Most fishes reproduce sexually, males fertilizing the eggs of females. Most fish individuals are one gender throughout life, either male or female (minnows, catfishes, salmons, black basses, perchlike fishes, tunas). Although it is often hard (for us) to tell the genders apart, in many species the difference is obvious, especially during the breeding season. Regardless, reproduction in fishes takes many twists and turns along paths not followed by amphibians, reptiles, birds, or mammals. Some fishes reverse sex, from male to female or from female to male. Individuals of other species are both male and female, including some that can self-fertilize.
Sex reversal occurs in at least 34 fish families. It is most common in marine fishes, especially on coral reefs. The prevailing pattern is for an individual to mature first as a female and then later switch to male (seabasses, wrasses, parrotfishes, gobies). In most species with this pattern, males fight over females, and large, dominant males fertilize the eggs of many females. Small males are by comparison unsuccessful reproducers, but every female mates. Hence there is little cost to being small and female, and little benefit to being small and male, which helps explain why the switch occurs. Cleaner wrasses (Labroides, Labridae) form harems of one large male and a peck order of up to 10 females. The largest female mates most with the male, on down the list. If the male dies, the largest female changes to male within two weeks.
In species that mature first as male, little fighting occurs over females (moray eels, loaches, snooks, porgies, threadfins, some damselfishes). Because larger females produce more eggs, the bigger the animal, the greater the advantage in being female. Small males still mate successfully and thus incur minimal cost; they wait until they are much larger to switch to female. Male-first species include the popular anemone or clownfishes (Amphiprion, Pomacentridae). Anemone fishes live in groups of two large and several small individuals in an anemone. Only the two largest fish are sexually mature, the largest one being female and the next largest being male. If the female dies, the male changes sex to female and the next largest fish in the group quickly matures as a male. If Finding Nemo had been true to life, Nemo’s dad, Marlin, should have become Nemo’s mother shortly after his original mother was eaten by a barracuda. Explain that to your little brother.
Hermaphroditism is the condition of being a functioning male and female at the same time. In hamlets (Hypoplectrus, Serranidae), fishes form long-lasting pairs. During spawning, individuals switch back and forth, one fish fertilizing the eggs of the other, then vice versa. One species of topminnow (Kryptolebias marmoratus, Rivulidae) is the only fish capable of fertilizing its own eggs. Self-fertilization produces clones of genetically identical offspring.
A few other unusual sexual patterns occur in livebearers and deep-sea anglerfishes. In livebearers such as some mollies (Poecilia, Poeciliidae), females mate with males but the male’s sperm only stimulate egg development without contributing any genes. All offspring are copies of the mother. Deep-sea anglerfishes (Ceratioidei) do not change sex but females may be 10 to 60 times larger than males. The males are small and parasitic, permanently fused to the side of the female, connected to her bloodstream. The female provides food and oxygen via her blood, and the male provides sperm when the female spawns. Finding a mate in the deep sea is challenging given the vast space involved, so this arrangement assures males and females are close (very close) when the time is right.
Most fishes (in fact, 98% of bony fishes) lay eggs. In about 2% of bony fishes and half of sharklike fishes, fertilization is internal in the female’s reproductive tract and young are born rather than hatched. Males deposit sperm in females via a penis-like structure that is a modified fin (pelvic fins in sharks, anal fin in guppies and goodeids). Embryos use nutrients from the yolk that was originally part of the unfertilized egg (many sharks, coelacanths, scorpaenid rockfishes). In others, including goodeids, bythitid brotulas, and embiotocid surfperches, the mother provides nutrition for the young in addition to the yolk.
In some sharks (e.g., lamnid White Shark), this nutrition involves a form of cannibalism. After using up their own yolk reserves, developing young eat unfertilized eggs or siblings. Many sharks have a placenta very similar to the one found in mammals, with nutrition coming from the mother. Young are born with a placental scar (yes, many baby sharks have a belly button).
