processes within living organisms. For example, they investigate viruses and bacteria, but also more complex life forms such as animals and human beings. They analyze structures on the level of molecules when studying them.
Biochemists can be found working in pharmaceutical companies, developing and testing new drugs. They also work in hospitals. They may conduct tests on blood samples and explore possible new treatments for diseases. When they work in the agricultural industry, they may genetically engineer new plants that can grow fast and are resistant to diseases.
ACTIVITY: The pH of Foods
Most people move through their everyday lives without thinking about whether the food they eat is alkaline or acidic. In fact, most people think of the measure of acidity, or the pH test, as something that belongs in a chemistry lab. You might be among the few who do test the pH of the food you eat. But another important question to ask is the following: Does the pH of food change when it’s in your body? In other words, does the pH of food assimilated in the body test differently than if you test the pH of that food outside the body?
You might recall that the numerical measurement of pH ranges from 0 to 14. A strongly acidic substance has pH=0, while a strongly alkaline substance has pH=14. Pure water sits right in the middle at pH=7 (neutral pH). If a substance has a pH less than 7, it is acidic. If its pH is higher than 7, it is alkaline. For example, our stomach acids have a pH range between 1 and 3. That number can increase to 4–5 when there is food in the stomach. The small intestines, however, have a pH that is equal to 8, making the environment in the small intestines alkaline. It is very important for your blood to have a pH range from 7.35 to 7.45, otherwise, it can be fatal.
In order to test the pH effect of food once you ingest it, use pH strips that have a pH range from 4.5–9.0 to test your saliva. Such strips come in small increments (smaller than one, usually increments of a half) allowing for more accuracy in readings. Also, those strips usually come with a color chart you can use to read the pH based on the color. Make sure to juice a fruit or vegetable before using it. Record your results in the table on the next page. Feel free to add more food items to the list of things you would like to test.
Materials Needed:
- pH testing strips ranging from 4.5 to 9.0
- Cow’s milk
- Coconut milk
- Vinegar
- Sugar in spring water (2 tablespoons of sugar in an 8-ounce glass of water)
- Spring water
- Carrot
- Cucumber
- Tomato
- Lemon
- Orange
- Sugar-sweetened fruit juice
NOTE: Make sure you use the fruit and vegetable items when they are freshly squeezed.
Procedure:
- Prepare your child by telling her that she can only test one item on the list at a time. No food or drink other than water should be taken at least 2 hours before testing the pH of the saliva. Otherwise the readings would not be accurate. Perhaps it’s best to test a couple of items every day.
- Have your child do this experiment first thing in the morning, before eating anything, even before brushing her teeth (the toothpaste can affect the measurement). Have her prepare the pH strip and the food she wants to test (in liquid form), then rinse her mouth with plain spring water.
- Next, tell her to touch one of the pH strips on her tongue, and immediately match her strip colors to the chart in order to identify her base pH value before putting any food in her mouth. Have her record that pH value on the table.
- Immediately after the previous step, have your child swish around in her mouth about 1 ounce of the liquid food she plans on testing that day in its pure form, then swallow it.
- Have your child wait 2 minutes before testing her saliva. Have her use a new pH strip to test her saliva after the liquid food she just ingested. Ask her to touch a new pH strip to her tongue and immediately match the new strip colors to the chart. Once she identifies the pH value of her saliva after ingesting this food, have her record that value in the third column on the following table.
- Ask your child the following questions: Did this food make her saliva more acidic or more alkaline? In other words, did her pH shift slightly toward becoming a little more acidic or more alkaline? Was this food acidifying or alkalizing? Have her record her answer in the last column of the table.
- Your child can test other items in the list; make sure she hasn’t eaten or drunk anything but except water for 2 hours prior to the test.
| Record of Food pH: | |||
|---|---|---|---|
| Food (in liquid form) | pH before ingesting the food | pH after ingesting the food | Acidifying or Alkalizing? |
Cow’s milk | |||
Coconut milk | |||
Vinegar | |||
Sugar in spring water | |||
Carrot | |||
Cucumber | |||
Tomato | |||
Lemon | |||
Orange | |||
Sugar-sweetened fruit juice | |||
Through this activity, hopefully your child will gain some insight into how different foods can change the chemistry of her body. It’s often surprising to find out that lemons have an alkalizing effect on the body once ingested, even though lemons test as an acid when they’re outside the body. It all depends on how the food is chemically assimilated in the body.
