5.1 Introduction
Glaucoma is one of the most common diseases and is a consequence of disorders in the anterior segment of the eye. It is the result of anomalies in the aqueous humor dynamics that lead to increasing intraocular pressure (IOP). This pressure pushes the lens and consequently, the vitreous humor, inducing a pressure on the retina. It can lead to damaging of the optical nerve with subsequent vision loss. In extreme situations, it can even lead to blindness.
The mathematical modelling of drug delivery from a device and its transport until the target tissue requires the knowledge of the physiology of the eye, mainly (1) the anterior segment (2) the dynamics of the aqueous humor, responsible for such anomalous pressure. The increase in IOP is due to an increase of the resistance to the fluid outflow, an increase of the aqueous humor production or even both. It is necessary to identify the physiologic processes involved in aqueous humor production and in its drainage.
- 1.
beta blockers and carbonic anhydrase inhibitors reduce eye pressure by decreasing the production of intraocular fluid;
- 2.
prostaglandin analogs induce a reduction of IOP, diminishing the resistance to aqueous humor outflow;
- 3.
alpha agonists induce a decrease in the production of fluid and also increase the aqueous humor drainage.
This article aims to contribute to the mathematical modelling of drug delivery from therapeutical contact lenses to treat glaucoma. We start by presenting the anterior segment of the eye and the dynamics of aqueous humor. The set of anomalous situations that lead to increasing intraocular pressure is then described. An overview on some therapeutic strategies that can be used to treat open-angle glaucoma is presented. The main part of this work concerns a mathematical model that describes the drug release from therapeutic lenses and its evolution in the cornea and anterior chamber. To simplify, the model is built under some assumptions on the phenomena involved as well as on the geometry of the anterior chamber. We conclude remarking that this work was published in the ECMI Annual Report 2016 [9].
5.2 Anterior Segment of the Eye and Aqueous Humor Dynamics
- 1.the epithelium (the outer layer);
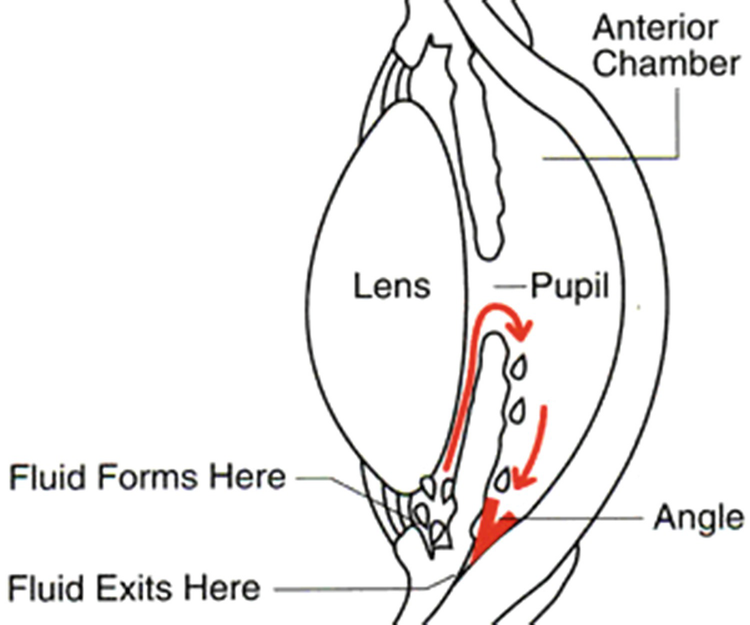 Fig. 5.1
Fig. 5.1Anatomy of the eye (http://www.theeyecenter.com/educational/005.htm)
- 2.
the stroma;
- 3.
the endothelium (the inner layer). It is coated by a tear film known as precorneal film, see Fig. 5.1.
- 1.
provides nutrients to the avascular tissues of the anterior segment;
- 2.
removes metabolic excretory products;
- 3.
stabilizes the ocular structure;
- 4.
contributes to the homeostasis of these tissues.
- 1.
the pressure difference between the trabecular meshwork, that induces a resistance to the outflow (porous structure), and the end of Schlemm’s canal, which is similar to the blood pressure (8–10 mmHg);
- 2.
the temperature difference near the cornea and lens.
5.3 Glaucoma
The closed-angle glaucoma can be induced by different causes that influence an iris dilation and its adheration to the lens.
