THE RATIONALIZATION OF MATTER
THE Memoirs of the Royal Academy of Sciences for the year 1783 (printed, however, in 1785) contain a paper, “Reflections on Phlogiston, to Serve as a Development of the Theory of Combustion and Calcination Published in 1777, by M. Lavoisier.” It opens with this paragraph:
In the sequence of Memoirs which I have communicated to the Academy, I have passed the principal phenomena of chemistry in review. I have insisted on those which accompany combustion, the calcination of metals, and in general all the operations in which there is absorption and fixation of air. I have deduced all the explanations from a simple principle. It is that pure air, or vital air, is composed of a particular principle peculiar to it, which forms the basis of it, and which I have named the oxygenic principle, combined with the matter of fire and heat. Once this principle is admitted, the principal difficulties of chemistry have seemed to dissipate themselves and to vanish, and all the phenomena may be explained in astonishing simplicity.
The “matter of fire and heat”—caloric, he later called it—has disappeared from the memory of modern chemistry, which has further simplified these views in recalling Lavoisier as the creator of the correct theory of combustion. In his own belief, his work had a more general significance, a deeper interest. He meant it to be the reform of a whole science. Every science has its orderer in the structure of history, one who first framed objective concepts widely enough to reorient its posture: Galileo for kinematics, Newton for physics, Darwin for biology. That high place Lavoisier claimed for himself in chemistry. And rightly, for the science owes him far more than its grasp on oxygen as what combines in burnings. It owes him, too, its form, that characteristic combination of material algebra and nomenclature in a language of which the syntax preserves, even now, an eighteenth-century analysis perpetually in act. Its equations balance. Its names say compounds. For Lavoisier read Condillac’s educational philosophy of science into laboratory investigations of combustion, heat, and gases, and fashioned his chemistry out of the combination.
The spirit of the philosophe re-entered science in the person of Lavoisier, and chemistry found its rationale in a treatise which is both synthesis and primer. The Elements of Chemistry has none of the inaccessibility of Newton’s Principia. Published in 1789, it was written to start the science and its students off together on the right foot of method. Lavoisier remembered with impatience the confusion that had presided over his own chemical education: the preliminary Newtonian pieties in no way borne out by the chaos of ingredients and recipes, the contrast between these verbal thickets and courses on mathematics and mechanics wherein consequences really did open out of postulates and definitions, the professor’s tacitly sharing his bewilderment by supposing that his pupils already knew what he was unsure how to teach. “These inconveniences are occasioned not so much by the nature of the subject as by the method of teaching it; and, to avoid them, I was chiefly induced to adopt a new arrangement of chemistry.” Given his philosophical background, given his generation, Lavoisier’s rearrangement of his science could scarcely have taken any guise other than a naturalistic pedagogy.
Nevertheless, the Elements of Chemistry is no mere discourse of method. It contains, too, the food on which that method fed, an account of the great experimental discoveries of Lavoisier’s lifetime, repeated and refined in his own incomparable laboratory, where if they were not all or any of them originated, there alone were they understood. For the incoherence of his chemical education was scarcely the fault of his teachers. They had not disposed of this information, which permitted Lavoisier to make a science of their lore. Nor had they yet been taught that weight is the chemist’s quantity. The chemistry laboratory of the early eighteenth century offered no scene of careful gravimetrics. It boiled over with investigations rather of the processes than the materials of nature.
INEVITABLY Boyle had failed to work the transformations of matter into an atomic chemistry. All ignorant of gases, chemists could never control their evidence, nor make anything determinate of the atomic hypothesis. Thus set back, eighteenth-century chemistry found its salvation, or its purgatory, in the ambiguous, the once-maligned phlogiston theory. Recently, historically-minded chemists, appalled by the complexities which beset their predecessors, have been taking an indulgent view of phlogiston. Eighteenth-century investigators grappled courageously with the phenomena of electricity, heat, and chemistry. Their annals teem with ineffable “principles” introduced by a science which aspired to nothing if not materialism as the physical bearers of otherwise inexplicable effects. Such was phlogiston, the creation of vitalism in G. E. Stahl (1660-1734), and transmuted through long usage into the rationale of Joseph Priestley and the experimentalists, whose fault it was to aspire to objectivity without having mastered quantities. Nevertheless, phlogiston did make sense of chemistry. It was the principle of combustion. Charcoal, sulphur, phosphorus burn almost to nothing because they are rich in phlogiston. The fumes form acids. Conversely, vitriol (sulphuric acid) plus phlogiston gives sulphur. Similarly, in smelting, phlogiston passes from charcoal to ore. In general, where there is, in fact, a gain or loss of oxygen, Stahl saw a loss or gain in phlogiston, that which deserts the burning mass to leave behind a formless pile of ashes. This, then, was a looking-glass chemistry, a reversed theory which, answering to its purposes in the youth of the science, became as difficult to overcome as any left-handed habit.
The role of such imponderable fluids in eighteenth-century science is a complex problem, insufficiently explored. But clearly distinctions are required between phlogiston, which was a figment, and (say) electricity which, like caloric and aether, has a history leading far. Of those two more later. Even with phlogiston, however, chemistry was well out of the mystic jungle of alchemy. It had become a science, but a qualitative rather than a metrical one. Some such stage occurs with more or less importance in the evolution of every science, during which it explains effects as real qualities occurring out in nature. So pre-Galilean mechanics explained motion by the impetus impressed, and pre-Newtonian optics saw colors as qualities of light really existent. So too, phlogiston, a help so long as it coordinated phenomena in rational fashion, became a hindrance only after 1765, when accommodation of the findings of gas chemistry began to complicate rather than sophisticate the theory.
The French, it often seems, formulate things, and the English do them. In the chemical revolution, at any rate, pneumatic chemistry was the achievement of the English experimental school, while theoretical chemistry expressed the French instinct for formal elegance. In 1727 Stephen Hales, an Anglican clergyman endeavoring to import Newtonian considerations into physiology and chemistry, had demonstrated an “air” to be “fixed” in many organic substances and in certain alkaline earths. In fact this was carbon dioxide. That gas was not, however, specifically identified as a chemical individual, something distinct from “bad air,” until researches which Joseph Black reported to the Philosophical (later Royal) Society of Edinburgh in 1755, and published the next year as Experiments upon Magnesia Alba, Quicklime, and some other Alcaline Substances.
Joseph Black, drawn from a medical training to chemistry, was of that intellectual circle which started into vitality around the universities in Glasgow and in Edinburgh. James Watt, David Hume, Adam Smith, Henry Home of Karnes, Dugald Stewart, Joseph Hutton, who founded igneous geology—they distinguished the northern kingdom, and though remote from the French style in gravity of mien, their radicalism brought Scotland closer in spirit to the Enlightenment than ever was England in her eighteenth-century complacency. Black began his researches as a thesis for a medical doctorate on magnesia alba (magnesium carbonate). On heating it (to get the oxide), he found a constant loss of weight, and found, too, that the residue dissolved in acids yielded the same salt as the original magnesia alba, though without effervescing. Limeburners had long produced quicklime from chalk by the same technique. Turning to this more common material, Black decided that the fire, far from adding some “principle” of causticity, simply drove off an elastic component of the alkaline earths, something aeriform as air itself. “Fixed air” he called this first found gas, thus perpetuating with Hales’s terminology an ambiguity which his exact research might otherwise have dispelled.
It was, indeed, with Black that the balance as the symbol of the chemist’s science displaced the still and the retort as symbols of the chemist’s craft. His theoretical conclusions are uninteresting—a naively Newtonian model of affinities running between corpuscles. But the scrupulosity of his gravimetric methods, his attention to the purity of his reagents, the patient cogency of each inquiry, the sound tactics of his experimental march—these were altogether admirable, and they were his own. For he is to be taken as the founder of quantitative rather than pneumatic chemistry. Though designed to identify carbon dioxide, his experiments read a little like one of those skillful plots in which the main character never appears, but is known by precise description of his effects on others, until ultimately he stands quite revealed. For gases are elusive.
Black took, for example, 120 grains of chalk, dissolved it in 421 grains of hydrochloric acid, and determined the weight of “fixed air” lost by effervescence to be forty percent. Then he took a second 120 grains of chalk, burned it to quicklime at a loss of forty-three percent, and found that to neutralize it—no effervescence this time—required 414 grains of acid. The quantities balanced within his limits of error, and demonstrated that chalk is forty percent fixed air. But Black in his classic Experiments seldom collected the fixed air itself, or studied its properties directly. Rather, he became a professor, and “a load of new official duties was laid on me, which divided my attention among a great variety of objects.” He retrieved it to formulate in later years the principles of latent and specific heat.
Ten years later, in 1765, the direct, quantitative study of gases was initiated in London by the Honorable Henry Cavendish, a nobleman, cousin to the Duke of Devonshire, and a scientific recluse and bachelor of the most extreme persuasion, and the most precise, the most finicking temper. His technique substituted mercury for water in the pneumatic trough of Stephen Hales, that little tub in which students still make their acquaintance with gas chemistry. He was able thereby to collect carbon dioxide without loss to solution. There, too, he imprisoned and identified the light “inflammable air” (hydrogen) which chemists had long elicited from vitriol (sulphuric acid) by its reaction with chips of iron or tin, and more recently of zinc.
Now the search became a chase. The most ardent in the hunt, and in his innocence one of the most attractive figures in all the history of science, was the Reverend Joseph Priestley of Birmingham. That industrial city, its practical, Puritan tradition softened now into Unitarianism and humanitarianism, made with London and Edinburgh the apex in the triangle of the British technical community. In March, 1772, Priestley presented his first paper on gases before the Royal Society. He announced the discovery of “nitrous air” (nitric oxide) and of “marine acid air” (hydrogen chloride). He had found, too, another “nitrous air” (nitrous oxide or laughing gas) which might be used to test the quality of common air. In modern terms, two volumes of nitrous oxide plus one of oxygen yield two volumes of nitric oxide. Since air is only one-fifth oxygen, the maximum contraction works out (as Priestley found empirically) at two volumes of air plus one of nitrous oxide to give 1.8 volumes of residual gases. Not only was the product smaller in volume than the sum of the reagents; it was twenty percent smaller than the air alone. And the degree to which this result was approached might be taken as a measure of the “goodness” of a sample.
