Rate laws take the form of rate = k[A]x[B]y.
- The rate orders usually do not match the stoichiometric coefficients.
- Rate laws must be determined from experimental data.
Zero-order reactions have a constant rate that does not depend on the concentration of reactant.
- The rate of a zero-order reaction can only be affected by changing the temperature or adding a catalyst.
- A concentration vs. time curve of a zero-order reaction is a straight line; the slope of such a line is equal to –k.
First-order reactions have a nonconstant rate that depends on the concentration of reactant.
- A concentration vs. time curve of a first-order reaction is nonlinear.
- The slope of a ln [A] vs. time plot is –k for a first-order reaction.
Second-order reactions have a nonconstant rate that depends on the concentration of reactant.
- A concentration vs. time curve of a second-order reaction is nonlinear.
- The slope of a
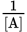 vs. time plot is k for a second-order reaction.
vs. time plot is k for a second-order reaction.