The aqueous humor is produced in the ciliary body involving complex phenomena that include ultrafiltration, diffusion and active transport (active secretion). The ultrafiltration occurs in the capillaries of the ciliary processes and it is a passive movement of water and water soluble substances across cell membranes, diffusion of solute takes place in the tissue between the capillaries and the posterior chamber in response to concentration gradient. Active transport occurs in nonpigmented epithelial cells and it is the main responsible for the aqueous humor formation, see [10, 15]. An abnormal production of the aqueous humor can lead to increased IOP.
In the human eye, 75% of the resistance to the fluid outflow is due to the trabecular meshwork and 25% occurs beyond the Schlemm’s canal. Trabecular meshwork’s resistance to the drainage of aqueous humor is due to the hydration of the trabecular meshwork structure that can cause obstruction of its structure. Such obstruction is also associated with the formation of deposits within this tissue. Recently, the region of the trabecular meshwork that is responsible by the IOP regulation was identified: the juxtacanalicular tissue, which is adjacent to Schlemm’s canal. To keep the aqueous humor flow channels open in the juxtacanalicular tissue, the extracellular matrix of this tissue presents a continuous remodelling. An interference in this remodelling process compromises the aqueous humor drainage and increases the IOP, see [14].
The hypothesis that the biomechanical properties of Schlemm’s canal contribute to the aqueous humor outflow was studied, for instance, in [2, 16]. It was observed that the pore formation is a mechanosensitive process: an increase of the biomechanical strain induces an increase of the porous density. Changing this biomechanical behaviour, it was observed that the porous formation decreases, leading to increased IOP.
5.4 Therapeutics Strategies to Open-Angle Glaucoma
To decrease the IOP it is necessary to attack the anterior segment of the eye fortress and introduce drugs in the anterior chamber. This fortress is defended by the tear fluid barrier, the tear film that coats the corneal epithelium, the permanent blink, the cornea (lower impermeable structure) and the blood-aqueous barrier. Eye drops are the most used ocular route to administer drugs. However the drug bioavailability in the anterior chamber is very low. The tear film turnover is one of the main contributors to this fact. The drug residence time in the corneal epithelium is equal to 5–6 min before being completely washed way. The permanent continuous blinking removes the mixture of drug solution with tear fluid from the corneal epithelium to the nasolacrimal ducts.
The low permeability of the corneal layers also contributes to the reduced amount of drug that reaches the anterior chamber. Less than 5% of the drug present in the eye drops reaches the ocular tissue. The use of the systemic rout to delivery drug into the anterior segment of the eye is also very inefficient. In fact, the blood-aqueous barrier restricts the entry of drugs from the blood stream into the posterior segment and consequently, to the anterior chamber. The poor eye drug absorption requires repeated applications during long periods to achieve drug concentrations in the anterior chamber within the therapeutic window.
Different drugs have been used to decrease the IOP and they depend on the specific pathology. For instance, if the increased IOP is due to an anomalous production of aqueous humor, β-blockers, α-agonists and carbonic anhydrase inhibitors lead to decreasing of aqueous humor inflow. Other approaches use prostaglandin analogs to enhance the uveoscleral outflow or muscarinic agonists to enhance the trabecular outflow, see [17].
Several approaches have been followed to avoid the limitations of classical topical administration, like, for instance, the use of viscosity enhancers, mucoadhesive and lens which aim at increasing the drug corneal residence time. Other strategies, like the use of penetration enhancers, prodrugs and colloidal systems aim to increase the corneal permeability, see [19]. The purpose of such strategies is to delivery drug into the anterior chamber at a sustained and controlled rate complying the drug concentration in the target tissue to therapeutic window .
To delay the drug delivery process such that the corneal drug residence time and loading increases, several authors propose to encapsulate the drug in polymeric particles that are dispersed in the lens, see [5, 11–13]. In this case, the drug can also be dispersed in the polymeric structure leading to increasing drug loading. Such drug can be in three states in the polymer: free, bounded or encapsulated. The dispersed drug, when in contact with the tear fluid, is immediately released followed by the delivery of the bounded drug. The release on the encapsulated drug is delayed by the particles structure and the corneal drug residence time increases significantly. One of the main advantages of such devices is the possibility to build lenses that deliver the drug with a pre-defined profile.