Priestley’s distinguishing characteristic was a kind of manual imagination, an experimental unconventionality. Had he known any chemistry, he is reported to have remarked, he never would have made any discoveries. Oxygen was the most famous, of course. He had obtained an “air” by heating mercuric oxide. In it he put a candle—which flared into a torch:
I cannot, at this distance of time, recollect what it was that I had in view in making this experiment; but I know I had no expectation of the real issue of it. Having acquired a considerable degree of readiness in making experiments of this kind, a very slight and evanescent motive would be sufficient to induce me to do it. If, however, I had not happened, for some other purpose, to have had a lighted candle before me, I should probably never have made the trial; and the whole train of my future experiments relating to this kind of air might have been prevented.
Such was Priestley’s engaging frankness about a “crucial experiment.” Ingenious and enthusiastic, he cannot, perhaps, be justly called incoherent, but certainly he was the most discursive of scientists. His Experiments and Observations on Different Kinds of Air fill six volumes; his theological and metaphysical writings twenty-six. But even his lengthiness cannot irritate. He always redeems our liking by taking us so utterly into his confidence. He will not, for example, draw quantitative conclusions about the respirability of one of his airs: “Not having had the precaution to set the vessel in a warm place, I suspect that the mouse died of cold.” But it did not signify; the creature had survived long enough to prove the sample “better” than common air.
IT IS difficult to imagine a more diametric contrast of intellectual personalities than that between Priestley and Lavoisier, the protagonists of the chemical revolution: the one ingenious, enthusiastic, naïve, and literal; the other skilled, reserved, sophisticated, and critical; the one a Unitarian preacher, generous, incautious, and predictable in the political radicalism of his kind; the other ambitious to serve himself and the state in the official tradition of French expertise, planning his career within an ordered framework as carefully as any series of experiments. And however engaging, Priestley’s scientific style did betray that want of judgment and elegance which is often the penalty attaching to the worthiest of radical educations. For eighteenth-century England divorced vigor of mind from urbanity of taste by excluding the dissenting community from the universities of Oxford and Cambridge.
Lavoisier, on the other hand, was educated in the most assured of cultures, at the Collège Mazarin in Paris. His mind emerged one of the finest critical instruments ever formed by French secondary education, that remarkable process which, continuing in the great lycées, instills in the French intelligentsia something of the Cartesian spirit, something of its imperative toward order and unity of doctrine. Paris was not the city to appreciate Priestley’s qualities. He tells in his Memoirs of an only visit there, of the skeptical French eyebrows raised on his assuring the gathering in some salon that, though a Unitarian, he counted himself a believing Christian. Much less could his hosts find common ground with Priestley’s haphazard, not to say happy-go-lucky, philosophy of discovery, nor accept
the truth of a remark, which I have more than once made in my philosophical writings, and which can hardly be too often repeated, as it tends greatly to encourage philosophical investigations; viz. that more is owing to what we call chance, that is, philosophically speaking, to the observation of events arising from unknown causes, than to any proper design, or pre-conceived theory in this business. This does not appear in the works of those who write synthetically upon these subjects; but would, I doubt not, appear very strikingly in those who are most celebrated for their philosophical acumen, did they write analytically and ingenuously.
Lavoisier’s milieu never assimilated the analytical to the ingenuous. The difference is notable in the approach to research. For as Lavoisier entered the world of science in the late 1760’s, through geology at first but drawn increasingly to chemistry, he did not move among preachers and pharmacists, noblemen and doctors. He moved amid the French Academy of Sciences, choosing his associates in its mathematical sections, and particularly Laplace, Lagrange, and Monge. These men judged ideas with rigor. They felt, and no doubt they expressed, the condescension for the old chemistry that a theoretical physicist might now feel for the weatherman or the veterinarian presuming on some solidarity of science. Physics had long since learned better than to make theories out of vague principles like phlogiston. Thus it was to the unsatisfactory state of theory, and not to capturing new gases, that Lavoisier turned his attention, and particularly to its skeleton in the cupboard. For if phlogiston were the principle of fire in bodies, then its elimination in combustion should diminish the mass. But it was well known that metallic “calxes” outweigh the virgin metal. Practitioners in the pharmacist’s shop, or in the teacher’s chair, never cared whether loose ends met, so long as the subject made sense. And Lavoisier was the first to undertake an exact, a systematic study of combustion.
He chose the most combustible of ordinary chemicals, and was rapidly rewarded. On 1 November 1772 he confided, as was his right, a sealed note into the hands of the permanent secretary of the Academy:
About a week ago I discovered that sulphur, in burning, far from losing weight, on the contrary gains it; that is to say that from a livre of sulphur one can obtain much more than a livre of vitriolic acid, making allowance for the humidity of the air; it is the same with phosphorus; this increase of weight arises from a prodigious quantity of air that is fixed during the combustion and combines with the vapours.
This discovery, which I have established by experiments that I regard as decisive, has led me to think that what is observed in the combustion of sulphur and phosphorus may well take place in the case of all substances that gain in weight by combustion and calcination: and I am persuaded that the increase in weight of metallic calxes is due to the same cause. Experiment has completely confirmed my conjectures: I have carried out the reduction of litharge [lead oxide] in closed vessels, with the apparatus of Hales, and I observed that, just as the calx changed into metal, a large quantity of air was liberated and that this air formed a volume a thousand times greater than the quantity of litharge employed. This discovery appearing to me one of the most interesting of those that have been made since the time of Stahl, and since it is difficult to prevent something from slipping out in conversation with friends which might put them on the track of the truth, I have thought it right to make this deposition into the hands of the Secretary of the Academy against the time when I shall publish my experiments.
At that time Lavoisier was twenty-nine years old. He refined his experiments. He studied and repeated those of Black, whose disciple he was in gravimetrics. He perfected his methods. His essential technique then consisted in the combustion of reagents under a glass bell by means of a burning glass. He was fortunate in his instruments, a fine lens belonging to the Academy, “le verre ardent du Palais Royal,” and an even more powerful glass belonging to one of the noble houses patronizing science, “le grand verre ardent de la Tour d’Auvergne.” With a clarity of mind which cannot be too much admired, Lavoisier saw what had to be done to create a modern science of chemistry out of that chaotic legacy in which were jumbled the Greek doctrine of elements, old Stoic vestiges of fire and flux, Paracelsian principles of salt, sulphur, and mercury, alchemistical distillations and purifications, mineralogical and metallurgical lore, and the proliferating laboratory discoveries of his own time. And to clear his mind—perhaps, too, to fix it—he wrote down in his laboratory register what he meant to do for the rest of his life. Scholars have not resolved an uncertainty about the date of this document, whether it was composed early in 1772 or early in 1773, just before or just after the experiments reported in the sealed note. The earlier date fits the pattern of his thought, the later of his researches. The issue poses in miniature, therefore, that most interesting problem of Lavoisier’s career: which came first? For whichever date may be correct, this document remains the most prescient, not to say a priori, program of research in all the history of science:
Before commencing the long series of experiments that I intend to make on the elastic fluid that is set free from substances, either by fermentation, or distillation or in every kind of chemical change, and also on the air absorbed in the combustion of a great many substances, I feel impelled to set down here some considerations in writing, in order to outline for myself the course that I ought to take.
It is certain that there is liberated from substances, under a great many conditions, an elastic fluid; but there are in existence several doctrines as to its nature….
It is important to be clear about the complexities that Lavoisier confronted. On the one hand, he was convinced that combustion and respiration involve combination with something atmospheric. On the other hand, “fixed air” (carbon dioxide), the one gas firmly identified in experiment, presents properties almost contrary to those required. It is rather the product than the food of respiration and combustion, and very different from common air.
These differences will be exhibited to their full extent when I shall give the history of all that has been done on the air that is liberated from substances and that combines with them. The importance of the end in view prompted me to undertake all this work, which seemed to me destined to bring about a revolution in physics and in chemistry. I have felt bound to look upon all that has been done before me merely as suggestive: I have proposed to repeat it all with new safeguards in order to link our knowledge of the air that goes into combination or that is liberated from substances, with other acquired knowledge, and to form a theory. The results of the other authors whom I have named, considered from this point of view, appeared to me like separate pieces of a great chain; these authors have joined only some links of the chain.
What those authors had mainly neglected was the source of air found in many substances. He, therefore, would repeat and extend all the experiments in which air is taken up, so that knowing the origin, he could trace what became of it. “The processes by which one can succeed in fixing air are: vegetation, the respiration of animals, combustion, in some conditions calcination, also some chemical changes. It is by these experiments that I feel bound to begin.” It is obvious in retrospect that he needed, first, to distinguish between reactions which “fix” carbon dioxide (vegetation, slaking) and those which require oxygen. Secondly, he had to establish the chemical similarity of burning, rusting, and breathing. Finally, he would recognize the atmosphere as a mixture containing oxygen as a distinctive member of the whole population of gases. For Lavoisier, it would prove the most interesting member by far.
Twenty years later Lavoisier had done the experiments he planned, all but those on respiration and chemical physiology, which he was about to undertake when interrupted by the guillotine. He had put the links together. He had created his body of doctrine and formed the theory of his science. He had worked the revolution, in chemistry at least. And perhaps it amounted to a revolution even in physics to recognize chemistry as a science continuous with itself.
What career has ever been planned in such perfection? It required money, of course, and Lavoisier was not rich. But he secured a place in the corporation of financiers which farmed the taxes, devoted a portion of each day to acquiring wealth, and acquired, too, his patron’s fourteen-year-old daughter as his wife. She was an heiress. She was intelligent. She knew English. She became interested in chemistry, and served as amanuensis in the laboratory. Laboratories were private enterprises in the eighteenth century. Lavoisier proposed an improved technique for making gunpowder, was given charge of the commission created in 1777 to practice it, and was installed in an official apartment and laboratory in the Arsenal at the charge of the state. Madame Lavoisier has left an account of his day which has recently come to light. It was written many years after the death he met in company with his colleagues of the tax farm. For at last accounts that proved an unprofitable association.
Each day Lavoisier sacrificed some hours to the new affairs for which he was responsible. But science always claimed a large part of the day. He arose at six o’clock of the morning, and worked at science until eight and again in the evening from seven until ten. One whole day a week was devoted to experiments. It was, Lavoisier used to say, his day of happiness. A few enlightened friends, several young men proud to be admitted to the honor of cooperating in his experiments, would gather in the laboratory in the morning. There they would lunch. There they would hold forth. There they would work. There they performed the experiments which gave birth to that beautiful theory which has immortalized its author. Oh, it was there that one had to see that man to understand him, gifted with so fine a mind, so sure a judgment, so pure a talent, so lofty a genius. It was by his conversation that one could judge the beauty of his character, the nobility of his thoughts, the severity of his principles. If ever any of the persons who were admitted into his intimacy should read these lines, the memory will not, I believe, retrace itself without emotion in their souls.
