13
Getting from Here to There: The War on Aging
![]()
 This book has presented, in as simple terms as possible, the biological details of what human aging is and how we can realistically set about defeating it. However, from what I’ve presented so far, you would be entirely justified in concluding that I’ve only made a case for the eventual development of therapies that could modestly delay aging. You might think that a realistic time frame for getting encouraging results in mice might indeed be a decade or so, but you’re probably already thinking about the problems that there would be in negotiating FDA obstacles and such like in translating these therapies to humans. And you may be concluding that the sort of timeframe I’ve been predicting for the arrival of widely-available therapies—a few decades with 50 percent probability—is too short by a factor of at least three. You’re probably also pretty skeptical about the degree of life extension that the techniques described in the last seven chapters could practically achieve, even in that extended timeframe: sure, they might be truly comprehensive if they worked absolutely perfectly, eliminating every scrap of their respective type of target damage, but we all know that no therapy is that perfect, and certainly not in its early versions. Thus, you’re probably thinking that I have fallen drastically short of making my well-publicized case that a lot of people alive today may well live to be one thousand—and you’d be absolutely right. Thus, in this chapter and the next I’m going to put the scientific details to one side and directly address these two important and legitimate concerns. I’m going to start with the time frame for widespread availability of the panel of therapies described in this book.
This book has presented, in as simple terms as possible, the biological details of what human aging is and how we can realistically set about defeating it. However, from what I’ve presented so far, you would be entirely justified in concluding that I’ve only made a case for the eventual development of therapies that could modestly delay aging. You might think that a realistic time frame for getting encouraging results in mice might indeed be a decade or so, but you’re probably already thinking about the problems that there would be in negotiating FDA obstacles and such like in translating these therapies to humans. And you may be concluding that the sort of timeframe I’ve been predicting for the arrival of widely-available therapies—a few decades with 50 percent probability—is too short by a factor of at least three. You’re probably also pretty skeptical about the degree of life extension that the techniques described in the last seven chapters could practically achieve, even in that extended timeframe: sure, they might be truly comprehensive if they worked absolutely perfectly, eliminating every scrap of their respective type of target damage, but we all know that no therapy is that perfect, and certainly not in its early versions. Thus, you’re probably thinking that I have fallen drastically short of making my well-publicized case that a lot of people alive today may well live to be one thousand—and you’d be absolutely right. Thus, in this chapter and the next I’m going to put the scientific details to one side and directly address these two important and legitimate concerns. I’m going to start with the time frame for widespread availability of the panel of therapies described in this book.
I use the phrase “the War on Aging” to describe a specific phase in the process leading to the defeat of aging. I define it as the period beginning with the destruction of the pro-aging trance and ending with the widespread availability of therapies that can add a few decades to the life span of people who are already middle-aged. First I’ll elaborate on this definition, then I’ll explain why I call it the war on aging, and finally I’ll explain why it has a good chance of lasting only fifteen to twenty years.
The pro-aging trance—the “rational irrationality” about aging that I described and critiqued in Chapter 2—will end only when its claim to rationality becomes unable to withstand even simple assaults, the sort that most people can understand. I believe that this will truly occur only when scientists obtain results in the laboratory—mainly with mice, I expect—that are so impressive that the majority of professional biogerontologists are finally prepared to say publicly that it’s only a matter of time before we can postpone aging by at least a few decades in humans. Science is in a very real sense the new religion: what individual scientists say can be doubted, but the public scientific consensus is gospel. The result that I think is needed is something that I’ve called “robust mouse rejuvenation” or RMR.
RMR is a mouse life-extension result, and it’s a rather precisely defined one. That’s what’s needed if we want scientists to set aside their paranoia about making predictions about what might happen in the future—we need to close all the major loopholes. By my definition, RMR will be achieved when:
- at least twenty mice of the species Mus musculus,
- from a strain whose natural average life span is at least three years,
- receive treatments starting only when they are at least two years old,
- and live to an average of five years of age, with all the extra time being healthy.
