34
PART 1 Organization of the Human Body
2.2
Chemical Reactions and Energy
LEARNING OUTCOMES

After reading this section, you should be able to
A.
Summarize the characteristics of synthesis,decomposition, reversible reactions, and oxidation-reduction reactions.
B.
Illustrate what occurs in dehydration and hydrolysisreactions.
C.
Explain how reversible reactions produce chemicalequilibrium.
D.
Contrast potential and kinetic energy.
E.
Distinguish between chemical reactions that releaseenergy and those that take in energy.
F.
Describe the factors that can affect the rate of chemicalreactions.
reactants are combined to form a larger, more complex product.An example is the synthesis of the complex proteins of the humanbody from amino acid “building blocks” obtained from food(figure 2.10
a
). Second, in other reactions, a reactant can be brokendown, or decomposed, into simpler, less complex products. Anexample is the breakdown of carbohydrate molecules into glucosemolecules (figure 2.10
b
). Third, atoms are generally associatedwith other atoms through chemical bonding or intermolecularforces; therefore, to synthesize new products or break down reac-tants, it is necessary to change the relationship between atoms.
Synthesis Reactions
A
synthesis reaction
is when two or more reactants chemicallycombine to form a new and larger product. The synthesis reac-tions occurring in the body are collectively referred to as
anabo-lism
(a˘-nab′ oˉ-lizm). These reactions produce the moleculescharacteristic of life, such as ATP, proteins, carbohydrates, lipids,and nucleic acids. The growth, maintenance, and repair of thebody could not take place without anabolic reactions.An example of a synthesis reaction is the combination oftwo amino acids to form a dipeptide (figure 2.10
a
). As the aminoacids are bound together, water results. Synthesis reactions inwhich water is a product are called
dehydration
(water out)
reactions.
As the atoms rearrange as a result of a synthesis reac-tion, old chemical bonds are broken and new chemical bonds areformed.
A
chemical reaction
occurs when atoms, ions, molecules, orcompounds interact either to form or to break chemical bonds.The substances that enter into a chemical reaction are called
reac-tants,
and the substances that result from the chemical reactionare called
products.
For our purposes, three important points can be madeabout chemical reactions. First, in some reactions, less complex
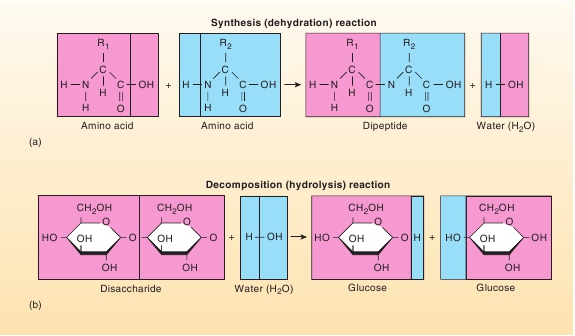
FIGURE 2.10
Synthesis and Decomposition Reactions
(
a
) A synthesis reaction in which two amino acids combine to form a dipeptide. This reaction is also a dehydration reaction because it results in the removal of awater molecule from the amino acids. (
b
) A decomposition reaction in which a disaccharide breaks apart into individual glucose molecules. This reaction is alsoa hydrolysis reaction because it involves the splitting of a water molecule.