Learning Objectives
After Chapter 7.4, you will be able to:
- Distinguish between endothermic and exothermic reactions
- Determine the enthalpy of a molecule or atom given reaction data:


After Chapter 7.4, you will be able to:


Most reactions in the laboratory occur under constant pressure (at 1 atm) in closed thermodynamic systems. To express heat changes at constant pressure, chemists use the term enthalpy (H). Enthalpy is a state function, so we can calculate the change in enthalpy (ΔH) for a system that has undergone a process—for example, a chemical reaction—by comparing the enthalpy of the final state to the enthalpy of the initial state, irrespective of the path taken. The change in enthalpy is equal to the heat transferred into or out of the system at constant pressure. To find the enthalpy change of a reaction, ΔHrxn, one must subtract the enthalpy of the reactants from the enthalpy of the products:
Equation 7.4
A positive ΔHrxn corresponds to an endothermic process, and a negative ΔHrxn corresponds to an exothermic process. It is not possible to measure enthalpy directly; only ΔH can be measured, and only for certain fast and spontaneous processes. Thus, several methods have been developed to calculate ΔH for any process.
The standard enthalpy of formation of a compound, ΔH°f, is the enthalpy required to produce one mole of a compound from its elements in their standard states. Remember that standard state refers to the most stable physical state of an element or compound at 298 K and 1 atm. Note that ΔH°f of an element in its standard state, by definition, is zero. The ΔH°f values of most known substances are tabulated. You do not need to memorize these values because they will be provided for you.
The standard enthalpy of a reaction, ΔH°rxn, is the enthalpy change accompanying a reaction being carried out under standard conditions. This can be calculated by taking the difference between the sum of the standard heats of formation for the products and the sum of the standard heats of formation of the reactants:
Equation 7.5
Enthalpy is a state function and is a property of the equilibrium state, so the pathway taken for a process is irrelevant to the change in enthalpy from one equilibrium state to another. As a consequence of this, Hess’s law states that enthalpy changes of reactions are additive. When thermochemical equations (chemical equations for which energy changes are known) are added to give the net equation for a reaction, the corresponding heats of reaction are also added to give the net heat of reaction, as shown in Figure 7.8.
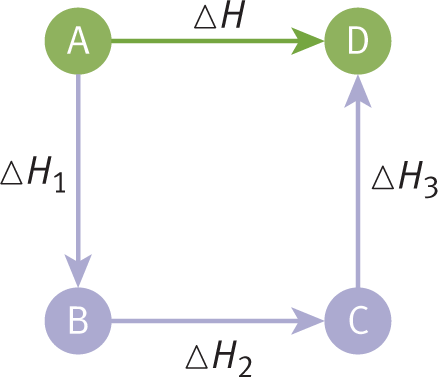
State functions are always path independent.
Hess’s law is embodied in the enthalpy equations we’ve already introduced. For example, we can describe any reaction as the result of breaking down the reactants into their component elements, then forming the products from these elements. The enthalpy change for the reverse of any reaction has the same magnitude, but the opposite sign, as the enthalpy change for the forward reaction. Therefore,
The ΔHrxn can be written as:
which is another way of writing
Consider the following phase change:

The enthalpy change for the phase change is called the heat of vaporization (ΔH°vap). As long as the initial and final states exist at standard conditions, the ΔH°rxn will always equal the ΔH°vap, irrespective of the particular pathway that the process takes. For example, it’s possible that Br2 (l) could first decompose to Br atoms, which then recombine to form Br2 (g), rather than simply boiling from the liquid to gaseous state. However, because the net reaction is the same, the change in enthalpy will be the same.
It is important to realize that Hess’s law applies to any state function, including entropy and Gibbs free energy.
When doing a problem like this on the MCAT, make sure to switch signs when you reverse the equation. Also, make sure to multiply by the correct stoichiometric coefficients when performing your calculations.
Hess’s law can also be expressed in terms of bond enthalpies, also called bond dissociation energies. Bond dissociation energy is the average energy that is required to break a particular
type of bond between atoms in the gas phase—remember, bond dissociation is an endothermic
process. Bond dissociation energy is given in the units
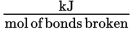 and is often given in tables on the MCAT in a format similar to Table 7.1.
and is often given in tables on the MCAT in a format similar to Table 7.1.
| Bond |
|
|---|---|
| O=O | 498 |
| C–H | 415 |
| H–H | 436 |
Bond enthalpies are the averages of the bond energies for the same bond in many different
compounds. For example, the C–H bond enthalpy
 is averaged from measurements of the individual C–H bond enthalpies of thousands of
different organic compounds. Note that bond formation, the opposite of bond breaking,
has the same magnitude of energy but is negative rather than positive; that is, energy
is released when bonds are formed. Remember that atoms generally form bonds to become
more stable (often by completing an octet). Thus, it makes sense that bond formation
is exothermic and bond dissociation is endothermic. The enthalpy change associated
with a reaction is given by
is averaged from measurements of the individual C–H bond enthalpies of thousands of
different organic compounds. Note that bond formation, the opposite of bond breaking,
has the same magnitude of energy but is negative rather than positive; that is, energy
is released when bonds are formed. Remember that atoms generally form bonds to become
more stable (often by completing an octet). Thus, it makes sense that bond formation
is exothermic and bond dissociation is endothermic. The enthalpy change associated
with a reaction is given by