There, in the laboratory, Lavoisier might, perhaps, be known. For us, it is more difficult. We may visit the instruments preserved in Paris at the Conservatoire des arts et métiers. We may admire the beautiful balances, the elegant pumps, the graceful bell-jars, which lent his results a precision to which no chemist had ever dreamed aspiring. Scholars may go to the Archives of the Academy to peruse the registers from his laboratory. They open with the program of 1772 (or 1773). They might still serve as object lesson in any laboratory of instruction. Every quantity is entered, over all the twenty years. His works are gathered in six volumes. His correspondence is just now in course of publication. Step by step, we may follow his career. Nevertheless, these voluminous, these utterly impersonal remains, they conceal, somehow, the most inaccessible of the great scientists. The man himself escapes us. Something is lacking in that too perfect career. It is not ambition. Every act bespeaks ambition. But outside the laboratory, at least, Lavoisier seems a cold man. Not that he avoided polemics, but his writings in this vein are chilly and clear. There is none of the heat of conflict, no welcome self-betrayal, only that “severity of principles.” The defense of phlogiston is too forlorn to tempt the historian. Lavoisier’s opponents never seem worthy of his steel. He never spared them for that. He controlled, it may be, everything but the power of his own mind. And the only thing he failed to foresee was the vulnerability of qualities like his, those of the expert, those of the intellectual, the man who knows and is right, at a time when the feelings of the common man (Rousseau’s common man, “who knows nothing and thinks none the worse of himself”) were at their most intense political pitch in all history, when in the metaphor Burke borrowed from Lavoisier’s science, the “fixed air had broke loose,… the wild gas was abroad.”
But that disastrous incompatibility apart, there was a passion in Galileo and Descartes, in Kepler and Newton, and even under the seemly Victorian guise of Darwin, which one misses in Lavoisier, whose superior lucidity does not quite fill the same office. Is this why, alone among the great founders, he was not a great discoverer? Did that clarity of mind exclude some element of imagination, or simple curiosity, some sense of sympathy for the unexpected inwardness of things? The question can only be conjecture. But there was that in the perfection of his experimental method which might be counted a limitation. The luminosity of the great research memoirs of the 1770’s and ’80’s, in which he executed the program of 1772, has been often and justly praised. In retrospect, to use his own figure, or pre-figure, each seems a link in the chain supporting the theory of combustion and the conception of chemical reactions supposed by that theory. Knowing the role of oxygen, the constitution of the atmosphere, the composition of water, we do not see how to withhold assent, although we know that some more qualified than we to judge, among them Priestley and Cavendish, never did feel compelled by that fine reasoning.
But to us it is persuasive. Does phosphorus emit or does it combine on burning? Weigh it, and the choice must come down with the balance. Does or does not the additional mass come from the atmosphere? Measure, and the diminished volume speaks the answer. Just so every experiment was designed with unprecedented elegance to answer some absolutely relevant question with a yes or a no. And there, perhaps, was the flaw. For Condillac’s logic, which Lavoisier applied, is that of a taxonomy, which is to say of a choice. It is a logic of the discovered, which makes no room for the psychology of discovery, for the adventure into the unknown.
Perhaps there is always a danger that it will impoverish inquiry to elevate the logic of existing science into precepts of method. Those magnificent experiments, they were almost laboratory exercises in the syllogism, deductions from known facts in accordance with maxims like conservation, rather than inquiries after the manner of Newton and his prism, or of Priestley and his tub of mercury, its bubbles yielding what next? Not so Lavoisier, whose experiments, how infinitely superior in design, served finally to clarify what was rightly in the premises of 1772. If so, this too would be consonant with his mathematical inspiration, deductive and rigorous rather than adventitious or random.
Chemistry profited, therefore, from the curious, the almost symbiotic relationship between Priestley and Lavoisier, however unwelcome to both. If Priestley’s lack of theoretical taste disqualified him from understanding his discoveries, Lavoisier’s lucidity disqualified him from making them. By his own program, combustion, calcination, and respiration, all involve fixation of something from the atmosphere. Lavoisier knew where to look, but not what to look for. Thus in this essential instance did all his method prove incompetent as an instrument of discovery. For it was Priestley who told him.
It is an affliction of immaturity in the historiography of science that many of its historians give themselves over to quarrels about priorities with as much, if not more, bitterness than the discoverers themselves, in whom the failing is more forgivable, if no less inelegant. By now the facts about oxygen seem tolerably clear. That gas betrayed its existence by a peculiar property of its oxide with mercury. On moderate heating, mercury will form its red oxide. On stronger heating, but at temperatures still well within the laboratory range, mercuric oxide will decompose. It was the one relatively common “calx” which might easily be reduced in the absence of charcoal. Only this reduction, therefore, would liberate oxygen instead of carbon dioxide.
Given all the activity in pneumatic chemistry and combustion, there must surely be a sense in which the discovery of oxygen was inevitable. In February, 1774, for example, Bayen in Paris roasted mercuric oxide—and reported the gas as fixed air! Nor was he the first. It is now known that at Upsala, Carl Wilhelm Scheele had been experimenting with oxygen, prepared from mercuric and also from silver oxide. “Fire air” he called it, but published nothing until later. Lavoisier had sent a copy of his first book, and Scheele did mention his discovery in the closing sentences of his letter of thanks, dated 30 September 1774. Lavoisier never acknowledged this rather cryptic communication. The phrasing would not command attention. And he must have received it just when Priestley was in Paris, exchanging experiences with his French colleagues, and shocking them by his religious belief.
Priestley first liberated oxygen on 1 August 1774, distinguished it from carbon dioxide by its insolubility and support of combustion, and mistook it for his familiar laughing gas. Shortly after, he tried the same technique with red lead. In October he was in Paris, and “As I never make the least secret of anything that I observe, I mentioned this experiment also, as well as those with the mercurius calcinatus, and the red precipitate, to all my philosophical acquaintance at Paris, and elsewhere, having no idea at that time, to what these remarkable facts would lead.” Nor did he suspect until the following March that he had a new and quite extraordinary air: “But in the course of this month, I not only ascertained the nature of this kind of air, though very gradually, but was led by it to the complete constitution of the air we breathe.” Trying all the means of testing air, quite at random at first, he found that his new air supported combustion, and respiration, even after the test for diminution with laughing gas: “Thinking of this extraordinary fact upon my pillow, the next morning I put another measure of nitrous air to the same mixture, and, to my utter astonishment, found that it was farther diminished to almost one half its original quantity.” Starting, then, with a fresh sample, he concluded from the laughing gas test that it was “between four and five times as good” as common air. And this, of course, was a way of saying, with characteristic indirection, that common air is no simple substance, that it contains about twenty percent by volume of “pure” or “dephlogisticated air.” So Priestley always called his great find. He was middle-aged and never changed his ideas. His new air supports combustion with such energy because its utter freedom from phlogiston gives it five times the capacity of ordinary air for blotting up that principle from burning bodies.
Lavoisier seized upon the mercuric oxide experiment more quickly than its author. He repeated it in November, 1774, and chose it to communicate the next spring at the Easter meeting of the Academy. This was always a formal occasion, when the public was admitted to the bar of science, and when, therefore, the Academy took care to offer something interesting. Lavoisier was appearing for the first time in a leading role. He had begun, he told his audience, with one ounce of oxide. After calcination under the lens of the Palais Royal for two and one-half hours, pure mercury was recovered to the amount of seven gros, eighteen grains. The difference was fifty-four grains, and the volume of “air” evolved seventy-eight cubic inches, “from which it follows, that supposing all the loss of weight is attributed to the air, each cubic inch must weigh a little less than two-thirds of a grain, which does not differ much from the weight of ordinary air.”
For once Lavoisier let eagerness betray him into wishful accuracy. He distinguished those seventy-eight cubic inches of gas from carbon dioxide, grasped at them as the object of his search, and mistook oxygen, not like Priestley for laughing gas, but for air itself: “And it is very likely that all metallic calxes, like that of mercury, would give only common air if they could all be reduced without addition,” For all the circumstances convinced him that what he had in his receiver “was not only common air, but that it was more respirable, more combustible, and consequently that it was more pure than even the air in which we live.” Lavoisier paid the price for this, almost his only venture into ambiguity. He had made, not perhaps a crucial experiment, but certainly a crucial mistake. He reported of the last of his tests that this air “was diminished like common air by an addition of a third of nitrous air.” Priestley had, therefore, one last contribution to make to the founding of the new chemistry. It was to set the founder right.
Priestley felt a certain pique on reading the Easter Memoir. Nowhere did it mention that Priestley had told of the gas evolved from mercuric oxide. Yet his pleasure in correcting Lavoisier’s misidentification is expressed with all the moderation of extreme good nature. He had got it right himself only in the meantime.
As a concurrence of unforeseen and undesigned circumstances has favoured me in this inquiry, a like happy concurrence may favour Mr. Lavoisier in another; and as, in this case, truth has been the means of leading him into error, error may, in its turn, lead him into truth. It will have been seen, in the course of my writings, that both these circumstances have frequently happened to myself; and indeed examples of both of them will be found in my first section concerning this very subject of dephlogisticated air.
It is pleasant when we can be equally amused with our own mistakes and those of others. I have voluntarily given others many opportunities of amusing themselves with mine, when it was entirely in my power to have concealed them. But I was determined to shew how little mystery there really is in the business of experimental philosophy, and with how little sagacity, or even design, discoveries (which some persons are pleased to consider as great and wonderful things) have been made.
Lavoisier was not, perhaps, one to be amused at a comedy of errors, much less if cast for a major role. He never alluded to this rebuke. But he gave over premature attempts at doctrine, and turned to occupying the ground for a new chemistry in a series of memoirs of which the strategy was as formal as that of an eighteenth-century military campaign.
THOSE decisive memoirs develop three lines which Lavoisier made converge on a new chemistry. The first explores the nature of acids. The second leads to the proposition that combustion, calcination, and respiration are all oxidation. The third advances the problem of heat. It is the second line which now seems central, having continued straight through the history of chemistry and grouped the science around its most frequent class of reaction. But if Lavoisier’s deviations on the constitution of acids and the “matter of heat” appear oblique, they are at least as interesting. Unlike the gay and spirited mistakes of the tolerant Priestley, they are the defects entrained by the most notable rationality to illuminate science since that of Descartes himself.