I thought about this definition pretty carefully before I publicized it, and it seems to be standing the test of time: no one has pointed out any way in which it could be achieved by “uninteresting” means, i.e., by means that would not convince knowledgeable scientists that a massive breakthrough had been made that was likely to be relevant to humans. The requirement to use at least twenty mice is so that we can be sure the age wasn’t a fluke or a bookkeeping error. The requirement to use Mus musculus is because other mouse species already live longer than Mus musculus but are less well characterised by scientists. The requirement to use a strain of that species that naturally lives to three, which is unusually long for that species, is to avoid any possibility that the mice have some specific congenital problem, something that normally kills them rather young, and that the treatment merely alleviates that defect rather than comprehensively affecting aging. And of course the requirement that the treatment begin only when the mice are already two-thirds of the way through their natural life span is to ensure that it has potential relevance to people who are already alive, read the newspapers, pay taxes—and vote.
My reason for calling the period that begins with the achievement of this milestone the War on Aging arises from the initial, essentially immediate reaction that I expect society to have to it. In order to describe this reaction, I must first describe a side effect of the pro-aging trance that determines society’s current reluctance to take aging seriously. I have a name for this, too: it’s the triangular logjam. See Figure 1.
Experimental biology, like any other area of science, costs money—really quite a lot of money. Most biology isn’t nearly as expensive as high-energy physics or astronomy, but it’s expensive enough that professors have to spend a hideous amount of their time fundraising. The overwhelming majority of the funds that support experimental biology come from the public purse.
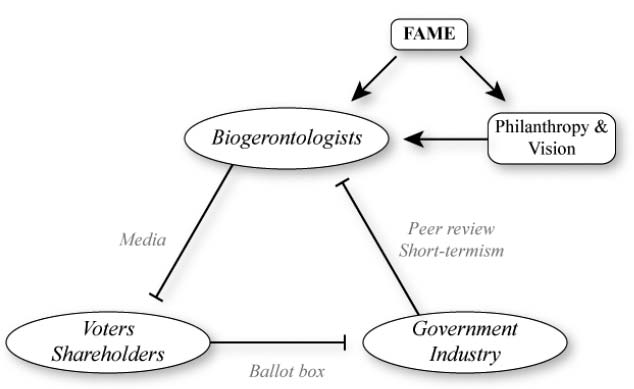
Figure 1. The triangular logjam impeding funding, and how philanthropy can unlock it.
Biogerontology is typical in the above regard, but it’s extremely unusual in one way: the public are absolutely fascinated by it, so biogerontologists get on the television all the time. I mean really, all the time. The difference in this regard between biogerontology and other biological fields—even really high-profile medical fields—cannot be overstated: even quite junior biogerontologists get more press attention than the world’s leaders in other areas. And of course, when given that chance, biogerontologists are just as keen as any other scientist would be to talk about their own research—which, necessarily, is the research that they were able to obtain the funding to perform.
Consider, for a moment, what else a biogerontologist might choose to talk about to the media. In particular, consider the possibility of talking about research avenues that the public consider distinctly suspect: defeating aging, for example. What are the attractions of discussing such topics? Well, your name might get quite widely known to the general public, and you might get more media exposure. But hang on: What is the media exposure for? Scientists are intensely preoccupied, as I just mentioned, with the miserable business of maintaining a funding stream for their laboratories. How, exactly, would a high media profile achieve this—or, conversely, make it harder?
In order to explain the answer to that question, I must make sure you are aware of a key feature of the way in which public funding for science is allocated. When scientists want to do a particular series of experiments, they write a detailed description of what they want to do, how long they think it’ll take and how much it’ll cost, and they send it to the appropriate government agency. But the government agency doesn’t then decide on its own whether the scientist can have the money. No: even though such agencies employ highly experienced ex-scientists as administrators of grant funds, those ex-scientists don’t have anywhere near broad enough expertise to be able to tell the difference between a good idea and a poor one across the whole range of scientific disciplines that they’re responsible for. So instead, they seek specialist advice from other scientists. This is called “peer review” and it’s an absolutely universal component of the process of evaluating applications for government grants to do science.
