CHAPTER 2 The Chemical Basis of Life
47
TABLE
2.8
Regulation
Transport
Protection
Contraction
Structure
Energy
Roles of Proteins in the Body
Enzymes control chemical reactions. Hormones regulate many physiological processes; for example, insulin affects glucose transport
into cells.
Hemoglobin transports oxygen and carbon dioxide in the blood. Plasma proteins transport many substances in the blood. Proteins in
plasma membranes control the movement of materials into and out of the cell.
Antibodies protect against microorganisms and other foreign substances.
Actin and myosin in muscle are responsible for muscle contraction.
Collagen fibers form a structural framework in many parts of the body. Keratin adds strength to skin, hair, and nails.
Proteins can be broken down for energy; per unit of weight, they yield as much energy as carbohydrates do.

the purpose of comparison, the molecular mass of water isapproximately 18, sodium chloride 58, and glucose 180, but themolecular mass of proteins ranges from approximately 1000 toseveral million.Proteins regulate body processes, act as a transportation system,provide protection, help muscles contract, and provide structure andenergy. Table 2.8 summarizes the role of proteins in the body.
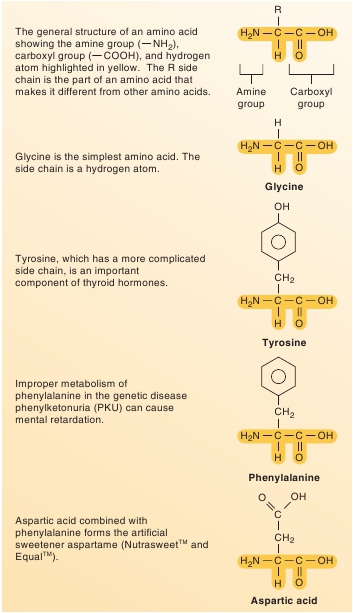
Protein Structure
The basic building blocks for proteins are the 20
amino
(a˘-meˉ ′ noˉ)
acid
molecules. Each amino acid has an amine (a˘-meˉn ′ ) group( NH
2
), a carboxyl group ( COOH), a hydrogen atom, and aside chain designated by the symbol R attached to the same carbonatom. The side chain can be a variety of chemical structures, andthe differences in the side chains make the amino acids differentfrom one another (figure 2.21).Covalent bonds formed between amino acid molecules dur-ing protein synthesis are called
peptide bonds
(figure 2.22). Adipeptide is two amino acids bound together by a peptide bond, atripeptide is three amino acids bound together by peptide bonds,and a polypeptide is many amino acids bound together by pep-tide bonds. Proteins are polypeptides composed of hundreds ofamino acids.The
primary structure
of a protein is determined bythe sequence of the amino acids bound by peptide bonds(figure 2.23
a
). The potential number of different protein mol-ecules is enormous because 20 different amino acids exist, andeach amino acid can be located at any position along a polypep-tide chain. The characteristics of the amino acids in a proteinultimately determine the three-dimensional shape of the protein,and the shape of the protein determines its function. A changein one or a few amino acids in the primary structure can alterprotein function, usually making the protein less functional oreven nonfunctional.The
secondary structure
results from the folding or bend-ing of the polypeptide chain caused by the hydrogen bondsbetween amino acids (figure 2.23
b
). Two common shapes thatresult are pleated (folded) sheets and helices (sing. helix, coil).If the hydrogen bonds that maintain the shape of the proteinare broken, the protein becomes denatured and nonfunctional.
FIGURE 2.21
Amino Acids