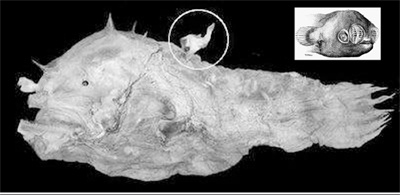
Male and female deep-sea anglerfishes differ in size and biology more than practically any other vertebrate. A 6.2 millimeter (0.25 inch) parasitic male Photocorynus spiniceps (Linophrynidae) (circled) is permanently fused to the back of a 46-millimeter (2 inch) female. Males of this species may be the smallest known sexually mature vertebrate. (Inset) a free living, 18 millimeter (0.75 inch) male of the Illuminated Netdevil (Linophryne arborifera, Linophrynidae), showing the greatly enlarged eyes and olfactory organ thought to be used in locating females that are four times longer. Photos courtesy of T. W. Pietsch
Some live-bearing fishes get around the problem of having too little internal room to produce many developing, relatively large young by staggering the beginning of development of different broods. Females of some poeciliid livebearers and three genera of halfbeaks (Dermogenys, Nomorhamphus, Hemirhamphodon; Zenarchopteridae) store sperm and then fertilize eggs in batches. One batch of eggs starts developing but the next batch is not fertilized until a week or so later, and so on. In this way, only one batch at a time is taking up much internal room because the more recently fertilized eggs are relatively small. The result of this process is that females can produce many relatively large babies at intervals of a week or so that would not be possible if all developed at the same time.
The number of eggs a female lays or produces depends on short-term and long-term factors. The most immediate influence is body size. Within a species, larger females lay more eggs. In some (salmons, cods, Haddock, Striped Bass, flounders), larger females lay larger, better eggs with more yolk, from which larger young hatch.
A cutaway view of embryos developing in the Butterfly Splitfin (Ameca splendens, Goodeidae). The drawing shows 13 embryos (8 heads and 5 tails) as they sit in the ovary of the mother. The fingerlike projections coming out of the ovary are structures that transport nutrition from the ovary to the developing young. Each embryo is about 3 centimeters (1 inch) long. Illustration by Julian Lombardi, from Wourms, Grove, and Lombardi, 1988; used with permission of the publisher
Ultimately, the number of young a female produces at any one spawning depends largely on their likelihood of survival. The number then is an evolutionary adjustment to past ecological conditions. Species that experience high levels of juvenile mortality tend to produce more and often smaller eggs. Most marine fishes produce larvae that are dispersed widely across the ocean, more than 99% of which die. These species typically produce many eggs. For example, a large female Atlantic Cod (Gadus morhua, Gadidae) may spawn 3 million eggs, a large Tarpon 12 million eggs, and a giant Ocean Sunfish (Mola mola, Molidae) 300 million!
Fishes in which hatchlings are relatively large or are cared for by the parents until they are large produce few eggs, as in sea catfishes (Ariidae) and Bowfin (Amia calva, Amiidae). The amount of yolk in an egg also influences egg number because larger eggs with more yolk take up more room inside the mother. Salmons produce relatively few, yolky eggs, on the order of a few thousand; in some small madtom catfishes (Noturus, Ictaluridae), only a few dozen large eggs are laid at one time and then guarded by the male. Coelacanths produce the largest eggs of all, up to 9 centimeters (3.5 inches) in diameter and these eggs hatch in the mother giving birth to live babies. Clutch size (number of young) is between 5 and 26. Live bearing fishes typically produce few young at any one time, again because live young take up more room inside a female than do eggs but they are born at a relatively large size that gives them a greater chance of survival.
In most fishes, eggs are produced and released in one spawning season, which takes a few months between early egg development and spawning. At higher latitudes and cooler climates, most fishes have only one spawning season per year in both fresh and ocean waters (centrarchid sunfishes, Yellow Perch, pickerels, gobies, kelpfish, Yellowtail). On coral reefs, fishes may spawn repeatedly throughout the year (wrasses, damselfishes), although some species are seasonal spawners (seabasses, snappers, rabbitfishes). Tropical freshwater fishes spawn seasonally, but seasons are defined by rainfall rather than temperature, and fishes spawn as rivers rise during the rainy season.
Live-bearing fishes hold their young for a month on average after fertilization. This time span varies greatly among species. The longest known “gestation” periods are among sharks, with Spiny Dogfish (Squalus acanthias, Squalidae) carrying young for up to 2 years, and Basking Sharks (Cetorhinus) perhaps as long or even longer. Coelacanths (Latimeria) are also thought to have a 3-year gestation period.
Once eggs have been spawned, it is usually only a matter of days before they hatch and swim about freely as larvae. Time until hatching varies greatly, being as little as 1.5 days in Asian carps, 3 to 4 days in centrarchid sunfishes, 1 or 2 weeks in North American and European minnows, a month in sculpins, and 2 or 3 months in Burbot, whitefishes, salmons, and Atlantic Cod. The longest incubation times occur in tropical freshwaters, where cyprinodontiform killifishes and rivulines (e.g., Nothobranchius, Aphyosemion), many of which are popular aquarium species, live for only 8 months. They spawn, deposit eggs in the bottom, and die. The eggs do not hatch until the next rainy season, which may be only 4 months away but can take as much as 5.5 years.