5.5 Mathematical Modelling of Drug Delivery from Therapeutic Lenses
Building a mathematical model that describes the drug delivery process from a specific device and its transport to the target tissue is a complex work that requires different tasks. Let us consider the case of a therapeutic lens used to treat open-angle glaucoma, that is to deliver a specific drug in the anterior chamber to decrease IOP. Different tasks can be identified in the mathematical modelling of this drug delivery process.
- 1.
the drug release from the polymeric structure;
- 2.
its clearance by the tear turnover;
- 3.
the drug transport through the different layers of the cornea;
- 4.
the drug transport and its drainage by the aqueous humor;
- 5.
the dynamics of the fluid, which includes the aqueous humor production in the ciliary body, its transport in the posterior chamber and in the anterior chamber, its drainage through the trabecular meshwork and uveoscleral route, and its transport in the Schlemm’s canal.
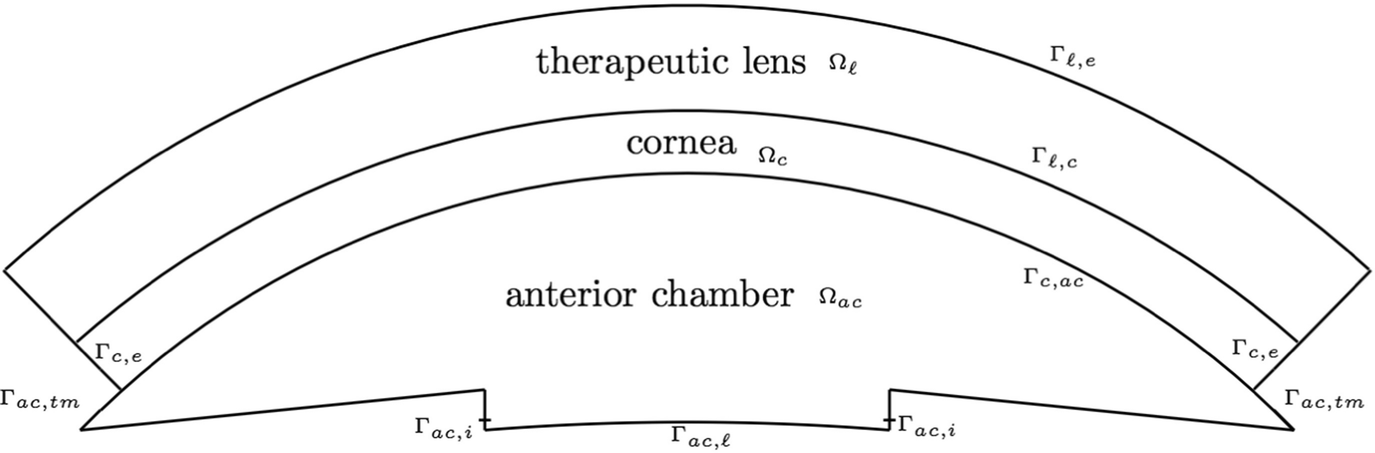
Spatial domain
We point out that the properties of the polymer used to construct the lens and particles entrapping the drug should be provided. We assume that the drug is dispersed in the polymeric lens presenting three different states, free, bounded and entrapped, while the cornea is composed by a single layer.
- 1.Ω ℓ—In the lens three different phenomena occur: the links between the polymeric chains and the drug molecules break and the bounded drug is converted in free drug that diffuses. Let λ b,f, λ e,f be the transference coefficients (1∕s) between bounded and free drug and entrapped and free drug, respectively, and D f,ℓ denote the free drug diffusion tensor (m 2∕s). Then the behaviour of the free and bound drugs is described by the diffusion equations
 (5.1)
(5.1)in Ω ℓ × (0, T], where T > 0 denotes a fixed time.
- 2.Ω c—Only the free drug is released from the lens and enters in the cornea where it diffuses. If D f,c represents the free drug diffusion tensor in the cornea then
 (5.2)
(5.2)in Ω c × (0, T]. Equation (5.2) is established assuming that the clearance of the drug occurs here being λ c the clearance rate (1∕s).
