After Chapter 4.5, you will be able to:
Perhaps the most useful information to glean from a balanced reaction is the mole ratio of reactants consumed-to-products generated. One can also generate the mole ratio of one reactant to another or one product to another. All of these ratios can be generated using the stoichiometric coefficients. In the formation of water (2 H2 + O2 → 2 H2O), for example, one can determine that, for every one mole of hydrogen gas consumed, one mole of water can be produced; for every one mole of oxygen gas consumed, two moles of water can be produced. Furthermore, mole-to-mole, hydrogen gas is being consumed at a rate twice that of oxygen gas.
Stoichiometry, an application of dimensional analysis, is often simplified to a series of three fractions. These fractions demonstrate an underlying three-step process:
Stoichiometry problems usually involve at least a few unit conversions, so take care when working through these types of problems to ensure that units cancel out appropriately to lead to the desired units of the answer choices. Pay close attention to the following problem, which demonstrates a clear and easy-to-follow method for keeping track of the numbers, calculations, and unit conversions.
Common conversions used in stoichiometry include:
Rarely are reactants added in the exact stoichiometric proportions shown in the balanced equation of a reaction. As a result, in most reactions, one reactant will be used up or consumed first. This reactant is known as the limiting reagent (or reactant) because it limits the amount of product that can be formed in the reaction. The reactants that remain after all the limiting reagent is used up are called excess reagents (or reactants).
When the quantities of two reactants are given on the MCAT, expect to have to figure out which is the limiting reagent.
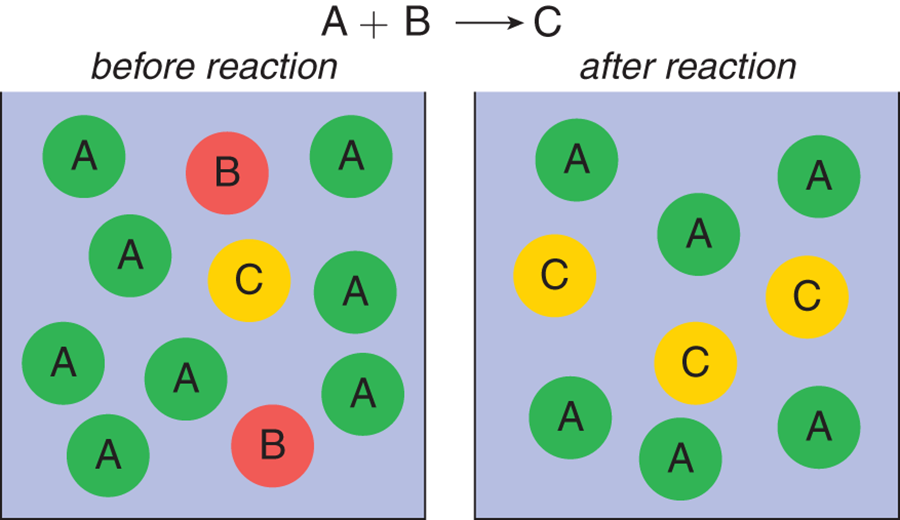
Figure 4.7 shows a reaction vessel that has significant amounts of reactants A and B, which react in equal amounts to produce product C. On the left, before the reaction, there is more reactant A than B. After the reaction is over, there is more product C but there is reactant A left over. Thus, reactant A is considered in excess, and reactant B is considered limiting.

For problems involving the determination of the limiting reagent, keep in mind two principles:
The yield of a reaction can refer to either the amount of product predicted (theoretical yield) or actually obtained (raw or actual yield) when a reaction is carried out. Theoretical yield is the maximum amount of product that can be generated as predicted from the balanced equation, assuming that all of the limiting reactant is consumed, no side reactions have occurred, and the entire product has been collected. Theoretical yield is rarely ever attained through the actual chemical reaction. Actual yield is the amount of product one actually obtains during the reaction. The ratio of the actual yield to the theoretical yield, multiplied by 100 percent, gives the percent yield:
An experimentally based passage that involves a chemical reaction may include a pseudo-discrete question that involves finding the percent yield.