On 26 April 1776, just a year after his Easter Memoir had exposed him to the humiliation of correction, Lavoisier read before the Academy a “Memoir on the Existence of Air in Nitric Acid, and on the Means of Decomposing and Reconstituting that Acid.” He reminded his colleagues of his early experiments on combustion of sulphur and phosphorus, and of the formation of acids by absorption of the vapors. Reflection had convinced him that air enters into all acids, and that they are differentiated by other principles specific to each. “The which, at first only a plausible conjecture, was soon converted into a certainty when I applied experiment to theory.” For Lavoisier’s memoirs take their structure and style from the pattern of his thought, and often begin with the conclusion. Nevertheless, he had learned his lesson. He specifies “not simply the air, but rather the purest portion of the air,” as that which enters into all acids and which “constitutes their acidity.”
The facts and experiments, he carefully recognizes, were all Priestley’s. Nevertheless, the conclusions he draws are so different, that “I hope that, if I am reproached for having borrowed my proofs from the works of this celebrated scientist, at least my property in the consequences will not be contested me.” He chose mercury to make his salt, since its reducibility without carbon eliminates the masking of results by carbon dioxide. Having, therefore, dissolved mercury in nitric acid, he evaporated the solution and heated the residue in two stages. First, he decomposed mercuric nitrate, and collected the nitric oxide. Then he roasted the mercuric oxide and collected oxygen. It is a mark of his purposive method that he distinguished the two gas fractions and held them separate. As always, he kept strict account of quantities. But perhaps the most interesting feature of this memoir is the deployment of yet a further element of sophisticated technique, one which became a hallmark in the memoirs to follow. Nitric acid once decomposed, the complement of the demonstration is “to reconstitute it by recombining the same materials, and in this I have succeeded:
Nitric acid … is nothing other than nitrous air, combined with an approximately equal volume of the purest part of common air, and with a considerable quantity of water. Nitrous air, on the contrary, is nitric acid deprived of air and water. No doubt there will be some who will ask at this point whether the phlogiston from the metal does not play some role in the operation? Without daring to decide a question so fraught with consequences, I shall only reply that, since the mercury emerges from the reactions in precisely the same state in which it entered, it does not appear to have lost or regained phlogiston—unless it be claimed that the phlogiston which has reduced the metal passed right through the walls of the vessels. But that would be to admit a particular kind of phlogiston different from that of Stahl and his disciples. It would be to return to the fire principle, to some fire combined with bodies, and that is a system of thought very much more ancient than Stahl’s, and very different in spirit.
And so Lavoisier flicks at phlogiston, lightly for now, hinting on the one hand that a test of the objective existence of a substance for chemistry is the possibility of taking it out of bodies, and then restoring it, handling it in short; inferring on the other hand that to evade this criterion is to fall back out of science onto Heraclitus and the Stoics, onto fire as activity and the world as flux and as becoming.
A year later, on 16 April 1777, Lavoisier read a second major paper, “Memoir on the Combustion of Kunckel’s Phosphorus.” It extends to phosphoric and sulphuric acid the analysis which makes “pure air” the principle of acidity. But the main point is to apply the double movement of analysis and reconstitution to the atmosphere, which “as I have already suggested several times, is composed of about one quarter of dephlogisticated, or eminently respirable air, and of three quarters of noxious, poisonous air, a type of gas of unknown nature.”
Lavoisier was busy all the while with a great number of minor experiments, of which record remains in the laboratory registers. He tried guinea pigs in bell jars of oxygen, and performed autopsies when finally they suffocated. He examined the changes in the blood stream on respiration. He made against Priestley a minor but a telling point—air expelled from the lungs has been converted to “fixed air” (carbon dioxide) and not to “phlogisticated air” (hydrogen) as in Priestley’s terms it should be. In one day, he deposited at the Academy twenty-six memoirs on acids, salts, and the incarnations of oxygen. His fidelity and exactness were beyond reproach. And they served a purpose higher than the purist’s.
By November, 1777, Lavoisier felt ready to advance an intermediate “Memoir on Combustion in General.” In the lucid preamble which always orients his reader to his purpose, he makes one of his most explicit and characteristic avowals on the role of theory:
Dangerous though the spirit of systems is in physical science, it is equally to be feared lest piling up without any order too great a store of experiments may obscure instead of illuminating the science: lest one thereby make access difficult to those who present themselves at the threshold; lest, in a word, there be obtained as the reward of long and painful efforts nothing but disorder and confusion. Facts, observations, experiments, are the materials of a great edifice. But in assembling them, we must not encumber our science. We must, on the contrary, devote ourselves to classifying them, to distinguishing which belong to each order, to each part of the whole to which they pertain.
In the case of modern chemistry, the facts might be ordered into four classes. 1) Combustion always evolves a “matter of fire,” apparent as light. 2) Bodies burn only in very few airs, perhaps only in “pure air.” 3) In all combustion, “pure air” disappears, while bodies gain weight by just that amount. 4) In all combustion, the product is transformed into an acid by further addition of “the substance which has increased its weight.” By the phlogiston hypothesis, combustible bodies are said to burn because they contain a principle of combustion, and to contain that principle because they burn. So combustion accounts for combustion. But “I dare venture the assertion in advance that the hypothesis I propose explains in a very happy, in a very simple manner, the principal phenomena of physics and chemistry.”
Now, Lavoisier turned to maneuver his acids into place. In November, 1778, he presented a paper complementary to that on combustion, “General Considerations on Acids.” As always, he reviewed the state of the question. Of old, chemists had pushed analysis only to the point of distinguishing oils, salts, earth, and water, conceived less as discrete types of matter than as quality-bearing principles. Eighteenth-century chemistry had immensely improved its description and theory of neutral salts. Henceforth, chemists must do “for the constituent principles of neutral salts, what the chemists our predecessors accomplished for the neutral salts themselves, which is to say to attack the problem of acids and bases and to push back the boundaries of such chemical analysis yet one more stage.” He feels himself ready for a first level of generalization on acids. And relying on his recent experiments, he advanced in a more important way than heretofore the hypothesis “that pure air, eminently respirable air, is the principle constituting acidity.”
So confident was Lavoisier of this consequence, this “truth which I regard as very solidly established,” that he took that step which was decisive for an eighteenth-century science, to be ventured only in the presence of a truth, the step of naming. All chemistry since has immortalized that error. “Henceforth I shall designate dephlogisticated air, or eminently respirable air in the state of combination or fixity, by the name of acidifying principle, or if the same signification be preferred in a word from the Greek, by the name of oxygenic principle”—that which generates acidity.
Nor had Lavoisier yet arrived at the notion of elementary oxygen. As he broke loose from the chemistry of principles, he carried with him for a little a diminishing residue of those old notions, more verbal than real. It is in chemical combination that oxygen is the acidifying principle. As a gas, the oxygenic principle is otherwise combined, with matter of fire—caloric—to put it in the aeriform state. But this was peripheral to chemistry. What was grave was that once again, in the full flush of theory, Lavoisier ignored a warning from that laboratory where he was the master of them all, more subtle this one than that which might have saved him the Easter Memoir, but a fact no less thorny. Acids, he wrote, are more complex than their neutral salts, and correspondingly troublesome to analyze. They demand great virtuosity of technique: “I hope, however, to be in a position to show in the future that there is no acid, with the possible exception of that from marine salt, which may not be decomposed and reconstituted, and from which one may not remove the acidifying principle and restore it at will.”
The possible exception, of course, is hydrochloric acid. The italics are not Lavoisier’s.
From the configuration of his work, it would appear that Lavoisier meant to press forward along the acid front. He was diverted by an unexpected opportunity. He himself had raised the question of what the product is when hydrogen burns? Finding none, he left the problem in abeyance. Nevertheless, the difficulty was real. A vanishing combustible was more helpful to an emission than to a combination theory of combustion. Then, in 1781 Priestley tried exploding inflammable air with common air by an electric spark. He was seeking to determine the weight of heat, and only mentioned that the inside of the vessels became dewy. In 1783 Cavendish repeated these experiments, on too small a scale to collect the dew, though he did discern that the reaction reduced the volume of the air involved by one fifth. Interested in the dew, he enlarged the scale, arranged to admit the two gases continuously into a receiver where the combustion was sustained, collected the moisture, and found it to be pure water.
The attendant polemics over priorities have been, if anything, even more ferocious than over the discovery of oxygen. In England James Watt, of the steam engine, in France Gaspard Monge, founder of descriptive geometry and then an impecunious instructor at the school of military engineering at Mézières, made the same discovery, independently, almost simultaneously, and in the case of Monge with no less clarity. Once again, therefore, the interesting question is not who made water, but who understood what had happened? For though the composition of water was the last of the great discoveries of the English pneumatic school, Cavendish interpreted his results as the condensation of water, not its synthesis. Indeed, the light weight of hydrogen encouraged certain of the English chemists, militant in their last ditch, to identify it for a time with phlogiston itself.
Lavoisier, on the contrary, instantly saw water, seemingly the simplest of substances and classically the most intuitive of elements, rather as the oxide of that gas which is—to anticipate the rest of nomenclature—hydrogenerative. He seized upon Cavendish’s results as upon a piece of tactical fortune. Or rather upon the word of those results, for this reached Paris before Cavendish had published, borne by Charles Blagden, who visited Lavoisier at the Arsenal in June, 1783. On the 24th, Lavoisier assembled a company of academicians, among them Laplace and Meusnier, and tried the experiment. He burned fifty pints of inflammable air with twenty-five of oxygen, and collected 660 grains of water. Next day Lavoisier and Laplace repeated the experiment and announced the results to the Academy. Monge communicated a memoir to the same effect in August. And now Lavoisier enveloped the question in his own method. Synthesis having come first, he proposed to demonstrate the reversibility of the results by analysis. Working with Meusnier, he wrapped a musket barrel in a sheet of copper (to prevent oxidation of the surface), and cradled it on an angle through a charcoal brazier. The lower end was connected through a condenser to a gas receiver. Drop by drop, water was admitted at the upper end, and the quantity compared to that condensed below. The difference “according to the theory which guides us” should be equal to the increase in weight of the gun barrel through oxidation of the bore added to the weight of hydrogen in the receiver. Unfortunately, the high temperatures, together with the smallness of the difference compared to the mass of the barrel, precluded real precision. Lavoisier found water to consist of one-third to one-sixth hydrogen by weight.
A memoir to this effect was read by Meusnier on 21 April 1784, just in time to be published (what with the chronic lag in the Academy’s schedule of publications) in the volume for 1781. In all his own writings on this subject, Lavoisier made very fleeting mention of Cavendish. One would suppose the findings to have been the continuation of his own abortive trials of hydrogen, and confirmations of the work of Monge. And perhaps one would be right, in principle if not in fact. For this dramatic demonstration that water is an oxide, though not a “crucial experiment” in the Baconian sense, was nevertheless decisive historically. in the campaign to exorcise phlogiston in favor of a positive concept of combustion as that chemical reaction in which oxygen combines.
