“If your experiment needs statistics, you ought to have done a better experiment.” – Ernest Rutherford
The Victoria University of Manchester in England boasted a research laboratory second only to the Cavendish Laboratory at Cambridge University. The Rutherfords, accompanied by their young daughter Eileen, arrived in Manchester in the spring of 1907. The atmosphere at Manchester was a change for Rutherford, as he wrote to a colleague, “I find the students here regard a full professor as little short of Lord God Almighty. It is quite refreshing after the critical attitude of the Canadian students.” Rutherford and his young German assistant, Hans Geiger, studied the alpha particles and proved that they were simply a helium atom with its electrons removed.
Like a drill sergeant inspecting his troops, Rutherford made regular rounds to each of the laboratories to check on the progress of his students. The students knew he was approaching as he often sang his off-key rendition of “Onward Christian Soldiers” in a thunderous voice. He would probe the students with questions such as, “Why don’t you get a move on?” or “When are you going to get some results?” delivered in a voice that rattled the student and the equipment. One of his students later commented, “At no time did we feel that Rutherford had a contempt for our work, although he might be amused. We might feel that he had watched this sort of thing before and this was the stage we had to go through, but we always had the feeling that he did care, that we were trying the best we could, and he was not going to stop us.”
In 1908, Rutherford was awarded the Nobel Prize in Chemistry “for his investigations into the disintegration of the elements, and the chemistry of radioactive substances”—the nuclear decay work that he had done back at McGill. As was the custom, Rutherford gave a speech at the Nobel award ceremony in Stockholm, Sweden, titled, “The Chemical Nature of the Alpha Particles from Radioactive Substances.” He ended his speech with the news of his latest discovery, “Considering the evidence together, we conclude that the alpha particle is a projected atom of helium, which has, or in some way during its flight acquires, two unit charges of positive electricity. It is somewhat unexpected that the atom of a monatomic gas like helium should carry a double charge. It must not, however, be forgotten that the alpha particle is released at a high speed as a result of an intense atomic explosion, and plunges through the molecules of matter in its path.”
The audience at the Nobel awards ceremony was filled with past award winners and dignitaries. At thirty-seven, Rutherford was a youngster, at least in this crowd. His large thin frame and head full of bushy blond hair stood out. After the formal ceremony there were banquets and celebrations, starting in Stockholm, then Germany, and finally the Netherlands. Later Rutherford recalled about that exciting period, “Lady Rutherford and I had the time of our lives.”
Rutherford continued to work on radioactive material and devised a method to quantify the amount of radioactivity a material possessed. Rutherford and Geiger used a scintillation counter to measure the amount of radioactivity produced. By counting the number of flashes on a zinc sulfide screen where a flash indicated a colliding subatomic particle, he and Geiger could tell that a gram of radium ejects 37 billion alpha particles per second. Thus, a unit of radioactivity was born, named after Pierre and Marie Curie, a “curie,” which represents 37 billion disintegrations per second. Rutherford would have his own unit of radioactivity named after him, the “Rutherford," which represents one million disintegrations per second.
At the time Rutherford arrived at Manchester, the inner workings of the atom were poorly understood. The overall masses and sizes of atoms were known. It was amazing that scientists were able to determine the sizes and masses of objects so small that they couldn’t be seen in the most powerful microscope. Even belief in the invisible world of the atom did take a leap of scientific faith.

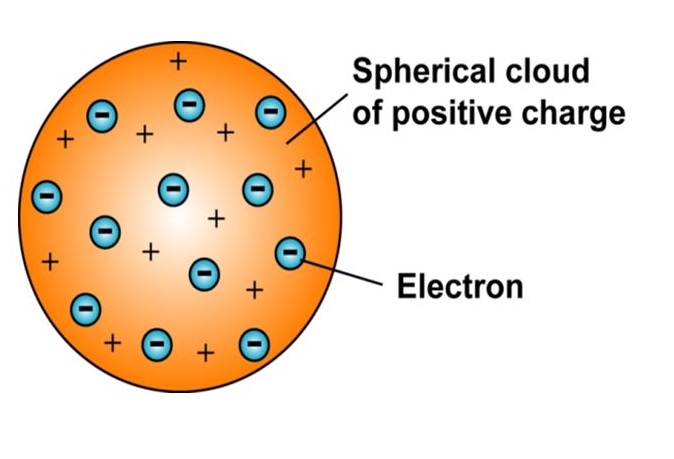
Figure - J.J. Thomson’s plumb pudding model of the atom.
Rutherford’s mentor at the Cavendish, J.J. Thomas, discovered a subatomic, electrically charged particle he called the “corpuscle," which we now call the electron. The lightest element, hydrogen, contains one corpuscle per atom; whereas, the heaviest of atoms, such as uranium, contain ninety or more. Since atoms are electrically neutral, it stood to reason that an equal number of positive charges must reside in the atom to offset the negative corpuses. Thomas and others put these facts and assumptions together and came up with the “plum pudding” model of the atom. In this picture of the atom, a sphere of smoothly spread positive charge, the “pudding,” was interlaced with numerous negative charged corpuscles, the “plums.” This model was the next conceptual leap from the indivisible spheres that originated in ancient Greece.