Eggs are scattered on the bottom among rocks (minnows, suckers); dug into sand or gravel (salmons, grunion); placed in a nest made of sand, pebbles, bubbles, or vegetation (minnows, sticklebacks, centrarchid sunfishes, Siamese Fighting Fish); released into the water (cods, wrasses, tunas); or deposited on vegetation or floating objects (herrings, flying fishes, Yellow Perch). Males fertilize the eggs while they are being laid or shortly after.
A few fish species use live invertebrates as a spawning site to protect the eggs against abundant egg predators. Snailfishes (Careproctus, Liparidae) lay their eggs inside the gill chambers of crabs. Bitterlings (Rhodeus, Cyprinidae) use freshwater mussels, a female depositing eggs into the gill chamber of the mussel, followed by a male who releases sperm over the inflow tube of the mussel.
Callichthyid catfishes, such as this Corydoras semiaquilus, are easy to keep in aquariums, serving as useful scavengers that clean up tank bottoms. Their breeding behavior is unusual, females drinking the sperm of males, passing the sperm live through their digestive tract, and then fertilizing their own eggs with the male’s sperm. Photo by Stan Shebs
In some African cichlids, females lay eggs on the bottom but quickly snap them up in their mouth. The female then nips at the anal fin of the male who releases sperm, fertilizing the eggs in the female’s mouth, where the eggs develop and are protected. In South American armored catfishes (Corydoras, Callichthyidae), a popular aquarium fish, the female places her mouth over the vent of the male and drinks his sperm. She passes the sperm rapidly through her digestive system. She then releases her eggs and holds them between her pelvic fins, and releases the male’s sperm to fertilize her eggs. The eggs are then deposited on the river bottom.
Spawning times of many fishes are highly predictable, depending on day length, water temperature, rainfall, and moon phase. Egg laying in seasonal spawners, discussed earlier, can therefore be predicted. Many fishers take advantage of this predictability, expecting the fish to come together in spawning groups as water temperature rises in the spring in temperate lakes or as a particular month approaches on coral reefs.
Predictable spawning locations are best known among coral reef fishes, including seabasses, snappers, croakers, wrasses, parrotfishes, and surgeonfishes. Seabasses and groupers (Serranidae) spawn year after year at the same places, fish traveling more than 100 km (60 miles) to gather on the spawning grounds. In parrotfishes and wrasses (Labridae) and surgeonfishes (Acanthuridae), the exact same coral heads will be used as traditional spawning sites, year after year, by different individuals.
Some fishes spawn only once in their lives, all at once. Salmons, lampreys, anguillid (freshwater) eels, and osmeriform southern smelts (Retropinnidae) and galaxiids of Australia and New Zealand are examples. Most fishes, however, spawn several times during a spawning season and several times during their lives (e.g., sharks, lungfishes, sturgeons, gars, tarpons, minnows, trouts, codfishes, seabasses).
The general name for baby fish is “fry.” Fish that have just hatched from eggs and are swimming around are usually called “larvae,” and if they still have their yolk sack attached they are called “yolk-sac larvae.” Salmons go through a series of development stages, each with a different name, such as alevin, parr, and smolt. Because many larvae are so different from the adults into which they grow, many fish larvae were thought to be entirely different species. Only when fish were raised in laboratories, or after all the different life stages were collected, did people link larvae with their adults, as in the gibberichthyid gibberfishes, whose larvae were once in their own family, the Kasidoridae. The larvae of several groups are still called by their older, family or generic names: amphioxides for lancelets, ammocoetes for lampreys, leptocephalus for all eels, querimana for mullets, rhynchichthys for squirrelfishes, and acronurus for surgeonfishes.
The relatedness of fish in a nest very much depends on whether one or both parents care for the young. In fishes that form pairs and where both mother and father take care of the eggs and fry, the young are full siblings with the same parents (cichlids, catfishes, wolf eels). Partial exceptions are seahorses and pipefishes (Syngnathidae). In these fishes, it is the male who becomes “pregnant” and cares for the young. Females lay eggs on the male’s belly or inside a pouch on the male’s belly. The male then cares for the eggs until they hatch out as baby seahorses or pipefishes. These fishes tend to be very faithful, mating with just one other individual for life.
When only one parent guards the nest, relatedness tends to vary. In fishes, it is usually the male that guards the eggs. A nest may then contain eggs from several females (darters, Percidae; sticklebacks, Gasterosteidae). In cardinalfishes (Apogonidae), the male broods the eggs in his mouth, but the eggs may come from several females. In some fishes, females prefer to lay their eggs in nests that already have eggs (Fathead Minnow, Threespine Stickleback, Painted Greenling, River Bullhead, Tessellated Darter, Browncheek Blenny). Male chubs (Nocomis) build pebble nests where female chubs, as well as females from other minnow species, deposit eggs, and the male guards all the eggs. Thus for these species—along with others that “dump” their eggs in the nest of another species (gars and minnows in sunfish nests, Golden Shiner in Bowfin and Largemouth Bass nests, cichlids in bagrid catfish nests)—the male guards young that are not full siblings but even belong to different species.