Selection to evaluate your colleagues’ ideas for experiments is an immense privilege and responsibility. It’s not something that junior scientists get to do very often; generally the most senior scientists are the ones who do it most.
Do you see the problem yet?
Science is about the testing and refinement of hypotheses and theories. In principle, the most important quality of a scientist should be their ability to accept, with an open mind, evidence that challenges theories that they had believed for many years. But scientists are human, and moreover they know that the scientists that produced the new evidence are also human. In particular, they know that when a result is reported that contradicts established conventional thinking, the new evidence is often found later on to have been the result of experimental error. Thus, it is generally pretty hard to get senior scientists to change their mind about things, even if your evidence is really strong. The legendary physicist Max Planck famously remarked in the 1920s that “Science advances funeral by funeral” and this is only barely an exaggeration: It can take well over a decade for really fundamental changes of understanding of aspects of science to become generally accepted. A famous example in biology is the mechanism of action of mitochondria, cellular components that we’ve heard a lot about in this book.1 And inevitably, this resistance to new ideas carries over heavily into how senior scientists evaluate grant applications.
So far, so unproblematic. After all, a modest degree of resistance to new ideas that you may not yet fully grasp is a good thing in some ways—we wouldn’t want the scientific consensus to flit from one new idea to another too easily either, because (as I just mentioned) new ideas are often wrong. But the inertia that exists in scientific thinking is generally greater than this happy medium. And unfortunately, it’s not just inertia of ideas, it’s inertia of reputations. Senior scientists who have been appointed by the government to evaluate their colleagues’ grant applications are, essentially by definition, members of the establishment. If they receive two applications of equal scientific merit, only one of which they have the resources to fund, and one is from a scientist who has a history of radical views about what science may shortly achieve, while the other is from a scientist who has never said anything outrageous in his life, you can bet that it’s the latter who will get the money.
And that’s not all. Reviewers of grant applications are, of course, given guidelines telling them what aspects of the applications’ scientific merit or demerit they should consider particularly important. One aspect that is invariably high on this list, if not at the very top, is feasibility: perceived likelihood that the investigator will complete the proposed experimental program within the time and budget requested and obtain results that will merit publication in a reputable scientific journal. Sounds pretty uncontroversial, doesn’t it?—but in fact this policy is a huge problem for science, because it is not (in practice) weighted by scientific significance. That’s to say: A proposal for a study that will almost certainly tell us something whatever its outcome will fare much better in peer review than a study that may well tell us nothing, even if what the second study might tell us is far more important than what any outcome of the first study would tell us. This bias in favor of low-risk, low-gain research at the expense of high-risk, high-gain research pervades the whole of science and is extremely strong in biogerontology.
Well, I’ve spent quite a while in this chapter denouncing the stubborn closed-mindedness of senior scientists, but I hope you’ve taken on board that in the past couple of paragraphs I’ve explained that it’s not entirely their fault: it’s really the fault of their paymasters, the public funding agencies, a.k.a. the government. Grant reviewers are also grant recipients, by and large (though they don’t review their own applications, of course). Thus, if the funding agency makes it known—either explicitly via written guidelines, or implicitly by their actions and off-the-record remarks—that they would prefer to fund middle-of-the road people to do dependable work than to fund troublemakers to do controversial work, the reviewers are hardly likely to dissent. It would be a very good thing if more such scientists did resist this sort of instruction, but realistically that’s too much to ask.
But actually…it’s not the government’s fault either. The real culprit is you, the public.