Equation 7.6
Because it takes energy to pull two atoms apart, bond breakage is generally endothermic. The reverse process, bond formation, is generally exothermic.
As the name implies, the standard heat of combustion, ΔH°comb, is the enthalpy change associated with the combustion of a fuel. Because measurements of enthalpy change require a reaction to be spontaneous and fast, combustion reactions are the ideal processes for such measurements. Most combustion reactions presented on the MCAT occur in the presence of atmospheric oxygen, but keep in mind that there are other combustion reactions in which oxygen is not the oxidant. Diatomic fluorine, for example, can be used as an oxidant. In addition, hydrogen gas will combust with chlorine gas to form gaseous hydrochloric acid and, in the process, will evolve a large amount of heat and light as is characteristic of combustion reactions. The reactions listed in the CH4 (g) example shown earlier are combustion reactions with O2 (g) as the oxidant. Therefore, the enthalpy change listed for each of the three reactions is the ΔHcomb for each of the reactions.
The glycolytic pathway, described in Chapter 9 of MCAT Biochemistry Review, is also a combustion reaction that utilizes a fuel (glucose) mixed with an oxidant (oxygen) to produce carbon dioxide and water.
The heat of combustion for this reaction is found in a similar fashion to that of Hess’s Law. Given the numerous reactions and pathways involved, we can determine the overall enthalpy of the reaction, as shown in Figure 7.9.
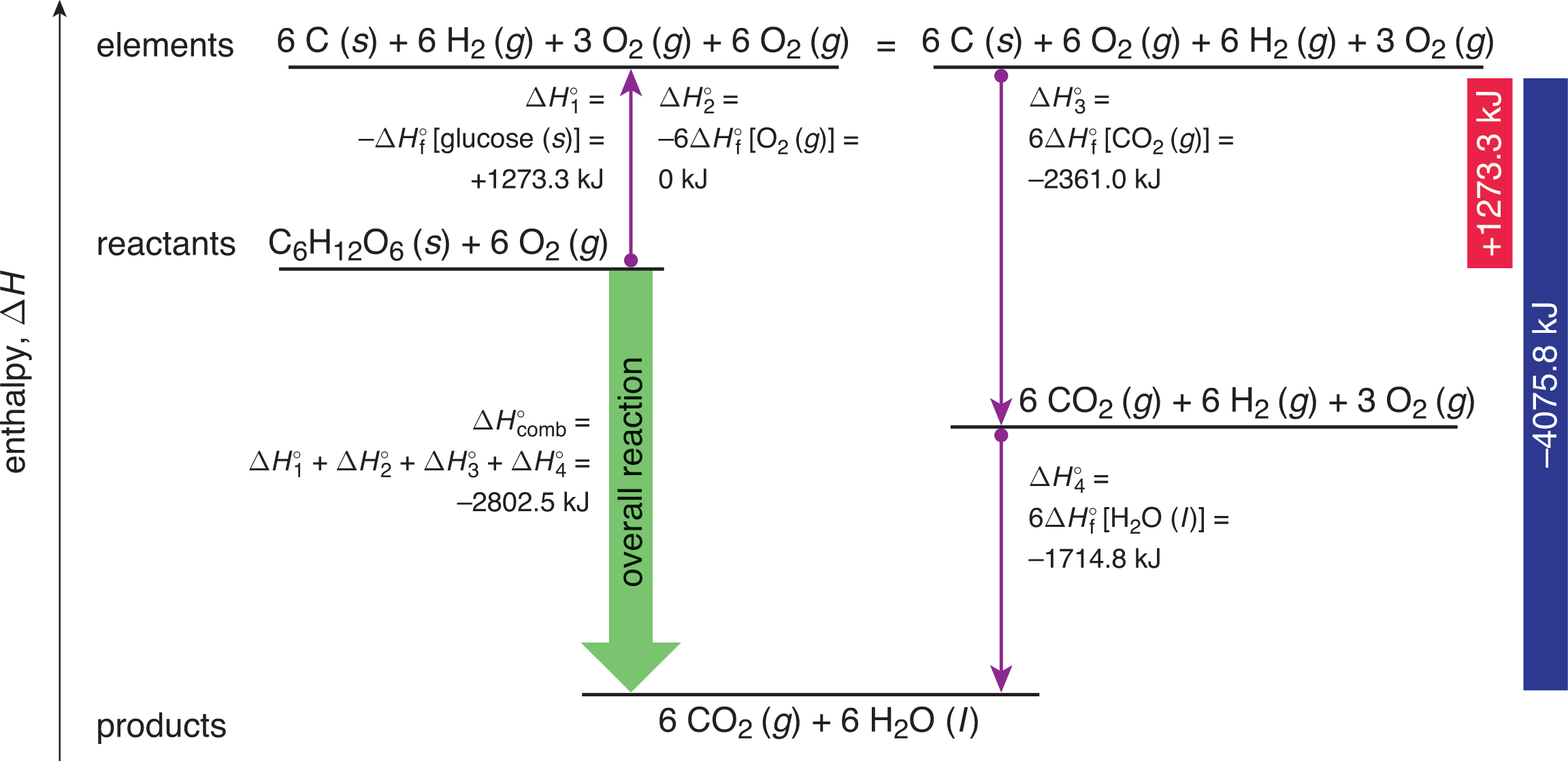
The larger the alkane reactant, the more numerous the combustion products.