Many baby fishes do not look anything like the adults of their species and were thought to be different species or even families. This “kasidoron” larva of a Gibberfish (Gibberichthys pumilus, Gibberichthyidae) was once placed in its own family, the Kasidoridae. The larva’s body is about 8 millimeters (0.3 inches) long, not counting all the trailing filaments. Inset is an adult, about 9 centimeters (3.5 inches) long. Photo courtesy of G. D. Johnson
The attractiveness to females of nests that already contain eggs leads to surprising behavior on the part of males. In sticklebacks, a male will steal eggs from the nests of other males and deposit the eggs in his own nest, making it more attractive to new females.
Other surprises occur. Even some live-bearing fishes give birth to young fathered by several males. A Bluntnose Sixgill Shark (Hexanchus griseus, Hexanchidae) that washed ashore near Seattle, Washington, contained 80 embryos. DNA tests showed that eight different males had fathered the babies. DNA testing also reveals that nests of fishes that usually contain full siblings have a few eggs with different fathers. These “aliens” exist because other males will rush into a nest when a pair is spawning and quickly deposit sperm, thus fertilizing a few of the eggs (centrarchid sunfishes, salmons, pupfishes, wrasses).
Once fish leave the care of their parents, they are often dispersed widely by currents before settling into their final habitats. It was generally believed that once this happened, siblings became separated. However, some recent studies using new genetic techniques have found that siblings may stay together much longer. Unicornfishes (Naso, Acanthuridae), European Eel (Anguilla anguilla, Anguillidae) and Kelp Bass (Paralabrax clathratus, Serranidae) are among the species that have been shown to stay together. Future research will undoubtedly discover more.
In fishes, the sex (or gender) of an individual is determined by its genes, its environment, or both. In most fishes, determination is entirely genetic, each individual becoming male or female solely depending on its genes. In many fish species, the genes determining sex are on sex chromosomes, similar to the XY chromosomes in mammals (skates, anguillid and conger eels, tetras, many catfishes, salmons, killifishes, livebearers, sticklebacks, cichlids, gobies). In mammals, including humans, the male has both X and Y chromosomes, whereas in fishes either the male or female is XY, depending on species.
Environmental factors that can influence sex determination include temperature, age or size, and social interactions. In Atlantic Silverside, (Menidia menidia, Atherinopsidae), larvae spawned in spring at low temperatures usually become female; those spawned in summer at higher temperatures become male. Males also result from higher development temperatures in some minnows, gobies, silversides, loaches, rockfishes, cichlids, and flounders. But sex determination, like so many reproductive characters in fishes, is hardly constant. Higher temperatures result in females in (some) lampreys, salmons, livebearers, sticklebacks, and seabasses.
The seabass family (Serranidae) is huge (maybe 475 species), and even within this group, very different patterns occur. Many seabasses mature first as female and then later change to male when they reach a certain size. In these species (such as Nassau Grouper and Red Hind), all of the largest individuals will be male. Fishing that targets the largest fish can then cause a population crash, because too few males are around to fertilize the eggs of females.
In other species, social conditions determine sex. Individuals change from one sex to the other depending on the balance of males and females in the population. In sex-changing wrasses (Labridae), the biggest fish are male. Smaller fish are female and stop growing until a large male dies, then the biggest female changes to male. An opposite situation occurs in anemonefish, where the biggest fish in an anemone is female, the next largest a male, and all the others are immature. Bump off the female and everyone moves up a notch, with the largest male changing to female.
In seahorses and pipefishes, the male carries the developing young in a special brood pouch on his belly, and females compete for access to males. This very pregnant male is about to give birth. Courtesy pics.64hd.com
Many fishes are good parents. About one-third of fish families include species that care for their young. Parenting ranges from guarding a nest containing eggs or young to brooding eggs and young in the mouth to producing body substances that nourish the young.
It is the male that usually does the work. Males construct and maintain nests, chase potential predators away, fan the eggs with their fins to keep oxygen concentrations high and get rid of silt, remove dead or diseased eggs, accompany young that are foraging and protect them from predators, and produce mucus that prevents growth of bacteria on eggs or that the young eat.