This should not be a surprise to you. It’s hardly a secret that governments in all democracies ultimately act to achieve one thing above all others: their own re-election. That will not be aided if the government spends, and is seen to spend, appreciable amounts of taxpayers’ money on what the public regards as blue-sky pipe dreams: research that will probably lead nowhere. If the public were scientifically mature enough to appreciate that, in the long run, the rate of scientific progress is slowed by this overcautious approach, their elected representatives would be able to exercise similar vision and to instruct grant reviewers accordingly. But the public do not adequately understand how science works, so this doesn’t happen. (A similar and even worse problem exists when it comes to medicine; I’ll explore that topic later in this chapter.) This is basically because the judgment of how likely a hypothesis is to be true, or of how likely an experimental result is to lead to further knowledge, is actually one of the most sophisticated and unteachable aspects of doing science.
In biogerontology, however, there is potentially a way out—and this brings me back full circle (or triangle!) to the thing that distinguishes biogerontology from all other scientific fields in terms of its interaction with the public: the sheer extent of that interaction. Though there is no hope of turning the public into scientists sophisticated enough to understand the merits of highly ambitious experiments, there is ample chance simply to tell them that such-and-such an experimental approach is well worth pursuing. There’s probably not enough chance of this for most scientific fields, but in the case of biogerontology it’s abundantly possible. So, why don’t biogerontologists do just that whenever they find themselves in front of a camera? I already told you: They don’t want to gain a reputation for irresponsibility among their peers, because to do so would be to jeopardise their own chances of being funded even for unambitious work.
So there you have it—the triangular logjam. Biogerontologists are cautious in what they say to the public, in order to protect their funding, which is provided by the government, which are cautious in what and whom they fund, in order to protect their votes, which are provided by the public, who are fatalistic about what’s even worth trying to achieve, because they see the biogerontologists saying only cautious things on the TV.
In order to defeat aging any time soon, I believe that an essential first step must be to break the triangular logjam. How can this be done?
Since I entered biogerontology, I’ve been chipping away primarily at one corner of the triangle: my fellow biogerontologists, especially the senior ones. Scientists are very politically aware, as described above, but they’re also honest and sincere people. Moreover—and this is a key point—hardly any biogerontologists suffer from the pro-aging trance themselves. They know full well how horrific aging is, and with very few exceptions they want an end to it just as much as I do. Thirdly, there aren’t very many of them, so personal contact is easy: I’ve known essentially everyone in the field personally for several years now. And finally, they’re all smart enough to have earned doctorates. All in all, if I have a good strong case that we may be much closer to fixing aging than people have hitherto realized, shouldn’t I be able to convince them—and even convince them to say so publicly?
Well, not quite—but nearly. As in any walk of life, what people say is important but what they don’t say is also important. Those of my colleagues to whom I have presented the SENS panel in detail have mostly concluded that it is not fantasy, even though it’s certainly very ambitious—but that hasn’t translated into explicit public calls for SENS to be funded. What it has led to, however, is a variety of demonstrations of tacit support. It started with coauthorship by five senior colleagues of the first paper on SENS, which arose from a workshop in 2000 (see Chapter 4); it’s continued with refusal of several eminent colleagues to coauthor a denunciation of SENS published in the respected journal EMBO Reports and orchestrated by some of the less open-minded members of the community;2 and most recently it’s included the remarkable development that some people who did sign that denunciation have taken the initiative to divorce themselves from it by publishing constructive responses to the SENS agenda,3,4 something that the EMBO Reports tirade specifically counselled against. While this may seem pretty tame as seen from the outside, I can assure you that it’s an about-face as thorough as one ever sees in science.
It’s obviously not enough, though. But it’s all I’m likely to get from my senior colleagues in biogerontology for the time being, i.e., until I can piece together enough funding to give SENS research serious momentum despite its radical nature. Thus, in the (hopefully brief!) meantime I must address the other points of the triangle, too.