- 3.Ω ac—In the anterior chamber the free drug diffuses and its transported by the aqueous humor to the trabecular meshwork. The evolution of c f is described by the following convection-diffusion-reaction equation
 (5.3)in Ω ac × (0, T]. In Eq. (5.3), D f,ac and λ ac (1∕s) represent the drug diffusion tensor and the drug clearance rate in the aqueous humor. As the aqueous humor is mainly composed by water, and its dynamics is mainly driven by the IOP, the velocity field v can be described by the incompressible Navier–Stokes equations
(5.3)in Ω ac × (0, T]. In Eq. (5.3), D f,ac and λ ac (1∕s) represent the drug diffusion tensor and the drug clearance rate in the aqueous humor. As the aqueous humor is mainly composed by water, and its dynamics is mainly driven by the IOP, the velocity field v can be described by the incompressible Navier–Stokes equations (5.4)
(5.4)in Ω ac × (0, T]. In system (5.4), p represents the intraocular pressure, ρ the density of the aqueous humor and ν its kinematic viscosity.
The velocity field v is time and space dependent if the drug molecules have a therapeutic effect in the trabecular meshwork. Otherwise the velocity does not change in time and then the system of equations (5.4) should be replaced by steady Navier–Stokes equations.
- 1.Let Γ ℓ,e be the exterior boundary of Ω ℓ, see Fig. 5.2. We assume that this surface is isolated, meaning that the drug mass flux is zero. Then
![$$\displaystyle \begin{aligned} {\mathbf{D}}_{f,\ell}\nabla c_f \cdot \boldsymbol\eta=0 \,\mbox{ on } \varGamma_{\ell,e}\times (0,T], \end{aligned} $$](../images/475466_1_En_5_Chapter/475466_1_En_5_Chapter_TeX_Equ5.png) (5.5)
(5.5)where η denotes the outward unit normal to Ω ℓ on Γ ℓ,e.
- 2.By Γ c,e we represent the exterior boundary of Ω c, see Fig. 5.2. As no drug mass flux occur on Γ c,e we have
![$$\displaystyle \begin{aligned} {\mathbf{D}}_{f,c}\nabla c_f\cdot \boldsymbol\eta=0 \,\mbox{ on } \varGamma_{c,e}\times (0,T], \end{aligned} $$](../images/475466_1_En_5_Chapter/475466_1_En_5_Chapter_TeX_Equ6.png) (5.6)
(5.6)where η denotes the outward unit normal to Ω c on Γ c,e.
- 3.On the fluid outflow boundary Γac, tm (see Fig. 5.2) we assume that the drug mass flux depends on a function A c(c f) that reflects the drug effect in the increasing of the porosity of the trabecular mesh. This function should increase as c f increases reaching a maximum threshold. Therefore we assume that
![$$\displaystyle \begin{aligned} \mathbf{J} \cdot \boldsymbol\eta=A_c(c_f)c_f \,\mbox{ on } \varGamma_{ac,tm}\times (0,T], \end{aligned} $$](../images/475466_1_En_5_Chapter/475466_1_En_5_Chapter_TeX_Equ7.png) (5.7)
(5.7)where J = −D f,ac∇c f + vc f, and η denotes the outward unit normal to Ω c on Γ ac,tm.
- 4.In the boundary Γ ac,ℓ ∪ Γ ac,i (see Fig. 5.2) we take
![$$\displaystyle \begin{aligned} \mathbf{J}\cdot \boldsymbol\eta=0 \,\mbox{ on } (\varGamma_{ac,e}\cup \varGamma_{ac,i})\times (0,T], \end{aligned} $$](../images/475466_1_En_5_Chapter/475466_1_En_5_Chapter_TeX_Equ8.png) (5.8)
(5.8)where η denotes the outward unit normal to this portion of the boundary.
- 1.In the inflow boundary Γ ac,i we assume that the normal component of the velocity is known
![$$\displaystyle \begin{aligned} \mathbf{v}\cdot \boldsymbol\eta=v_{in} \,\mbox{ on } \varGamma_{ac,i} \times (0,T]. \end{aligned} $$](../images/475466_1_En_5_Chapter/475466_1_En_5_Chapter_TeX_Equ9.png) (5.9)
(5.9) - 2.There are several approaches to define the boundary condition when the pressure is known. One of them is to consider
![$$\displaystyle \begin{aligned} (\nu\boldsymbol\nabla \mathbf{v} - p\boldsymbol I)\boldsymbol\eta=-p_0\boldsymbol\eta \,\mbox{ on } \varGamma_{ac,tm} \times (0,T], \end{aligned} $$](../images/475466_1_En_5_Chapter/475466_1_En_5_Chapter_TeX_Equ10.png) (5.10)
(5.10)where p 0 denotes the pressure in Schlemm’s canal which is taken equal to the blood pressure and I is the identity matrix.