Some males go to extremes to care for their young. In seahorses and pipefishes (Syngnathidae), females lay their eggs on or in a brood pouch on the male’s belly, where the young develop. The pregnant male helps the developing young with salt excretion, oxygen uptake, and some nutrition until they reach a relatively advanced stage of development. In nurseryfishes (Kurtidae), males develop a downward-bent hook on their foreheads where the eggs are attached and carried until hatching. The Spraying Characin or Splash Tetra (Copella arnoldi, Lebiasinidae) deposits its eggs out of water on the undersides of leaves, as much as 10 centimeters (4 inches) above the water surface. The male guards the eggs and keeps them moist by splashing water on them with flips of his tail.
A 15 centimeter (6 inch) male Australian Nurseryfish (Kurtus gulliveri, Kurtidae) from Northern Australian streams carries eggs on a hook that projects from its forehead. How the eggs get there remains a mystery. Drawing from Weber, 1913.
In cichlids and catfishes, the mother or father or both participate in parental duties. Many cichlids and catfishes hold eggs and young in their mouths. Mothers flick their fins to attract free-swimming fry when danger approaches, at which time the mother inhales the young into the safety of her mouth. The Cuckoo Catfish (Synodontis multipunctata; Mochokidae) of Lake Tanganyika, Africa, takes advantage of this mouth-brooding behavior. It lays its eggs on the bottom as a female cichlid is picking up her own eggs in her mouth. The young catfish are protected by the female, grow faster than the baby cichlids, and eventually eat some of the cichlid fry that are their “nestmates.”
Some African cichlids get help with babysitting. These “non-parental care givers” are usually young from a previous breeding period. They remain with the parents and feed and defend new young or defend and maintain the territory. In turn, they may someday inherit a good nesting spot (Lamprologus, Neolamprologus, Julidochromis).
The usual pattern of growth in fishes is for very rapid growth early in life, followed by slower—but continual—growth later. This pattern of continued growth differs from other vertebrates (amphibians, reptiles, birds, mammals) that reach a maximum size and stop growing. Although a minnow will never grow as large as a marlin, both will get larger every year as long as their needs for food, clean water, oxygen, and other necessities are met. If conditions are particularly good, growth will be faster.
Growth does however slow and even stop occasionally. Growth is greatly slowed or even halted during winter or drought. If a fish is attacked by a predator and injured, or is stressed by pollution or competitors, or caught and released by a fisherman, growth will stop while repairs are made and the individual’s physiology returns to normal. During the reproductive season, fishes stop putting energy into body growth and instead invest in eggs, sperm, fighting over territories or mates, defending young and territories, etc. Again, body growth slows or stops until breeding activities are over.
You can tell a fish’s age from its scales. This scale is from a Chinook Salmon (Oncorhynchus tshawytscha) caught during its second summer of growth. Winter growth bands are slower and closer together, forming darker regions, whereas summer growth lines are further apart where growth is faster. Adjacent winter and summer growth bands taken together equal one year’s growth and are referred to as an annulus (from “annual,” or one year). Photograph courtesy of Oregon Department of Fish and Wildlife
It is relatively easy to tell how old a fish is because of the continual growth described in the preceding question. As they grow, fishes add layers of bony substances and proteins to their scales and bones. A fish’s scales can therefore be read and the fish aged much like a tree can be aged by its rings (except you usually need a microscope to count growth rings on fish scales). Where distinct warm and cold seasons occur, such as in North America and Europe, a fish adds a pair of rings to its scales and vertebrae and ear bones and fin spines every year. Each ring pair appears as dark lines and light lines. The dark lines are where the added bony layers are close together due to slow growth, such as in winter. The light lines are the layers that are added much faster, such as during the summer. Dark lines of slow growth are also added during the breeding season. These are called “spawning checks” and can be used to tell how many times a fish bred.
It is more difficult to determine the age of a fish from the tropics where temperatures are fairly constant because all the lines on the scales look the same. In tropical areas that experience wet and dry seasons, distinct dark (dry season) and light (wet season) lines may be added. But if drought and rainfall occur irregularly, the lines are not very useful. And because growth slows as a fish gets older, the growth lines on the scales get closer and closer together, making counting more difficult. As a result, we can know how old young fish are but our counting becomes less accurate as a fish gets older.
It may surprise you to know that the age of very young fish can be known in days, not just years. This precision is possible because young fish lay down layers to their ear bones every day. These layers can be seen under very strong microscopes such as electron microscopes. Electron microscope techniques also allow us to sample the chemicals in these rings, which can then be used to determine where the fish lived during the earliest part of its life. Different habitats contain different chemicals, leaving behind a chemical history of sorts. The biography of a young fish can almost be read like a book.