It’s just conceivable that the government could be influenced directly. There are visionaries in government, and just occasionally they find a way to realize those visions. But in order to have any real chance on Capitol Hill or its counterparts in other countries, you really have to know the minds of the major players well—and that is something you don’t pick up overnight. Thus, I’ve continued to leave that strategy to others—and I’m delighted to say that the ball seems to be slowly being picked up, most notably with the splendid “Longevity Dividend” initiative, a new effort spearheaded by the veteran lobbyist Dan Perry of the Alliance for Aging Research in collaboration with three gerontologists.5 Whether they have much chance of success remains to be seen, but I emphatically wish them luck.
As I’ve become more prominent, however, I have been able to start addressing the third corner of the logjam: the public. You may recall that I started this book with a somewhat cantankerous complaint to the effect that if it weren’t for the pro-aging trance I would be able to get on with the actual science and technology of defeating aging. Well, that’s certainly true—but once given the chance, I have thrown as much energy into my advocacy and outreach efforts as I was already throwing into the science. Apart from anything else, I’m aware that the public are a source of funds in their own right, as well as a source of pressure on governments to alter their funding priorities.
The pro-aging trance dominates the nature of my interaction with the media, and via them with the public. The overwhelming majority of my time in interviews is occupied in discussions of the desirability of defeating aging, rather than the feasibility. But the good news, which I encounter mercifully often, is that it generally takes only a little bit of probing to reveal that the ultimate basis of my interlocutor’s concern is their reluctance to accept the feasibility. It is this that convinces me so thoroughly that the achievement of robust mouse rejuvenation will consign the pro-aging trance to history in the twinkling of an eye.
![]() The Intensity of the War on Aging, and Its Consequent Likely Duration
The Intensity of the War on Aging, and Its Consequent Likely Duration
In order to give you a sense of what the world is likely to be like after RMR is achieved, I’m going to review some basic epidemiological and biomedical facts about three well-known viruses—HIV, CMV, and avian flu—and then examine an interesting scenario.
HIV has become one of the world’s major killers. Belatedly, drugs that can suppress it and prevent it from progressing to full-blown AIDS are gradually becoming available in the developing world—still nowhere near in the quantities needed, but maybe soon even in those quantities. In the developed world, however, HIV is in a meaningful sense under control. It’s possible to live with HIV for decades without any symptoms whatsoever, by the regular administration of expensive but (in the West) affordable drugs. What we still don’t have, of course, are two key things:
- a drug to eliminate HIV from the body;
- a vaccine to stop it from infecting people.
CMV, cytomegalovirus, is not one of the world’s major killers. Well, not obviously. In people with a normal immune system, it is completely suppressed and causes no symptoms at all. (My “not obviously” qualification arises from the fact that this suppression gradually wears down the immune system during aging, so that eventually people become more susceptible to more aggressive infections such as pneumonia; in this sense CMV is indirectly life-threatening. For more details on this and what we need to do about it, see Chapter 10.) But it is incredibly widespread: most Westerners are infected with it.
Avian flu is big news as I write these words (mid-2007), because for the past few years we have been somberly informed that it could soon mutate into a form that would cause a pandemic and potentially kill tens of millions or even hundreds of millions of people. All that needs to happen is for the avian flu virus to acquire genetic changes so that it can easily be passed from humans to other humans, the same as more familiar (and much less deadly) flu viruses can do. Such mutations are rare, but not astronomically rare; this could happen any time. Vaccines for avian flu are under development, but how well they will work depends on what mutations the virus picks up in the process of becoming human-to-human transmissible, and anyway vaccines often don’t work so well on the elderly, who will be most at risk. Hence the possible death rates.
I’ve summarized these viruses as background for a scenario that I now want to explore in some detail, by way of an analogy with the situation after RMR has been achieved. I needed to lay out this background so that you appreciate that the scenario is reasonably realistic; I don’t think I’ll have any trouble convincing you that it’s a valid analogy.
Let’s suppose that HIV mutated to become transmissible by air, just like flu.
That’s it. That’s the situation I want to explore. Nothing else changes: the drugs to suppress HIV still work, they’re still pretty expensive, and vaccines for HIV are still far away from development.