- 3.On ∂Ω ac∖(Γ ac,i ∪ Γ ac,tm) the normal component of the velocity is null
 (5.11)
(5.11)on (Γ c,ac ∪ Γ ac,e) × (0, T].
- 1.Interface between the lens and cornea:
 (5.12)
(5.12)on Γ ℓ,c × (0, T], where η denotes the outward unit normal to Ω ℓ on Γ ℓ,c. Here c f,ℓ and c f,c represent the drug concentrations in the lens and cornea, respectively, and A ℓ,c (m∕s) denotes the partition coefficient on Γ ℓ,c.
- 2.Interface between the cornea and anterior chamber:
 (5.13)
(5.13)on Γ c,ac × (0, T], where here c f,ac denotes the drug concentration in the anterior chamber, η the outward unit normal to Ω c on Γ ℓ,c and A c,ac (m∕s) represents the partition coefficient on Γ c,ac.


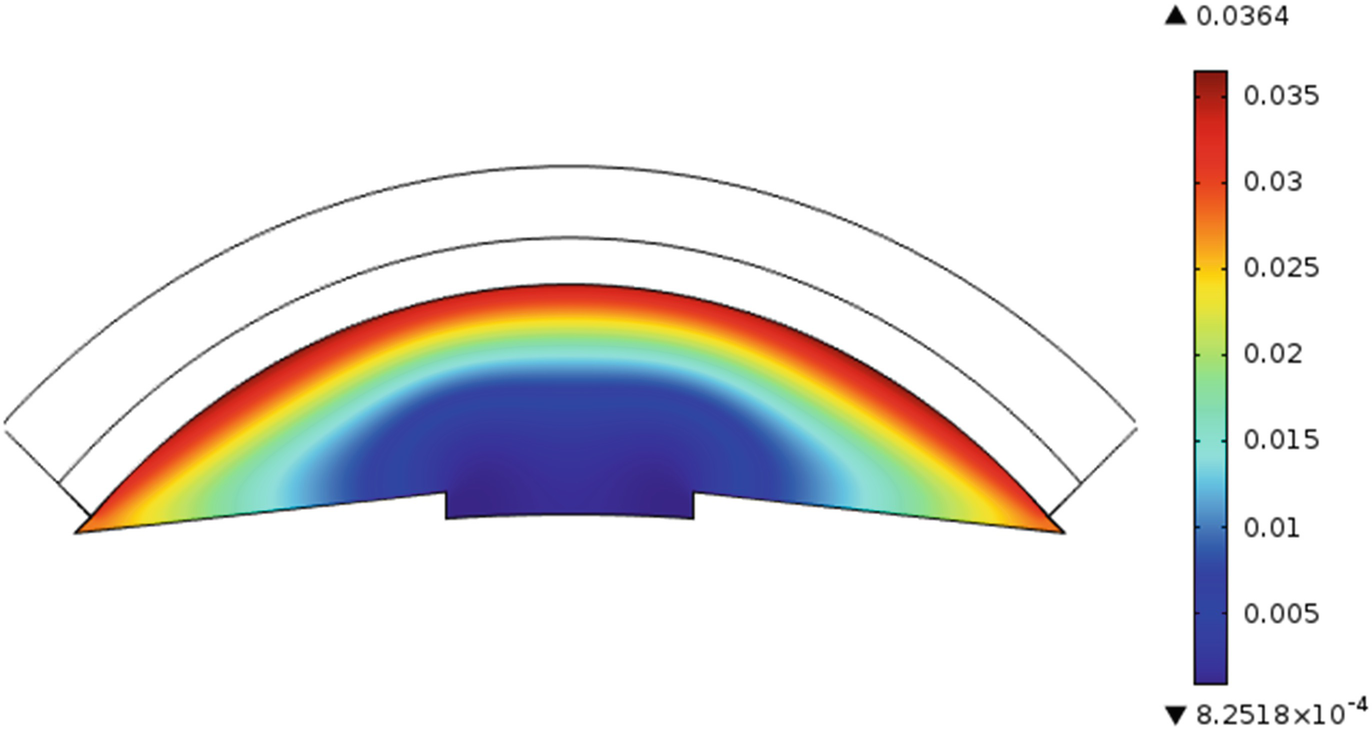
Drug distribution in anterior chamber after 20 min for a lens
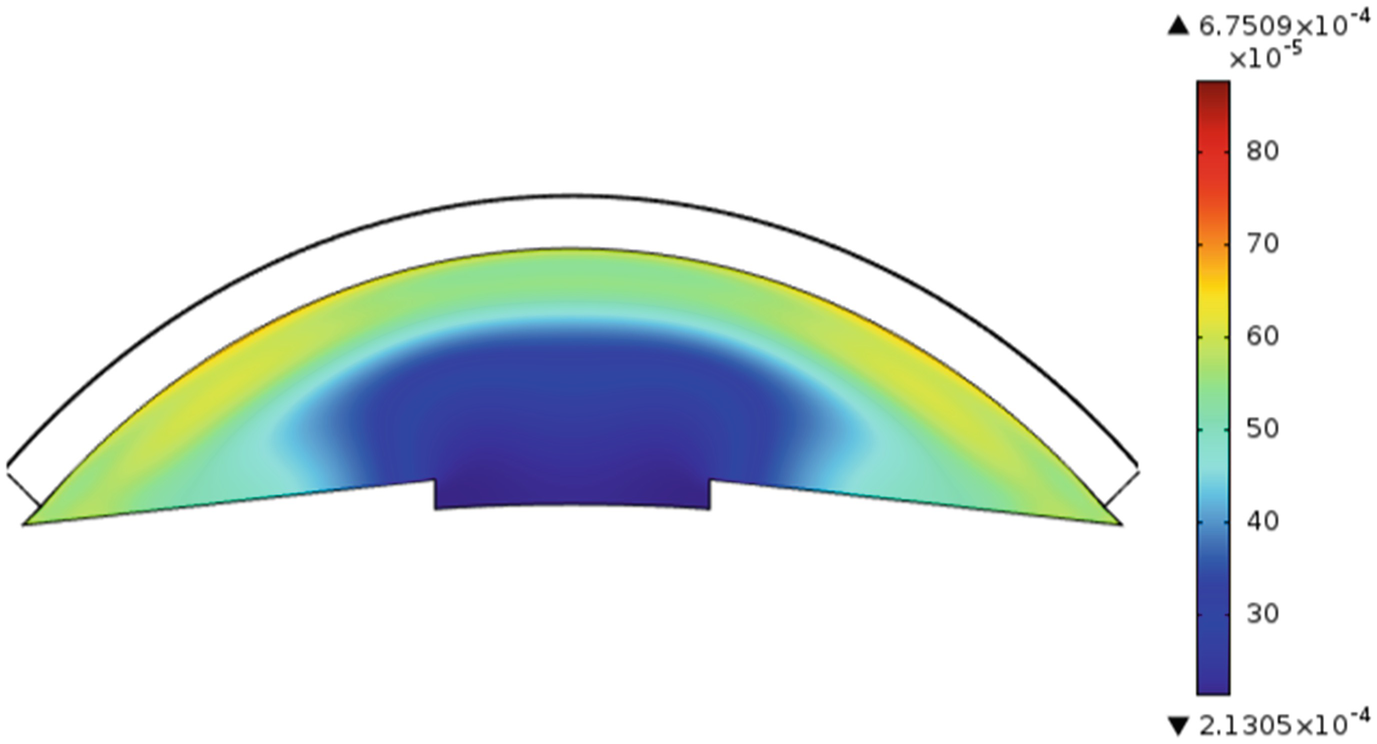
Drug distribution in anterior chamber after 20 min for an eye drop
From the plots we can infer that the amount of drug that reaches the anterior chamber with lens is higher than for drops due to the initial loading. Moreover the drug release from a therapeutic lens is a slower process due to the polymeric barrier for the dispersed and entrapped drugs.
5.6 Conclusions
This work aims to contribute to the mathematical modelling of the drug delivery from a drug delivery device—the therapeutic lens—used to decrease IOP in a glaucoma scenario. The model is established under several simplifying assumptions in what concerns the geometry of the spatial domain and the phenomena involved.

There is a compromise between the complexity of the mathematical model and its utility to predict the IOP evolution in different scenarios. In fact, the number of parameters needed increases with the complexity and some of them are not known.
This work was partially supported by the Centre for Mathematics of the University of Coimbra—UID/MAT/00324/2019, funded by the Portuguese Government through FCT/MEC and co-funded by the European Regional Development Fund through the Partnership Agreement PT2020.