Thus it fell out that Lavoisier’s rationalization of chemistry around the combining role of oxygen appears in retrospect as the linear continuation of his early experiments on combustion. This chapter opened with the first paragraph of the Reflections on Phlogiston of 1785. In reality, that beautifully argued, that truly magisterial memoir, represents the completion of the case, the summing up and not the opening argument. It came as no surprise. Lavoisier had not, after all, been able to restrain his impatience with the old doctrines, his intolerance for untidy facts. The self-denial imposed after the Easter Memoir weakened as his confidence grew. Expressions of scorn for phlogiston chemistry escaped him in each successive memoir. Now, the assault is frontal:
Chemists have made of phlogiston a vague principle, which they in no way define rigorously, and which in consequence is adaptable to any explanation they please. Now this principle has weight, and again it is weightless; Now it is free fire, and again it is fire combined with the element earth; Now it penetrates right through the pores of vessels, and again it finds bodies impenetrable. It explains at once causticity and its opposite, translucence and opacity, colors and the absence of colors. It is a veritable Proteus, changing form at every instant.
It is time to bring chemistry to a more rigorous way of reasoning: It is time to strip off everything merely systematic or hypothetical from the rich store of facts to which that science is every day adding: It is time, in short, to exhibit the stage to which chemical knowledge has attained, so that those who follow us may start from that point and proceed with confidence to the advancement of the science.
Wielding Occam’s Razor, Lavoisier cut phlogiston right out of the heart of chemistry, right out of its consciousness. And the chemical revolution consisted in the extension of Lavoisier’s conception of combustion to all chemical reactions. That act made of chemistry a modern science. Forgotten was the lingering animistic instinct for cosmic digestion as the archetype of chemical processes. No longer was the allegiance of chemistry ambiguous as between the two great camps of organic and physical science. Its object became the positive combinations and separations of materials rather than qualities, its events reactions rather than fluxes, coctions, and processes. Henceforth, the chemist weighs amounts. He does not distill out principles. For more intimately in chemistry than in any other science, the foundations of objectivity were embedded in a quantitative conscience.
Scientists have sometimes written that Lavoisier formulated the law of conservation of matter. The reality was simpler. He assumed it. It was for him what it had been for the ancient materialists, a precondition but no finding of his science: “We must lay it down as an incontestable axiom,” says the Elements of Chemistry, “that in all the operations of art and nature, nothing is created; an equal quantity of matter exists both before and after the experiment.” From this principle, he writes again in the Memoir on Water, derives “the necessity to perform experiments with more exactness, and on a larger scale.”
Social scientists, for their part, sometimes like to see science drawing inspiration from society and politics. It has even been suggested that Lavoisier derived his chemical “philosophy of the balance sheet” from the accounting practices of the corporation which farmed the taxes. The economic interpretation of creativity takes special pleasure in Lavoisier’s hobby of agricultural reform. With the Frenchman’s feeling for the soil, with the theorist’s interest in new techniques, he bought a manor at Fréchines, not far from Blois. There he realized the physiocrat’s dream of a model farm. Madame Lavoisier tells how an account was opened for every field, and a record kept (as in the laboratory) of seed, fertilizer and labor, of yield, price and ultimate return. But surely the historian need not go thus far afield to find the origin of Lavoisier’s input-output chemical procedure. Lavoisier, a Parisian, had studied Joseph Black before he became a gentleman farmer or a pillar of the tax farm. His was the spirit of the philosophe, shining the bright light of scientific method into dark corners of routine.
Philosophe and scientist, he turned from the Academy, before whom he had read Reflections on Phlogiston, to the public whom he wished to educate, repudiating in eighteenth-century style the erring past along with error itself, placing his confident hopes for his science rather in that still malleable youth destined to pay the first installment on posterity’s debt to the Enlightenment. The closing sentences of that memoir invoke this future:
I do not expect my ideas to be adopted all at once. The human mind gets creased into a way of seeing things. Those who have envisaged nature according to a certain point of view during much of their career, rise only with difficulty to new ideas. It is the passage of time, therefore, which must confirm or destroy the opinions I have presented. Meanwhile, I observe with great satisfaction that the young people who are beginning to study the science without prejudice, and also the mathematicians and physicists, who come to chemical truths with a fresh mind—all these no longer believe in phlogiston in Stahl’s sense. They regard that whole doctrine as a scaffolding more embarrassing than useful for continuing the edifice of chemical science.
The pattern of 1772 still determined Lavoisier’s career. First he had discerned the shape of the new science. Then he had spent the fifteen intervening years in the laboratory comprehending “chemical truths.” Now those truths must be disseminated and clothed in rational dress. In 1787 the public meeting of the Academy fell on 18 April. Lavoisier chose the occasion to present a “Memoir on the Necessity of Reforming and Perfecting the Nomenclature of Chemistry,” Nothing, he remarked in a private note, seemed to him more urgent for the advancement of the sciences.
That method which it is so important to introduce into the study and teaching of chemistry is closely linked to the reform of its nomenclature. A well-made language, a language which seizes on the natural order in the succession of ideas, will entail a necessary and even a prompt revolution in the manner of teaching. It will not permit professors of chemistry to deviate from the course of nature. Either they will have to reject the nomenclature, or else follow irresistibly the road it marks out. Thus it is that the logic of a science is related essentially to its language.
Consistently with the purpose, at once pedagogical and methodological, Lavoisier stepped back into the company of his colleagues to let the enunciation of chemical linguistics appear as the work of a school, a French school. In no circumstance was the chemical revolution more intimately related to the Enlightenment. Chemists had often wished to specify their materials more precisely than by the picturesque names of old tradition. But the first to propose a sweeping reform was Guyton de Morveau, trained in the categories of the law rather than of science, and experienced in the affairs of the Parlement of Dijon earlier than in the laboratory, to which he came by way of the industrial, literary, and philosophical aspirations of a provincial man of affairs. In the 1770’s the publisher Panckoucke put in hand a revision and systematization of Diderot’s Encyclopaedia. In this Encyclopédie methodique each department of science and affairs was to have a subsidiary collection to itself. Guyton took charge of the chemical volumes. His proposal for a systematic re-ordering of chemical terminology had the object, therefore, of reducing that science to the encyclopedic state.
Guyton came to Paris to consult the chemists of the Academy. Lavoisier tells how the magnitude of the project was borne in upon them all, and of how Guyton, “destined to be, in a sense, the spokesman for French chemists,” generously sacrificed his private intellectual property in the project to the better part of a collaboration. “Only,” Lavoisier continues, “after having reviewed all the parts of chemistry several times, after having meditated profoundly on the metaphysics of language, and on the relation of ideas to words, have we ventured to form a plan.”
They published their Method of Chemical Nomenclature, as a symposium, in 1787. The tenor is far less doctrinaire than its philosophic inspiration might lead the reader to expect. Common names of so-called simple substances—iron, sulphur, ammonia—were retained wherever they did not entrain really false ideas. If they did—“inflammable air” for example—a word was substituted, drawn usually from a Greek stem, so as to express the most characteristic property of the body in question. The principle was both mnemonic and pedagogic. Students were to be accustomed from the outset to admit no word without attaching an idea. Many “simple” substances would, no doubt, yield to analysis. But so long as the science was organized around a method of naming, rather than a dogmatic nomenclature, its future need not be embarrassed. And indeed, the scheme did provide for the possibility of reading, for example, sodium chloride for salt, potassium nitrate for nitre.
As to compound substances, a classification had first to be agreed on. “In the natural order of ideas, the name of class and genus is that which evokes the properties common to a large number of individuals, while the name of species is that which leads to the idea of the properties peculiar to certain individuals. That natural logic pertains to all the sciences. We have tried to apply it to chemistry.” Thus, acids are composed of two substances. One constitutes acidity. As a group, acids assume the status of a class or genus. The other is peculiar to each acid. The specific name must differentiate as modifier. But certain acids, those formed by sulfur for example, contain their two principles in varying proportion: they will be distinguished by varying the suffix, sulfurous or sulfuric, according to the proportion of oxygen. So, too, would oxides, salts, and bases find their places, and the very few carbides and sulphides then known to chemical science. Nor is it necessary to insist on the principles which have guided chemical nomenclature ever since.
Nomenclature, conservation, quantity, economy, synthesis-analysis, rationality, enlightenment in truths of nature—such were the materials which in 1789 were assembled in the Elements of Chemistry, Lavoisier’s first general work, and perforce his testament. And it has seemed best to let the organization of this chapter itself exemplify a Lavoisier analysis: decomposing, distinguishing those elements, and recomposing in the mind of history and—one hopes—in its understanding and even in its sympathy.
THAT sympathy will go out to Lavoisier more naturally, perhaps, in his failures, and in his death, than in his precisions or his triumph.
Reading about combustion, heat, and acidification in Lavoisier’s memoirs, the modern chemist will, of course, follow the strand of combustion. It is the warp thread about which his science has woven its fabric. Of the two lines that led astray, the theory of acidification has seemed the more venial a mistake. Most acids do contain oxygen, after all, even if it is in the wrong place, and the notion of acidification as super-oxidation has been indulged as a natural over-extension of theory. But caloric has been handled with some severity. It was Lavoisier’s belief that heat is the manifestation of a subtle, elastic fluid—light too, perhaps, though as a chemist Lavoisier was more interested in heat. On the strength, or rather on the weakness, of this view, the most recent of Lavoisier’s historians, Maurice Daumas, questions the reality of a distinction between Lavoisier’s chemical doctrine and that of “principles” which he supplanted. Caloric, imponderable and permeating, was no more positive than phlogiston. “In spite of his genius,” says Daumas in the final sentence of an illuminating book, “Lavoisier could act and think only as a man of the eighteenth century.”
But not, one feels, in the retrograde sense which M. Daumas (in this connection only, it is true) implies; and it will, perhaps, be conformable to the tactic of that century to argue toward a reversal of his emphasis from an etymological analysis of Lavoisier’s two cardinal principles. For the word “oxygen” honored in the breach the caution which presided over the systematic nomenclature of 1787, ensuring that names should be noncommittal against the chance of future discoveries. Ignoring the inconvenience of hydrochloric acid, Lavoisier fell into mortal sin against fact. He put himself (if the historian may borrow the logician’s banality) in the position of a naturalist questing in the Antipodes who should ignore his fleeting glimpse of a black swan in deference to the proposition that swans are white.