STEM Career Choices
Pharmacologist
Pharmacologists are concerned with studying the effects of chemicals and pharmaceutical drugs on cells. They study these responses in human and animal cells. The main concern of a pharmacologist is not just developing new drugs, but making sure they are safe to use, and measuring the correct doses to administer.
Pharmacologists design experiments to test drugs. They may also carry out clinical trials to test the newly formulated drugs on humans. They collect data, then analyze it. This allows them to find the harmful effects of the drugs in order to explore ways to minimize or eliminate them. Pharmacologists often work in teams with other research scientists.
ACTIVITY: Separating Salt from Water
Table salt is a common presence in most modern diets. Food without a proper amount of salt just doesn’t taste the same. Before electricity was invented, when people didn’t have refrigerators, meat was cured with salt in order to preserve it over a long period of time.
But where does salt come from? Is it grown on trees? Of course not. It comes from salty bodies of water. But salt doesn’t have to come from deep within the ocean; it’s fairly easy to remove salt from seawater. Ancient people did it thousands of years ago, so the process doesn’t require much technology. In fact, you can even try it at home.
Materials Needed:
- Salt (unless you live near an ocean and have access to saltwater from the ocean)
- Water
- Large Teflon pan
- Teakettle, or cooking pot
Procedure:
- If you’re using ocean water, have your child pour half a gallon of ocean water into the Teflon pan. It’s best to do this experiment in the summer when it’s hot, otherwise the salt will take a long time to be extracted. (If you are using ocean water, skip the next two steps.)
- If you don’t have access to ocean water, then warm up half a gallon (8 cups) of water in the teakettle or pot, and pour it into the Teflon pan.
- Ask your child to add 1⁄2 cup of table salt into the warm water, and stir it until all the salt has dissolved.
- Have your child place the Teflon pan outside in a sunny place, where it can get sun for a good portion of the day. If she’s doing this experiment indoors, have her place the pan in front of a window that gets a lot of sun. Inform your child that she’ll have to be very patient, as it may take days for the water to evaporate from the pan and leave the salt behind.
- Remind your child to check on her pan at least once every day, so she can observe the changes that take place over time. What does she find after all the water has evaporated? Is it a whitish powder?
The powder left behind after the water evaporates is salt. Salt is a chemical compound that’s composed of one sodium atom and one chlorine atom. The name of this compound is sodium chloride. In the periodic table of the elements, sodium has the symbol Na, and chlorine has the symbol Cl, so sodium chloride would be chemically expressed as NaCl. When salt is dissolved in water, the sodium dissociates from the chlorine, forming ions. The sodium becomes a positive ion, Na+, while the chlorine becomes a negative ion, Cl–. The reason salt dissolves in water is because the positive sodium ions (Na+) are attracted to the negative side of the water molecules, while the negative chlorine ions (Cl–) are attracted to the positive side of the water molecules.
STEM Words to Know
periodic table of the elements
The periodic table of the elements organizes all 118 known atoms in a big grid. Starting at the top left corner of the table, hydrogen is listed as the lightest of all atoms. Moving from left to right along each row, each atom gets heavier by one proton than the one before it. The elements are arranged in certain locations, depending on specific characteristics of each. The modern periodic table traces its roots back to Russian chemist Dmitri Mendeleev. Mendeleev classified the 63 elements known by 1871 according to certain patterns he noticed. He used his table to predict properties of more elements that were discovered later.
If you had to mix salt with water to make saltwater, then the white powder is the table salt you used, which is sodium chloride (NaCl). If you used ocean water, then that powder may have more than just sodium chloride. There may be other minerals present in the ocean water that are now part of your salt. If you obtained the ocean water from an area where the ocean is clean and not polluted, you can use the salt you just made.
On average, 1 gallon of ocean water would give you a little more than 1⁄2 cup of salt. So if you live near an ocean and you want to have locally made salt, you can’t get more local than right in your own home.
