72
PART 1 Organization of the Human Body
Specificnon-lipid-soluble
Non-lipid-solublemolecules
Concentrationgradient
1
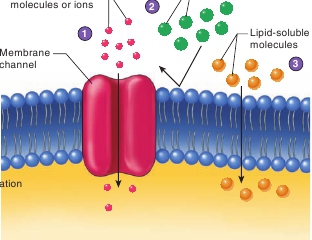
Certain specific non-lipid-soluble molecules or ions diffuse throughmembrane channels.
2
Other non-lipid-soluble molecules or ions, for which membranechannels are not present in the cell, cannot enter the cell.
3
Lipid-soluble molecules diffuse directly through the plasmamembrane.
osmotic pressure of a solution can be determined by placing thesolution into a tube that is closed at one end by a selectively perme-able membrane (figure 3.12). The tube is then immersed in distilledwater. Water molecules move by osmosis through the membraneinto the tube, forcing the solution to move up the tube. As the solu-tion rises into the tube, its weight produces hydrostatic pressure,which moves water out of the tube back into the distilled water sur-rounding the tube. At equilibrium, net movement of water stops,which means that the movement of water into the tube by osmosisis equal to the movement of water out of the tube caused by hydro-static pressure. The osmotic pressure of the solution in the tube isequal to the hydrostatic pressure that prevents net movement ofwater into the tube.The osmotic pressure of a solution provides information aboutthe tendency for water to move by osmosis across a selectively per-meable membrane. Because water moves from less concentratedsolutions (fewer solutes, more water) into more concentrated solu-tions (more solutes, less water), the greater the concentration of asolution (the less water it has), the greater the tendency for waterto move into the solution, and the greater the osmotic pressure toprevent that movement.
Predict 4
Given the demonstration in figure 3.12, what happens to osmoticpressure if the membrane is not selectively permeable but instead allowsall solutes and water to pass through it?
Membrane
PROCESS FIGURE 3.11
Diffusion Through the Plasma
Predict 3
Mr. Smith is suffering from chronic renal failure, characterized by agradual decrease in his kidneys’ ability to perform their normal functions.Over the past few months, blood tests have indicated an increasingconcentration of urea in his blood, which is a sign that the concentrationof urea in the extracellular fluid is also increasing. Urea, a toxic wasteproduced inside cells, diffuses across the plasma membrane into theextracellular fluid. The kidneys eliminate excess urea in the urine. Explainwhy the extracellular concentration of urea is increasing, and predict howthe intracellular concentration of urea is changing.
Osmosis
Osmosis
(os-moˉ ′ sis) is the diffusion of water (solvent) across aselectively permeable membrane, such as a plasma membrane(figure 3.12).
Selectively permeable
means that the membraneallows water but not all the solutes dissolved in the water to diffusethrough it. Interestingly, the permeability of some kidney cells towater can be regulated. Researchers found that the cells of kidneyshave
aquaporins,
or water channel proteins, that open and close toadjust membrane permeability to water. Water diffuses from asolution with proportionately more water, across a selectively per-meable membrane, and into a solution with proportionately lesswater. Because solution concentrations are defined in terms ofsolute concentrations, not in terms of water content (see chapter 2),water diffuses from the less concentrated solution (fewer solutes,more water) into the more concentrated solution (more solutes,less water). Osmosis is important to cells because large volumechanges caused by water movement disrupt normal cell function.
Osmotic pressure
is the force required to prevent water frommoving by osmosis across a selectively permeable membrane. The
Three terms describe the osmotic pressure of solutions. Solu-tions with the same concentration of solute particles (seechapter 2) have the same osmotic pressure and are referred to as
isosmotic
( ˉ
l
′ sos-mot ′ ik). The solutions are isosmotic even if thetypes of solute particles in the two solutions differ from each other.If one solution has a greater concentration of solute particles, andtherefore a greater osmotic pressure than another solution, the firstsolution is said to be
hyperosmotic
(h ˉ
l
′ per-oz-mot ′ ik) comparedwith the more dilute solution. The more dilute solution, with thelower osmotic pressure, is
hyposmotic
(h ˉ
l
-pos-mot ′ ik) comparedwith the more concentrated solution.Three additional terms describe the tendency of cells to shrinkor swell when placed into a solution (figure 3.13). If a cell placedinto a solution neither shrinks nor swells, the solution is said to be
isotonic
( ˉ
l
-soˉ-ton ′ ik). In an isotonic solution, the shape of the cellremains constant, maintaining its internal tension or tone, a condi-tion called
tonicity
(toˉ-nis ′ i-teˉ). If a cell is placed into a solution andwater moves out of the cell by osmosis, causing the cell to shrink,the solution is called
hypertonic
(h ˉ
l
-per-ton ′ ik). If a cell is placedinto a solution and water moves into the cell by osmosis, causing thecell to swell, the solution is called
hypotonic
(h ˉ
l
-poˉ-ton ′ ik).An isotonic solution may be isosmotic to the cytoplasm.Because isosmotic solutions have the same concentration of sol-utes and water as the cytoplasm of the cell, no net movement ofwater occurs, and the cell neither swells nor shrinks (figure 3.13
b
).Hypertonic solutions can be hyperosmotic and have a greater con-centration of solute molecules and a lower concentration of waterthan the cytoplasm of the cell. Therefore, water moves by osmosisfrom the cell into the hypertonic solution, causing the cell toshrink, a process called
crenation
(kreˉ -naˉ ′ shu˘n) in red blood cells