The word “caloric” entailed no such fatality. Unlike oxygen, it was coined in a spirit which combined the precepts of Condillac with a Newtonian prudence which knows bodies by their effects. Guyton explained the term in a footnote to the Nomenclature. It denotes that which is perceived as heat without implying anything about its nature. Nor will its function appear as a derogation from Lavoisier’s chemical wisdom. For caloric does not act as a chemical agent. It is excluded, absolutely excluded from the practice of chemistry, by the most elementary consideration of method. Like light, heat passes right through the walls of laboratory vessels. The bounds of the experiment are no boundaries for heat. It may not be weighed. Its effects are like some action which remains unknown to common law, not because it is nonexistent, but because of legal conventions. Just so a science which found its metric in weighed masses might not take cognizance of caloric.
Surely this consideration must restore all the sharpness of the distinction on which Lavoisier himself insisted. For Priestley as formerly for Stahl, and indeed for the whole school which Lavoisier set his face against, phlogiston constituted the essence of theory. Nor could this concept be outgrown. It had to be turned on its head. No mathematical statement of the quantities embraced by phlogiston could possibly be abstracted from the question of its nature. Not so caloric, which (like Newton’s aether) entered permissively rather than constitutively into the structure of theory. Caloric disappeared from science in the nineteenth century. Nevertheless, the theory of oxidation, and by extension the conception of chemical reaction, remain what they were left by Lavoisier, and not by Priestley.
Drawing out the analogy with aether may help. In a note of 1774 Lavoisier had conjectured that under proper conditions of pressure and temperature, any body may assume each of the three states of matter. What transforms a solid to a liquid, and over a higher threshold a liquid to a gas, is permeation by caloric, which counters Newtonian attraction:
Thus, [according to the Elements of Chemistry] the particles of all bodies may be considered as subject to the action of two opposite powers, the one repulsive, the other attractive, between which they remain in equilibrio. So long as the attractive force remains stronger, the body must continue in a state of solidity; but if, on the contrary, heat has so far removed these particles from each other as to place them beyond the sphere of attraction, they lose the adhesion they before had with each other, and the body ceases to be solid.
If (to render his thought by a terminology which was not his) the gaseous state argues permeation by heat as anti-attraction, then caloric its medium was anti-aether. And Lavoisier introduced a medium for the same reason as Newton had done: not as a constituent of positive theory, but as a suggestion designed to situate theory in an intelligible picture of the world. Newton came to that last, closed the Principia with the aether, and sorely puzzled his readers. Caloric, on the other hand, comes first in the Elements of Chemistry. That book is nothing if not good pedagogy, and Lavoisier opened it with the most familiar of physical phenomena, the expansive effects of heat and the complete reversibility of those effects.
Caloric, then, belonged rather to Lavoisier’s picture of the universe than to his chemistry. It played the part, not of a chemical body, but only of a physical or better a mathematical body—in the same sense in which Newton left gravity a mathematical and not a mechanistic force. Throughout his career Lavoisier’s predilection was for the intellectual style of his mathematical colleagues. Studying heat, he went beyond consultation to collaboration with the strongest mind among them, with Laplace. On 18 June 1783, Laplace read to the Academy their “Memoir on a New Method of Measuring Heat.” That method employed the ice calorimeter. It took advantage of Joseph Black’s principle of latent heat to measure the specific heats of various bodies by the weight of ice melted across a measured range of cooling. Insulation was insufficient to permit good results except when the external temperature was near the freezing point, and continuation of the experiments had to await the return of a winter colder than is usual in France. Nevertheless, they made a number of determinations, and their memoir was the fountainhead of calorimetry.
And what is very instructive is that those measurements succeeded, and those calculations were performed, in the face of a fundamental disagreement between the two collaborators on the nature of heat:
Physicists are divided on the nature of heat. Some regard it as a fluid permeating all nature, which penetrates bodies to a greater or lesser degree in proportion to their temperature and capacity. It may combine with bodies, and in this state it ceases to affect the thermometer or to flow freely from one body to another. It is only in the free state, which permits it to reach equilibrium in bodies, that it forms what we call free heat.
Other physicists think that heat is only the result of imperceptible motions in the molecules of matter. Everyone knows that all bodies, even the densest, are filled with innumerable pores or little cavities, of which the total volume may considerably surpass that of the matter they surround. This leaves the parts freedom to oscillate in all directions, and it is natural to think that these parts are in continual agitation, which if it increases to a certain point, may disintegrate and decompose the body. It is this internal motion which, following the physicists of whom we speak, constitutes heat.
They do not say (though from other sources it is known) that Lavoisier was the partisan of heat as caloric, and Laplace, the Laplace who completed Newton in celestial mechanics, of heat as motion. Surely, their dilemma is bound to appeal to our own science, which must live with its principle of complementarity, even as Newton in his day had lived with the duality of light, now periodic and again corpuscular. So, too, Lavoisier and Laplace never needed to resolve this issue in order to fulfill their object. Their calorimeter would know no distinction between conservation of caloric and conservation of kinetic energy—vis viva in the terminology of the time.
We shall not decide between the two preceding hypotheses. Some phenomena seem favorable to the latter. Such, for example, is that of the heat produced by friction between two solid bodies. But there are others which are more simply explicable in the former. Perhaps, they both take place at once. But however that may be, since only these two hypotheses are possible on the nature of heat, then we should admit the principles common to both. Moreover, according to the one or the other, in simple mixtures of bodies, the quantity of free heat is a constant.
And in the Elements Lavoisier, speaking now only for himself, develops the advantages of this mode of treatment:
Wherefore, we have distinguished the cause of heat, or that exquisitely elastic fluid which produces it, by the term of caloric. Besides that this expression fulfills our object in the system which we have adopted, it possesses this further advantage, that it accords with every species of opinion, since, strictly speaking, we are not obliged to suppose this to be a real substance; it being sufficient, as will more clearly appear in the sequel of this work, that it be considered as the repulsive cause, whatever that may be, which separates the particles of matter from each other, so that we are still at liberty to investigate its effects in an abstract and mathematical manner.
Any science of matter must concern itself with two sorts of problems: on the one hand the configurations of particles, and on the other the propagation of phenomena in space. It was to serve the latter necessity that Lavoisier introduced caloric. It is a continuous medium for the streaming of heat. And there, perhaps, we may leave it for the moment, in conceptual equilibrium with aether its complement, there where the nineteenth century was to adopt these notions, aether to be identified with fields of force and vanish into relativity, caloric to merge rather into the continuum of energetics and to be swept away on the great, irreversible stream of thermodynamics. Formulating the Second Law of that enigmatic science in 1822, Sadi Carnot still handled caloric as flowing from a real reservoir of heat down a continuous gradient.
Strategically, therefore, caloric was no retreat from science. It might, indeed, be argued—this book in fact does argue—that it was the essential instrument by which Lavoisier achieved objectivity for chemistry. That achievement is a precise intellectual act in the history of each science. Criticism should be able to say what constituted it, even when the scientist is himself too pressed into his problems to be so explicit, or so self-conscious. And Lavoisier made between caloric and heat (most signally the heat of combustion) that distinction which Newton drew between aether and light, which Galileo interposed between change and motion, and which Darwin was to make between the origin and preservation of organic variations. Thus he projected old qualities of matter out from chemistry onto caloric, or read them into the perception of its effects. For though no chemical agent, caloric was physically innocent, serving conservation laws rather than some mystical or romantic, some personal or sympathetic nature.
NOR IS IT caloric which will open in the armor of Lavoisier’s rationality some chink through which our sympathy may slip. Only in the theory of acidification does the appealing light of a really interesting error break through the fault in that tightly articulated career. In 1782 appeared a final memoir in the series on acids, “General Considerations on the Dissolution of Metals in Acids.” But for the accidental decisiveness of water, it is this memoir (it seems clear) which would have prepared the ground for Reflections on Phlogiston. And in that case, the Lavoisier theory of oxidation would appear in history as the wrong conception of acidification instead of the right one of combustion.
The opening paragraphs announce the imminence of a definitive attack upon phlogiston. They review his doctrine on combustion and calcination (rusting) as oxidation: “But what is not yet sufficiently known, and what I propose to prove in this memoir, is that a perfectly similar humid calcination occurs when metals dissolve in acids, that in all such cases there is decomposition of the acid or the water, and that there is united with the metal a quantity of the oxygenic principle approximately equal to that which the metal is capable of removing from the air in a dry calcination.” To exemplify that proof, Lavoisier returned to his early experiments on the “air” in nitric acid, repeated them with all the sophistication now at his command, and argued the results with unwonted urgency. Once again he dissolved mercury in acid. He neutralized the residual acid to determine by the difference what amount had reacted with the metal. He knew the percentage of oxygen contained in nitric acid. He determined the increment of weight upon the metal. And he found the last two quantities to answer. Just so did his precision seal him into an error springing from another source than weights and undetectable by any balance.
But what is most interesting is Lavoisier’s mode of representing these results, so correct in quantity, so wrong in principle. In order to see what he was about, one has to follow him into some detail. It is obvious, he writes, that acidification of a metal involves many variables—heat, concentration, chemical affinities, etc.—each of which is a force acting with characteristic energy. Therefrom results a problem complex and difficult of solution:
Better to exhibit the state of the question in this respect, and in order to show at a glance the result of what happens in metallic solutions, I have constructed formulas of a sort, which could at first be taken for algebraic formulas, but which have not the same object, and do not derive from the same principles …
Let any metallic substance be |
|
Let any acid be |
|
Let water be |
|
Let the oxygenic principle be |
|
Let nitric oxide be |
|
Let nitric acid be |
|
Then, we will have, for the general expression of any metallic solution
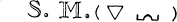
This general formula will vary according to the nature of the acid and that of the metal. Thus, for example, to express the solution of iron in nitric acid, we will have

But, since nitric acid is itself a compound, we must substitute the proper values, and the formula then takes the form:
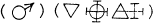
Let the quantity of iron be a, it is clear that it will require a determinate quantity of acid to dissolve it, that there is a relation between the quantity of acid and that of iron; and that in designating this relation b, I shall have a b as the quantity of acid required for this solution.