STEM Career Choices
Toxicologist
Toxicologists are concerned with studying the harmful effects of chemical compounds on the cells in living organisms. We live in a world that is highly saturated with chemical hazards such as pesticides, air pollutants, and other chemicals in the water we drink. This makes the work of toxicologists extremely important to our health and well-being.
Toxicologists develop better methods to test the biochemical effects of exposure to certain chemical material that may cause diseases. They may work in industries such as cosmetics, food, drugs, and agricultural products. They may also work in the public service to establish safety regulations for such products.
ACTIVITY: Ice versus Dry Ice
You’ve probably seen, or at least heard of, dry ice. It’s said that dry ice can keep things much cooler than ice made from water. You might have even been to a Halloween party where someone used dry ice to make eerie floating fog.
Now you’ll get a chance to investigate the difference between regular ice and dry ice. What is “dry” about dry ice? Doesn’t all ice get wet when it begins to melt?
Materials Needed:
- 1 bag of dry ice
- Insulated gloves for handling dry ice
- Container to put the dry ice in
- 2 small bowls
- Spoon
- 2–3 ice cubes from the freezer
- 2 different color balloons
Procedure:
- Have your child put a few cubes of ice from the freezer into one of the bowls.
- Using the insulted gloves, assist your child in placing the dry ice into a container. Caution: always use insulated gloves when handling dry ice. Place a small chunk of dry ice into one of the small bowls.
- You’ll probably have to wait a while until the ice from your freezer starts melting in the bowl. But check out the bowl of dry ice. What do you notice? If you wait long enough for the ice from the freezer to totally melt in the bowl (an hour or two), what’s left in that bowl?
- Use the insulated gloves again. Stretch open the neck of one of the balloons, and have your child to use a spoon to scoop up some of the dry ice and drop it into the open mouth of the balloon. Tie the balloon shut and set it on your counter.
- Have your child place a couple of ice cubes from your freezer into the other balloon, then tie it shut and set it on the counter close to the balloon with dry ice.
- Wait a while until the ice from your freezer starts melting. When you come back to check out the ice in each of the balloons, how do you find each balloon? Do you find both of them still at the same size as when you tied and left them on the counter?
When you came back to check the two bowls of ice, you probably found one with water from the melted ice cubes, while the other one was empty! Where did the dry ice go? Did it evaporate? Actually, yes. Dry ice undergoes sublimation rather than evaporation. Some substances go directly from being a solid to a gas, without passing through a liquid state. When this happens, it is said the substance has undergone sublimation. Frozen carbon dioxide (dry ice) is one such example.
But what about the two balloons? The one with regular ice just ended up having water in it once the ice melted. The one with dry ice ended up being inflated! Why is that? Well, when water is frozen, it occupies roughly a similar size as (or just slightly larger than) liquid water. That’s why that balloon stays about the same size. However, the balloon with dry ice gets bigger because the dry ice underwent sublimation, turning from a solid into a gas. Dry ice is none other than frozen carbon dioxide gas, like the gas you expel from your lungs every time you exhale. Gases expand to take up a lot more space than solids do, because gases are much less compacted than solids. Gases have a much lower density than solids. That’s why the balloon inflates.
STEM Q&A
Q: What is the difference between evaporation and sublimation?
A: A substance like water can go from being a solid (ice) to a liquid (water) then to a gas (water vapor) when the water evaporates. When a substance goes through the intermediate liquid state as it transitions from a solid to a gas, then it is said that the liquid undergoes evaporation as it turns into gas. When a substance undergoes sublimation, it goes directly from a solid to a gas without ever becoming a liquid in between.
The temperature of water when it freezes is 32°F, while the temperature of dry ice is about –109°F! That’s why you should use insulated gloves when handling dry ice. Otherwise you can get a freeze burn.
STEM Career Choices
Lab Technician
Lab technicians in chemistry work alongside chemical engineers and chemists to assist them in testing chemical material. They use laboratory equipment to conduct experiments often designed for research and development. They may set up lab equipment, prepare chemical solutions, perform chemical experiments, collect and analyze data, and write reports to present their findings.
The work of chemistry lab technicians is always in a laboratory, whether they work in industry, in an academic setting, or in any other institution. Most jobs for chemical lab technicians require a two-year associate degree. They receive their training at the job site.