In this scenario, it’s essentially certain that almost everyone in the world would have HIV within a couple of years. Not everyone gets flu in a pandemic, of course, but the difference is that once you get flu, if you don’t die you mount an immune response that actually works, i.e., that eliminates the virus from your body. Thus, any given individual is infectious only for a rather short period (and after they’ve recovered, they can’t be infected again, either). In the scenario we’re looking at, once you get it you have it forever, and you’re infectious forever. There will be no hiding place.
Pretty apocalyptic, isn’t it? (Luckily, virologists think that in actual fact this scenario is vastly more unlikely than the corresponding mutation for avian flu.)
Well, hang on—is it so apocalyptic? We do have these drugs…
Let’s look at a few round numbers. In the United States, roughly one person in every 250 has HIV, according to the Populations Reference Bureau—that’s about one million people. The drug treatment to keep HIV under control costs about $30,000 per year, so that adds up to about $30 billion per year. Thus, if everyone in the United States had HIV, we’d be talking about $30 trillion per year. But the actual cost of production of these drugs is far, far lower: generic forms of them are being synthesized in India and sold (still at a profit, mind) for only $300 per year, and even lower prices are in the offing. So actually we’re looking at only $300 billion per year—$1 billion per day—to keep everyone in the United States healthy even if they all have HIV.
Now, a couple of points. First, you might think that “only $300 billion per year” is a pretty curious use of the word “only.” Well, think again, because that’s almost exactly what the United States is currently spending on the war in Iraq. (I’m not commenting here on the relative merits of these expenditures, you understand—I’m just pointing out that we have a precedent of an unexpected expense of the same size that is not bankrupting the nation.) Second, you might be against the infringing of patents, so you might object to my slashing the cost by a factor of a hundred. But is your belief in the patent system stronger than your belief in stopping your neighbors—or yourself, or your family—from coming down with AIDS and dying horribly? Ask yourself honestly: If this scenario actually happened, and one major party campaigned on a manifesto to raise taxes by $300 per year for the average person and to spend that money on generic drugs to prevent AIDS, and the other party campaigned on a commitment either to raise taxes by $30,000 per year for the average person or not to provide the drugs at all, who do you seriously think would get elected?
I hope I’ve convinced you what would happen in the above scenario: we would find the resources to treat everyone. We’d probably find the resources to treat everyone in the developing world, too, just as we’re now stirring ourselves to treat everyone who needs such drugs in the developing world today.
Now, let’s look at society’s post-RMR view of aging in the same way. I suspect you can quickly see the similarities. Everyone has aging. The therapies we’ll be looking to make available will be suppression therapies, which we will have to take for as long as we live (though much less frequently than those with HIV need to take their drugs). Within that limitation, however, the therapies will work: people’s aging won’t progress. But the therapy will be very expensive. (In the first instance that expense will be mainly for funding of research, training greatly increased numbers of medical personnel, building additional drug synthesis facilities and such like. The same figure I discussed above, $1 billion per day, is as good an estimate as any.)
So let’s ask the opposite question: what are the differences between a post-RMR world and a universal-HIV world? I would say that there are really only two:
- the therapies won’t yet exist at the time RMR is achieved;
- our acceptance that human aging can probably be defeated fairly soon will be new, while the universality of aging is age-old: this is the reverse of the situation with HIV in the above scenario.
I would argue that neither of these differences has any real chance of causing society to behave any differently in the aftermath of RMR than in the universal-HIV scenario. The nonexistence of the therapies is really no different than the nonexistence of enough antiretroviral drugs, which would certainly be the initial situation in the scenario I’ve described: we will work to develop those therapies as fast as possible, just as we would work to scale up production of antiretrovirals as fast as possible. The idea that the order of events could matter seems equally far-fetched; if everyone has a life-threatening health condition and we have a shot at making it no longer life-threatening, we’ll clearly strive to do so.