Further, it is clear that a quantity a b of nitric acid contains a certain proportion of water, which I could
call ..................... |
|
A certain proportion of |
|
oxygenic principle, ............. |
|
A certain proportion of |
|
nitric oxide .............. |
|
Finally, to prevent excessive effervescence, a certain amount of water must be added. Thus the formula becomes:
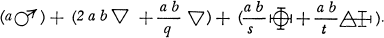
This expression represents the solvent and the solute before mixing. But then [to paraphrase for brevity] the metal takes from the acid that amount of oxygen which will saturate it. For each metal, this quantity bears a constant proportion to the quantity of the metal. Since a is the quantity of metal, 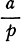 may represent the quantity of oxygen needed to saturate it. Clearly, when solution is complete, this quantity should be added to that of iron in the formula, which then becomes
may represent the quantity of oxygen needed to saturate it. Clearly, when solution is complete, this quantity should be added to that of iron in the formula, which then becomes
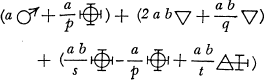
This gives Lavoisier all his parameters, and we will not follow the successive steps, in which he subtracts the air evolved as approximately equal to the weight of oxygen absorbed by the iron, supposes for simplicity that the quantity of acid used is unity, and replaces his coefficients with the actual quantities of an experiment, to end with this expression:
One step more, and this might seem the first chemical equation. But instead of placing the formulas for reagents and products on either side of an equality sign, Lavoisier wrote them as two sums to demonstrate the equivalence of mass before and after.
Is this not the most tantalizing memoir in the history of chemistry, and in Lavoisier’s the most appealing? Here alone, one gets a sense of modesty. He never claims for these expressions the dignity of algebra. “We are still very far from being able to introduce mathematical precision into chemistry, and I beg, therefore, that no one consider these formulas … as more than simple annotations, of which the object is to ease the labors of the mind.” (But compare this disclaimer with what he says of algebra itself in the Method of Chemical Nomenclature: “Algebra is the analytical method par excellence: it was invented to facilitate the labors of the mind, to compress into a few lines what would take pages to discuss, and to lead, finally, in a more convenient prompt and certain manner to the solution of very complicated questions.”)
Here alone, one gets a sense of true novelty, of a Lavoisier who is groping beyond the program of 1772 toward the mathematicization of chemistry. These embryonic chemical equations, they clothe his experiment in a far more structural formalism than that of nomenclature, a formalism like that in which Laplace and he had just quantified exchanges of heat and differentials of temperature in their joint Memoir on Heat. And surely it is a fair likelihood that Lavoisier found in that collaboration the idea of expressing chemical quantity in “the same abstract and mathematical manner.” “To know the energy of all these forces,” he concludes, “to succeed in giving them a numerical value, to calculate them, that is the goal which chemistry should propose itself. We are moving by slow steps. But it is not impossible that we shall win through.”
Here alone Lavoisier touches our fellow feeling by his very mistakes, for here they appear, not as the retribution properly awaiting an overbearing dogmatism, but as risks freely run in an adventure of the mind. Here he plays the innovator rather than the lawgiver. And those mistakes, indeed that failure in this memoir, emerge as inevitable defects of the very great qualities of order and reason which put his science on its objective footing.
For it has not been appreciated how Lavoisier’s life ended in an intellectual as well as a personal tragedy, how in both aspects he fell victim to grave faults in that eighteenth-century philosophy of science which he had made his own. That philosophy prepared the reforming spirit in the Revolution which took his life. It is true that the democratic resentment which then raged around the Academy, whose spokesman he had made himself, erupted rather out of popular romanticism, playing on that sentimental vulgarity which lurks in Baconian utilitarianism, never far beneath the surface. Nevertheless, the educational philosophy of science—which was to develop into positivism—had also made its compromise with progress and utility. It had dressed out the method of Newtonian physics in the logic of Baconian natural history, which classifies the forms and species of things. Naming (“Codifying,” as Bentham would say, “like any dragon”) it had reached in practice for the naturalist’s easy instrument of classification, in preference to the theoretical physicist’s exacting instrument of abstraction and mathematicization.
Now it should be apparent how much more closely Lavoisier’s error over acids was entrained by his philosophy of science than was his hypothesis of caloric. Let us read him in the Elements on ordering by nomenclature:
To those bodies which are formed by the union of several simple substances we gave new names, compounded in such a manner as the nature of the substances directed; but, as the number of double combinations is already very considerable, the only method by which we could avoid confusion was to divide them into classes. In the natural order of ideas, the name of the class or genus is that which expresses a quality common to a great number of individuals: the name of the species, on the contrary, expresses a quality peculiar to certain individuals only.
These distinctions are not, as some may imagine, merely metaphysical, but are established by nature. “A child,” says the Abbé de Condillac, “is taught to give the name tree to the first one which is pointed out to him. The next one he sees presents the same idea, and he gives it the same name. This he does likewise to a third and a fourth, till at last the word tree, which he first applied to an individual, comes to be employed by him as the name of a class or a genus, or an abstract idea, which comprehends all trees in general. But, when he learns that all trees serve not the same purpose, that they do not all produce the same kind of fruit, he will soon learn to distinguish them by specific and particular names.” This is the logic of all the sciences and is particularly applied to chemistry.
Is it not obvious, from this model of botanical linguistics, that what we finally have to do with, even in Lavoisier, is a chemical taxonomy? Most notably does this appear in the collective Method of Chemical Nomenclature. Into the heart of that book is folded a tabulation of chemical substances arranged to exhibit the order of the science on a single sheet. This, too, of course, is the object of the Mendeleev Periodic Table, to which every modern chemistry class may lift its eyes should it grow bored with its professor. But Guyton’s principles are altogether different. His table has five columns, each subdivided to juxtapose the old name against the new. Column I divides the “simple substances” into five classes. There are only fifty-five of these individuals in the entire chemical population. In the first class fall four principles, which have in common only their extreme activity: light, caloric, oxygen, and hydrogen. Class II comprises twenty-five acid radicals, Class III sixteen metals, Class IV five earths, and Class V three alkalis. Finally, resisting classification, is a miscellany of seventeen organic composites which seem to act as simple substances.
But it is in the organization of the columns that the modern student may see at a glance how, siren-like, Lavoisier’s beautiful principles had led him past the theory of combustion, past the objectification of his science, down a wrong path to a dead end. Column II tabulates the physical action of caloric in putting certain simple substances into the gaseous state: thus oxygen gas is the combination of the oxygenic principle with caloric, and so, too, of hydrogen, nitrogen, and ammonia. Column III assembles the combination of each simple substance with oxygen. Here fall water, all the acids of course, and the old “calxes” now become metallic oxides. To the acids correspond in Column IV the “gaseous oxides”—nitrous gas, carbonic acid gas (the old fixed air), etc. Finally Column V contains the very few composites which do not traverse the acid state: sulphides, carbides, and the metallic alloys.
Thus, the chemical population is tabulated according to two criteria of reference, two “coordinates” if one wished for the moment to grant Lavoisier’s claims for this process as a true analysis. (One would be wrong to do so, except in sympathy.) The one, the horizontal arrangement of the table, finds the place of each simple substance, each species, in the natural order. The other, the vertical arrangement by columns (neglecting Column II which has to do with physical state rather than chemical combination) relates every compound to the mode by which some simple substance combines with oxygen. Oxidation becomes, therefore, the critical reaction. One might even call it the privileged reaction, in analogy to the place made by Galileo for circular motion in his not dissimilar rationality, his really quite comparable determination to order a science within the closed scheme of the intelligible. Just so did Lavoisier’s science fall victim to the ultimate incompatibility of the algebraic and the taxonomic procedures. That incompatibility vitiated the entire philosophy of science of the Enlightenment. There it lurked under those good intentions, concealed as in Lavoisier’s case by the commitment to education and humanitarianism. The preface to the Elements contains a remark about his predecessors which might, perhaps, be taken as his own epitaph: “All these chemists were carried along by the influence of the genius of the age in which they lived, which contented itself with assertion without proofs; or, at least, often admitted as proofs the slightest degrees of probability, unsupported by that strictly rigorous analysis required by modern philosophy.”
Aspiring to that rigor at the end of his career, Lavoisier turned in the last memoir on acids to the mathematicization of chemistry—and that fine project aborted for having been conceived on the wrong ontology. He grasped at the wrong door. The final terms of his analysis were not such that numbers of general bearing could be assigned them. He took his coefficients from the weights of the reagents in each experiment. Even if he had formally equated reagents to products, instead of simply comparing the sums, his equations still would have had no generality. For what he was numbering were the forms of matter, not its particles. And throughout all history, from Aristotle on, the mathematicization of forms has yet to succeed. Historically speaking, only extension on the one hand, and parts on the other, atoms and the void, had yet been brought to the test of number, the one by true analysis, never by classification, the other by geometric synthesis, both together by analytical geometry. And this is too bad. For Lavoisier believed in atoms. But
All that can be said upon the number and nature of elements is, in my opinion, confined to discussions entirely of a metaphysical nature … I shall, therefore, only add upon this subject that if by the term elements we mean to express those simple and indivisible atoms of which matter is composed, it is extremely probable that we know nothing at all about them; but, if we apply the term elements, or principles of bodies, to express our idea of the last point which analysis is capable of reaching, we must admit, as elements, all the substances into which we are capable, by any means, to reduce bodies by decomposition.
He believed in atoms. But he never thought to count them, never thought to match or mate them, not even in imagination. It is not only too bad: it is very ironic. Lavoisier treated caloric mathematically. But not matter. Matter he only weighed and classified.
Tragic would be too melodramatic a word for this wrong turning, although Lavoisier, who could little bear to be wrong, might not have thought so. But thus to end in a blind alley was at least an indignity. It was an indignity that Lavoisier left an epilogue to be played out, on another stage, in a different vein. That new chemistry, so proudly and so justly claimed by Lavoisier as a French science, was consummated not in the elegant Gallic style of Condillac, not according to Lavoisier’s own standards of theoretical distinction, but in the concrete English manner of Boyle, and under the banner of Newton.
For if Boyle was the founder of atomic physics, John Dalton, not Lavoisier, was the first recognizable physical chemist.
THAT EPILOGUE is quickly told in its essentials. It has to do, of course, with the foundations of chemical atomism. Without that theory the development of modern chemistry is simply unthinkable. But in the over-arching structure of the history of science, the atomic theory goes beyond the chemical. For chemistry is the science which first clothed the ancient atomic assertion with scientific meaning, which made it a theory and not just a philosophical policy. Lavoisier’s mind did not run much on these matters. Nevertheless, what decisively vindicated his conception of chemical combination, and his assumption of conservation, was its reducibility to atomism and its consequent accessibility to numbers. For the Mendeleev Table and the concept of valence depend upon atomic number rather than upon chemical typology: oxide forming, acid forming, base, or whatever.
