CHAPTER 27 Water, Electrolyte, and Acid-Base Balance
1023
Predict 4
Predict the effect of aldosterone hyposecretion on body fluid pH.
45.
Name the factors that cause an increase and a decreasein H
+
secretion.
46.
What is the purpose of buffers in the urine? Describe howthe ammonia buffer system operates.
47.
What is acidosis? What are the two types, and whatcauses each? How does the body compensate for each ofthe changes?
48.
What is alkalosis? What are the two types, and whatcauses each? How does the body compensate for eachof the changes?
The secretion of H
+
into the nephron can decrease the filtrate pHto approximately 4.5. A filtrate pH below 4.5 inhibits the secretionof additional H
+
. The H
+
that passes into the filtrate is more than theamount required to decrease the pH of an unbuffered solution below4.5. Buffers in the filtrate combine with many of the secreted H
+
.The buffers in the filtrate include HCO
3−
, phosphate ions (HPO
42−
),and
ammonia
(
NH
3
). HCO
3−
and HPO
42−
enter the filtrate throughthe filtration membrane, and NH
3
diffuses across the wall of tubulecells into the filtrate. These ions combine with H
+
secreted by therenal tubule, lowering its concentration (figure 27.15).NH
3
is produced in renal tubule cells when amino acids, suchas glutamine, are deaminated. Subsequently, NH
3
diffuses fromthe tubule cells into the filtrate and combines with H
+
in the fil-trate to form
ammonium ions
(
NH
4+
; see figure 27.15). The rateof NH
3
production increases when the pH of the body fluids hasbeen depressed for 2–3 days, as occurs during prolonged respira-tory or metabolic acidosis. The elevated ammonia productionincreases the buffering capacity of the filtrate, allowing the secre-tion of additional H
+
into the urine.HCO
3−
, HPO
42−
, and NH
3
constitute major buffers within thefiltrate, but other weak acids, such as lactate in the filtrate, alsocombine with H
+
and increase the amount of H
+
that can besecreted into the filtrate.
ASSESS
YOUR PROGRESS
42.
What happens to blood pH when blood CO
2
levels go upor down? What causes this change?
43.
What effect do increased CO
2
levels or decreased pHhave on ventilation? How does this change in breathingaffect blood pH?
44.
Describe the process by which tubule cells move H
+
intothe tubule lumen and HCO
3–
into the extracellular fluid.
Acidosis and Alkalosis
The normal pH of the body fluids is between 7.35 and 7.45.
Aci-dosis
(as-i-d ō ′sis) results when the pH falls below 7.35;
alkalosis
(al′k ă -l ō ′sis) occurs when the pH rises above 7.45.Metabolism produces acidic products that lower the pH of thebody fluids. For example, CO
2
is a by-product of metabolism thatcombines with water to form H
2
CO
3
. Likewise, anaerobic respira-tion produces lactate, protein metabolism produces phosphoricand sulfuric acids, and lipid metabolism produces fatty acids.These acidic substances must continuously be eliminated from thebody to maintain homeostatic pH. Failure to eliminate the acidicproducts of metabolism results in acidosis. Excess elimination ofthe acidic products of metabolism results in alkalosis.The major effect of acidosis is depression of the central nervoussystem. When blood pH falls below 7.35, the central nervous systemmalfunctions. The individual becomes disoriented, and possiblycomatose as the condition worsens.A major effect of alkalosis is hyperexcitability of the nervoussystem. Peripheral nerves are affected first, resulting in spontaneousnervous stimulation of muscles. Spasms, tetanic contractions, andpossibly extreme nervousness or convulsions result. Severe alkalosiscan cause death as a result of tetany of the respiratory muscles.Although buffers help resist changes in the pH of body fluids,the respiratory system and the kidneys regulate the pH of the body

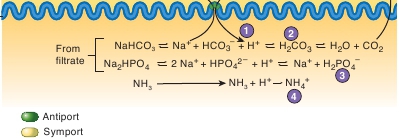
1
Hydrogen ions secreted into thefiltrate are buffered.
2
Hydrogen ions can react with HCO
–3
that enters the filtrate to form H
2
CO
3
,which is in equilibrium with H
2
O andCO
2
.
3
Hydrogen ions can react with HPO
2 –4
that enters the filtrate to form H
2
PO
4–
.
4
Hydrogen ions can react with NH to
3
form NH
4+
.
Interstitialfluid
Basalmembrane
Tubule cellcytoplasm
Apicalmembrane
Na
+
HCO
3–
+ H
+
H
2
CO
3
H
2
O + CO
2
Lumen
PROCESS FIGURE 27.15
Hydrogen Ion Buffering in the Filtrate
The secretion of H
+
into the filtrate decreases filtrate pH. As the concentration of H
+
increases in the filtrate, the ability of tubule cells to secrete additionalH
+
becomes limited. Buffering the H
+
in the filtrate decreases its concentration and enables tubule cells to secrete additional H
+
.