![]() Why “the War on Aging”?
Why “the War on Aging”?
I think you can probably see by now my reasons for calling this period “the War on Aging.” In the early 1970s, there was an initiative called “the War on Cancer” that involved a sharp and sustained hike in the funding for cancer research fueled by the hope that cancer could be cured within as few as five years.6 The war on cancer was not as abject a failure as some people tend to suggest—without that funding we would not have advanced nearly as fast as we have in our understanding of cancer, so there’s little doubt that that initiative will have brought forward the true defeat of cancer quite substantially—but it was a complete misnomer, for one simple reason: the amount of money involved was really quite small, imperceptible to the U.S. taxpayer. As summarized above, the war on aging will be extremely expensive—not imperceptible at all. And yet, it is clear that the public will embrace the necessary tax rises: it will be quite obviously impossible to get elected except on a manifesto commitment to attack aging with all available resources. This is a mind-set that has previously been seen only at one type of stage in a wealthy nation’s history: wartime.
![]() Hippocrates and Gelsinger
Hippocrates and Gelsinger
In closing this chapter, I want to touch on one final aspect of the war on aging—one which completes my case that it may well last only 15-20 years.
In 1999, a teenager named Jesse Gelsinger died of anaphylactic shock in a trial of a gene therapy procedure at a hospital in Philadelphia.7 This was the first such incident of its kind, and it sent the gene therapy world into its own form of anaphylactic shock. The bottom line was that essentially all gene therapy trials worldwide were suspended for about a year. We don’t know how much delay that will eventually turn out to translate into in terms of the development of safe and effective gene therapy, but the chances are pretty good that it’ll be a few weeks at least, and given the enormous breadth of applicability of gene therapy, that could mean thousands of lives lost—maybe even hundreds of thousands if it delays the defeat of aging. Bearing this in mind, was the suspension of trials for so long a proportionate response?
The U.S. Food and Drug Administration (FDA) would answer in the affirmative, as would their counterparts around the world. Regulation of experimental drugs and therapies, whether it be in terms of what results are needed or how they are obtained, is based on one abiding principle above all others: the minimization of risk that the therapy might make the patient worse. Specifically, this minimization of risk explicitly counts for more, much more, than maximizing benefit. In this way, the FDA is following a principle that has existed since medicine’s earliest days: the famous edict of Hippocrates, primum non nocere, or “first do no harm.” (Note that this phrase is actually not part of the Hippocratic Oath, the set of principles by which medical professionals swear to abide as part of their qualification process.)
I take the view, quite simply, that Hippocrates has had his day. The avoidance of harm was a rational strategy to adopt during the early days of medicine, when people very often recovered spontaneously from what their doctors thought were fatal conditions simply because the doctors had inadequate diagnostic tools. In such a situation, the psychological effect of possibly causing harm, whether it be the effect on the doctor or on the patient’s loved ones, legitimately skews the objective cost-benefit analysis of a given treatment. In the modern world, however, such recoveries are relatively very rare. I therefore believe that the 10:1 (at least) ratio of lives lost through slow approval of safe drugs to lives lost through hasty approval of unsafe drugs8 is no longer acceptable.
Furthermore, I believe that in the turbulence of the War on Aging, the general public will also come to the view that it is unacceptable. This will lead, in a matter of months from the achievement of RMR, to a root-and-branch revision of the laws and regulations governing clinical trials and approval of drugs and therapies. A fair guess is that drugs will be approved for universal use (via prescription) after a degree of testing that approximates today’s Phase 2. People will die as a result; the 10:1 ratio mentioned above will probably reduce to 2:1. And people will be happy about this change, because they’ll know that it’s wartime, and the first priority—even justifying considerable loss of life in the short term—is to end the slaughter as soon as humanly possible. I am the first to acknowledge that, without such a change of priorities, my prediction that the war on aging may well last only fifteen years would be totally absurd. But with that change, only the pace of research will be limiting.