After Lavoisier’s death, in the emergence of French science from the Revolutionary torment, discussion turned to the mechanism of reaction, the nature of the chemical bond, and ultimately to the structure of matter. The first important suggestions were advanced by Berthollet. It is extremely unfair to that really notable laboratory chemist that he should appear in this history of scientific ideas as the author of those that were wrong. In 1801 he did, it is true, adumbrate the law of mass action. He did not express it in quite the modern form, but he had it clear from his experiments that the rate and extent of reactions are functions, not just of chemical properties, but of the amounts of the reagents, of the concentrations. From there, he saw the problem of chemistry as one of affinities, (the word is much older). He would seat the science in the relative reactivity of chemical agents. Then on the basis of specific affinity and relative concentration, the chemist would predict the amount of one reagent that will combine with another. For example, the amount of oxygen which will combine with a given quantity of copper depends on the relative affinities, which are fixed, and the concentration of the oxygen. There was, in Berthollet’s view, an infinite series of oxides of copper, or indeed of any substance, up to certain saturation points. Nor was this view unsupported by evidence. Not to mention amalgams and glasses, copper and iron do oxidize gradually. There was even a certain warrant in Lavoisier for predicting the extent of a reaction according to the circumstances of the reagent. His tabulation lists alloys under Column V, and elsewhere he alludes to a variable composition of nitric acid up to the saturation point of oxygen.
Nevertheless, Berthollet’s view was disputed by Proust, drawing on the same evidence, but disagreeing in principle. For despite occasional inconsistences in Lavoisier’s own usage, it was implicit in his chemistry that a given compound always contains its components in a fixed proportion by weight. Proust made the distinction, the first to do so clearly, between mixture and true combination, and explained the apparent variation in metallic oxides as the mixture of two oxides of definite but differing proportions, ferrous and ferric oxide, for example. And he laid down, as an axiom of chemistry rather than as a finding, the principle now known as the Law of Definite Proportions. That law accords well with scientific instincts. It is fair to say that it was displacing Berthollet’s conception even before Dalton explained it by the hypothesis that every chemical combination consists in discrete, characteristic atom-to-atom linkages.
Like Priestley’s, John Dalton’s milieu was the earnest Nonconformity of the English Midlands. Manchester was his home instead of Birmingham, the Society of Friends his religion instead of Unitarianism. The son of a weaver, his circumstances were even humbler. His mind, too, was formed by self-education. It was a strong mind, ingenious rather than distinguished. He expressed it quite without elegance or charm.
Some obscurity hangs about the genesis of Dalton’s atomic theory. Nor is it likely to be dispelled, since certain of his private papers were a casualty of the second World War. Nevertheless, it is quite sure that he came to it through physical rather than chemical considerations. The New System of Chemical Philosophy of 1808 drew together findings of the previous five or six years. Like Lavoisier’s Elements it opens with a discussion of heat, not however because of the familiarity of the phenomena, but because thermometry, expansibility, specific heats, and states of matter were what interested Dalton. He reached the atomic hypothesis of combining matter only on page 213, and then rather by way of explaining than introducing it. His first book had dealt with meteorology. Nor did he ever become adept in the laboratory. Even by the standards of the time he tolerated a very generous margin of error. He had never studied chemistry until he began to consider gases, probably in search of models of atmospheric phenomena on a laboratory scale. Neither does one experience in his writings the inwardness of that science, as in reading Priestley or Lavoisier. On the other hand, his scientific style gives a notably graphic instance of thinking in models. And the success of the chemical atomic theory testifies very persuasively to the advantage, on occasion, of trying a fresh model.
The advantage lay in the model and in Dalton’s fidelity to the mechanical philosophy in Boyle’s sense. For compared to the deep researches of the French school, the problems he resolved with it seem almost trivial. In 1802 and 1803 he was investigating the solubility of different gases in water. A colleague, William Henry, had just announced the law that the weight of gas dissolved is directly proportional to the pressure, and in the case of a mixture, to the pressure of that gas alone. Relying on certain rather inconclusive experiments, and upon the mechanical hypothesis, Dalton supposed that the separation of gas particles one from another in the vapor phase bears the ratio of a small whole number to their interatomic distance in solution. Henry’s law follows as a consequence if this ratio is a constant for each gas at a given temperature.
Trying his solubilities further, Dalton found that light gases and elementary gases are least absorbable, and that solubility increases with density and chemical complexity. Yet not knowing Avogadro’s hypothesis that equal volumes of gas contain equal numbers of molecules, he could not go directly from density to relative atomic weights. Instead, he turned to chemical combining equivalents to estimate the relative weights of gas atoms. Thus did he import the atomic hypothesis into chemistry, together with his “rule of greatest simplicity.” According to this assumption, if two elements combine in only one compound, they form a binary compound AB; if in several, the first is binary and the next two ternary (AB2, A2B). Taking hydrogen as 1, his information gave him 5.66 for oxygen, 4 for nitrogen, 6.66 for water, etc. These results were much improved in later years, until he arrived at 8 for oxygen.
Apparently Dalton lived with this picture for some years before he saw it as applying primarily to chemical combination. The immediate use he made of it extended it rather from the solubility to the mixture of gases. In Proposition 23 of Book II of the Principia Newton had derived Boyle’s law from the definition that an elastic fluid is composed of small particles repelling each other by a force inversely as the distance. But a difficulty had arisen. All unknown to Newton, there are at least three gases in the atmosphere. Why is oxygen not a bottom layer, water vapor in the middle, and nitrogen bringing up the top? Dalton worried this problem for several years. He tried combining his atoms into composites, but they would never come out even. He tried making each atom a little world with an atmosphere of caloric around it, evening things up to bring all the atoms to the same specific gravity by making the affinity for heat proportional to weight. But this conflicted with the tendency of caloric to seek its own level. Therefore—this is a real step, of course—he gave up heat as a repulsive mechanism. Then it occurred to him that perhaps atoms repel only their own kind. Kinetics would then explain diffusion. From this he supposed that it is because of differences in size and weight that atoms elect only their own species to repel, “the particles of one kind being from their size unable to apply properly to the other at their common surfaces of contact.” This is obscure enough, but again it led Dalton back to chemistry. Once again that science could answer his questions. Not as to size, but chemistry does deal with weights:
This led the way to the combinations of gases, and to the number of atoms entering into such combinations, the particulars of which will be detailed more at large in the sequel. Other bodies besides elastic fluids, namely liquids and solids, were subject to investigation, in consequence of their combining with elastic fluids. Thus a train of investigation was laid for determining the number and weight of all chemical elementary principles which enter into any sort of combination one with another.
And a plate in the Chemical Philosophy shows how that must be (See facing pages 256 and 257).
In this New System of Chemical Philosophy, Dalton simply drops the unanswerable question of particle size, and concentrates on weights and numbers.
Now, it is one great object of this work, to show the importance and advantage of ascertaining the relative weights of the ultimate particles, both of simple and compound bodies, the number of simple elementary particles which constitute one compound particle, and the number of less compound particles which enter into the formation of one more compound particle.
This plate contains the arbitrary marks or signs chosen to represent the several chemical elements or ultimate particles.
Fig.
1 Hydrog. its rel. weight |
1 |
2 Azote, |
5 |
3 Carbone or charcoal, |
5 |
4 Oxygen, |
7 |
5 Phosphorus, |
9 |
6 Sulphur, |
13 |
7 Magnesia, |
20 |
8 Lime, |
23 |
9 Soda, |
28 |
10 Potash, |
42 |
11 Strontites |
46 |
12 Barytes |
68 |
13 Iron |
38 |
14 Zinc |
56 |
15 Copper |
56 |
16 Lead |
95 |
17 Silver |
100 |
18 Platina |
100 |
19 Gold |
140 |
20 Mercury |
167 |
21. An atom of water or steam, composed of 1 of oxygen and 1 of hydrogen, retained in physical contact by a strong affinity, and supposed to be surrounded by a common atmosphere of heat; its relative weight = |
8 |
22. An atom of ammonia, composed of 1 of azote and 1 of hydrogen |
6 |
23. An atom of nitrous gas, composed of 1 of azote and 1 of oxygen |
12 |
24. An atom of olefiant gas, composed of 1 of carbone and 1 of hydrogen |
6 |
25 An atom of carbonic oxide composed of 1 of carbone and 1 of oxygen |
12 |
26. An atom of nitrous oxide, 2 azote + 1 oxygen |
17 |
27. An atom of nitric acid, 1 azote + 2 oxygen |
19 |
28. An atom of carbonic acid, 1 carbone + 2 oxygen |
19 |
29. An atom of carburetted hydrogen, 1 carbone + 2 hydrogen |
7 |
30. An atom of oxvnitric acid, 1 azote + 3 oxygen |
26 |
31. An atom of sulphuric acid, 1 sulphur + 3 oxygen |
34 |
32. An atom of sulphuretted hydrogen, 1 sulphur + 3 hydrogen |
16 |
33. An atom of alcohol, 3 carbone + 1 hydrogen |
16 |
34. An atom of nitrous acid, 1 nitric acid + 1 nitrous gas |
31 |
35. An atom of acetous acid, 2 carbone + 2 water |
26 |
36. An atom of nitrate of ammonia, 1 nitric acid + 1 ammonia + 1 water |
33 |
37. An atom of sugar, 1 alcohol + 1 carbonic acid |
35 |
Thus Dalton completed the chemical revolution. The principle of simplicity is wrong, of course. For Dalton, water was HO, and oxygen had the atomic weight of eight. Many of his gravimetric determinations were inaccurate. But though often wrong in fact, Dalton was right in principle. And we may stop here, therefore, short of Gay-Lussac’s law of combining volumes, which by the rule of simplicity Dalton could never accept, short too of Avogadro’s Hypothesis which reconciled the differences, and far short, finally, of the valence theory which gave Mendeleev the numbers he needed for that tabulation of chemical elements which is truly an analysis of their properties.
All that is interesting, and of gathering technical significance. But chemistry moved into its strategic place in the progress of science with Dalton. He it was who brought Newton and Lavoisier face to face, eliminating the mediation of Condillac. We are done now with the educationists in science. “The novice,” says Dalton, brushing him aside in a footnote rather than writing for him, “will all along understand that several chemical subjects are necessarily introduced before their general history and character can be discussed.” All the terrible difficulties that Lavoisier had overcome, in the theory of combustion and reaction, in the quantitative technique, in the rationalization of the language—all these Dalton simply took for granted, along with the chemical information that accompanied them. That was his right, of course, as of any scientist. And to them he brought old wisdom straight from his reading of seventeenth-century corpuscular philosophy:
It has been imagined by some philosophers that all matter, however unlike, is probably the same thing, and that the great variety of its appearances arises from certain powers communicated to it and from the variety of combinations and arrangements of which it is susceptible….
This does not appear to have been his [Newton’s] idea. Neither is it mine. I should apprehend there are a considerable number of what may properly be called elementary particles, which can never be metamorphosed one into another.
For in Dalton it is for the first time obvious why it is better to think of streams of particles than streaming fluids.
They can be numbered.