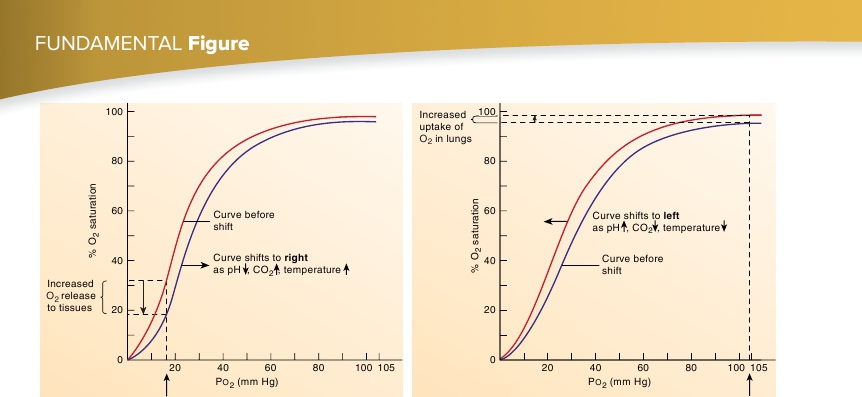
P O 2 in tissue
(a) In the tissues, the oxygen-hemoglobin dissociation curve shifts to the right.
As pH decreases, P CO2 increases, or temperature increases, the curve( red
) shifts to the right ( blue
), resulting in an increased release of O
2
.
P O 2 in lungs
(b) In the lungs, the oxygen-hemoglobin dissociation curve shifts to the left.
As pH increases, P CO2 decreases, or temperature decreases, the curve( blue
) shifts to the left ( red
), resulting in an increased ability ofhemoglobin to pick up O
2
.
FIGURE 23.18
Effects of Shifting the Oxygen-Hemoglobin Dissociation Curve
to the right. Under these conditions, as much as 75–85% of the O
2
is released from the hemoglobin. In the lungs, however, the curveshifts to the left because of the lower CO
2
levels, lower tempera-ture, and lower acid levels. Therefore, hemoglobin’s affinity forO
2
increases, and it becomes easily saturated (figure 23.18
b
).During resting conditions, approximately 5 mL of O
2
aretransported to the tissues in each 100 mL of blood, and cardiacoutput is approximately 5000 mL/min. Consequently, 250 mL ofO
2
are delivered to the tissues each minute. During exercise, thisvalue can increase up to 15 times. Oxygen transport can beincreased threefold because of a greater degree of O
2
release fromhemoglobin in the tissue capillaries, and the rate of O
2
transport isincreased another five times because of the increase in cardiacoutput. Consequently, the volume of O
2
delivered to the tissuescan be as high as 3750 mL/min (15 × 250 mL/min). Highly trainedathletes can increase this volume to as high as 5000 mL/min.
affinity for O
2
. Thus, hemoglobin releases more O
2
. A potent trig-ger for increased BPG production is low blood O
2
. For example,barometric pressure is lower at high altitudes than at sea level,causing both the partial pressure of O
2
in the alveoli and the percentsaturation of blood with O
2
in the pulmonary capillaries to belower. Consequently, the blood holds less O
2
for delivery to tis-sues. BPG helps increase O
2
delivery to tissues because higherlevels of BPG increase the release of O
2
in tissues (the oxygen-hemoglobin dissociation curve shifts to the right). On the otherhand, when blood is removed from the body and stored in a bloodbank, the BPG levels in the stored blood decrease. As BPG levelsdecrease, the blood becomes unsuitable for transfusion afterapproximately 6 weeks because the hemoglobin releases less O
2
tothe tissues. Banked blood is, therefore, discarded after 6 weeks ofstorage.
Predict 9
Predict 8
In carbon monoxide (CO) poisoning, CO binds to hemoglobin, therebydecreasing the uptake of O
2
by hemoglobin. In addition, when CO bindsto hemoglobin, the oxygen-hemoglobin dissociation curve shifts to theleft. How does this shift affect the ability of tissues to get O
2
? Explain.
If a person lacks the enzyme necessary for BPG synthesis, does he orshe exhibit anemia (a lower than normal number of red blood cells) orerythrocytosis (a higher than normal number of red blood cells)? Explain.
Fetal Hemoglobin
Fetal hemoglobin is a unique type of hemoglobin found in fetusesonly (see chapter 19). As fetal blood circulates through the pla-centa, O
2
is released from the mother’s blood into the fetal blood,and CO
2
is released from fetal blood into the mother’s blood. Fetalhemoglobin is very efficient at binding O
2
for several reasons:
Effect of BPG
As red blood cells metabolize glucose for energy, they produce aby-product called
2,3-bisphosphoglycerate
(
BPG;
formerly calleddiphosphoglycerate). BPG binds to hemoglobin, which reduces its
852