Chapter 13: Evolution in Populations
Lesson 13.1: Genetics of Populations
Lesson Objectives
- Analyze the relationship between Darwin’s work and Mendel’s discoveries.
- Explain the goal of population genetics.
- Describe the relationship between genes and traits.
- Differentiate between genes and alleles.
- Connect alleles to variations in traits.
- Distinguish environmental effects on gene expression from allelic variations in genes.
- Describe the relationship between mutations and alleles.
- Explain the causes and random nature of mutation.
- Compare rates of mutation in microorganisms to those in multicellular organisms.
- Analyze the ways in which sexual reproduction increases variation.
- Relate mutation and sexual reproduction to natural selection.
- Explain why populations, but not individuals, can evolve.
- Define a population’s gene pool.
- Distinguish between a population’s gene pool and a gene pool for a single gene.
- Analyze the usefulness of the gene pool concept.
- Explain how to determine allele frequencies.
- Define evolution in terms of allele frequencies.
- Discuss what is meant by a population which is fixed for a certain gene.
- Show how allele frequencies measure diversity.
- Evaluate the significance of a change in allele frequency.
Introduction
If you have ever taken something apart to find out how it works, you will understand biologists’ delight in exploring, since Darwin's findings, how evolution actually works. Darwin gave us the keys to unlocking this mystery by describing natural selection and common ancestry as a scientific explanation for the similarities and differences among the millions of Earth’s species – living and extinct. However, his theory depended on the ideas that traits could vary, and that variations were heritable – and even Darwin was puzzled as to how this might work. Another biological giant, Gregor Mendel, was a contemporary of Darwin’s, but his now-famous work with pea plant inheritance was not widely known or appreciated until after both scientists had died. During the 20th century, “re-discovery” of Mendel’s work stimulated extensive research in genetics, and the identification of DNA as the universal genetic material of life, brought “heritable variation” into sharp focus. A branch of the new field of Population Biology (discussed in the Populations Chapter) finally combined what Mendel and Darwin had begun separately - the exploration of population genetics and the evolution of populations.
Genetics of Populations
You have studied genetics, DNA, and Darwinian evolutionary theory in previous chapters. You have had the opportunity to learn far more about biology than either Darwin or Mendel could imagine. You are prepared, then, to join biologists in exploring how evolution works. You know that biologists have massive amounts of evidence that natural selection and evolution happen. This chapter will explore what we know about how molecules, genes, and populations change – with the ultimate goal of understanding how the “Origin of Species” produced the vast diversity of life on Earth.
Genes and Alleles
As you’ve learned, each cell of an organism contains all the information needed to build the entire organism in the DNA of its chromosomes (Figure below). A gene is a segment of DNA which has the information to code for a protein (or RNA molecule) (Figure below). For many genes, the protein product controls or at least contributes to a particular trait. For example, enzymes are proteins which are important in the biochemical reactions controlling many cellular processes.
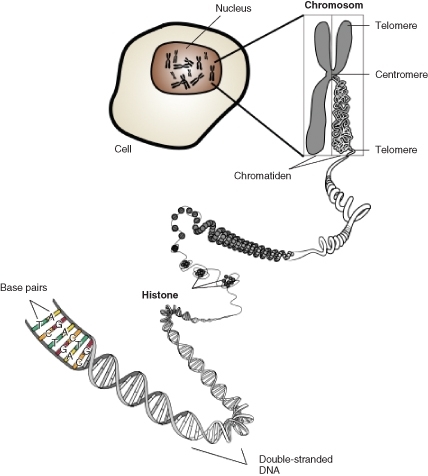
Figure 13.1
The source of variation required for natural selection is not traits or characters, as Darwin predicted, but the underlying genes, which were not discovered until Mendels work became known. Genes are segments of DNA located at a particular place on a chromosome. The genes sequence of bases codes for a protein - often an enzyme that catalyzes a particular chemical reaction in the cell. The chemical reaction determines or contributes to a physical trait or behavior.
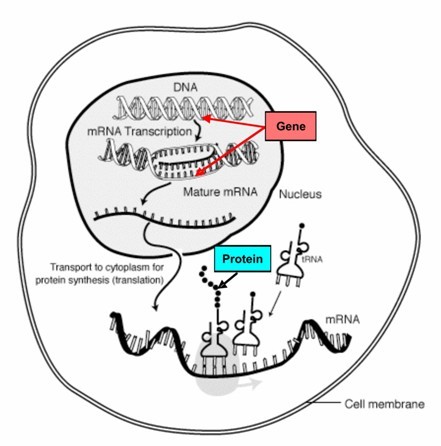
Figure 13.2
A gene is a segment of DNA which codes for a protein. Proteins, in turn, determine traits. Changes in genes (mutations) cause changes in proteins, which in turn produce variation in traits.
For example, the gene for the enzyme tyrosinase controls the chemical reaction which makes melanin, a brown-black pigment which colors the skin and hair of many animals, including humans. The gene is the “recipe” for tyrosinase; tyrosinase is the protein, and the trait is coloring of the skin or fur. The rabbit in Figure below has brown fur because its DNA contains a gene for tyrosinase.

Figure 13.3
Most wild rabbits have brown fur because their DNA contains a gene which codes for tyrosinase an enzyme which makes the pigment, melanin.
Genes often have different forms – slightly different nucleotide sequences – known as alleles. In some rabbits, an alternative form of the gene for fur color makes non-functional tyrosinase. A change in the sequence of As, Ts, Cs, and Gs changes the sequence of amino acids in the protein and alters or destroys its activity. This allele is recessive, and can cause lack of pigment – an albino rabbit (Figure below). We will refer to the dominant gene for brown fur as B, and the recessive gene for albinism as b.

Figure 13.4
If a rabbit receives two copies of the mutant allele for tyrosinase, it will lack pigment altogether a condition known as albinism. Although these rabbits are obviously domesticated, mutations leading to albinism do occasionally occur in wild populations.
Recall that Mendel showed that humans and rabbits have two copies of each gene, or "heritable unit" – one from each of our parents. The two alleles we received make up our genotype. For simple Mendelian traits, our genotype determines our physical appearance, or phenotype, for that trait. A rabbit having two copies of the mutant, recessive gene cannot make tyrosinase – or melanin; its genotype is bb, and its phenotype is albino. A rabbit having either one or two copies of the gene for tyrosinase makes melanin; its genotype is either Bb or BB, and in either case, because B is dominant, its phenotype is brown. Brown rabbits which have different alleles (genotype Bb) are heterozygous, and brown rabbits with identical alleles (BB) are homozygous. Albino rabbits with identical recessive alleles (bb) are also homozygous.
Keep in mind that the environment can influence the expression of a gene – its transcription and translation to produce a protein. By preventing or promoting the expression of certain genes, the environment can cause variation in traits, but note that this influence is not at all goal-directed and does not result in heritable change. If you do not water or fertilize your garden, the plants will be short and stunted, but their small size is not a heritable change. Let’s return to rabbits to see how this kind of variation happens at the level of genes and molecules.
A third allele for rabbit fur coloration codes for a form of tyrosinase which is temperature-sensitive. At low temperatures, the enzyme makes melanin normally. However, higher temperatures denature the enzyme in the same way that cooking changes egg white; at higher temperatures, the enzyme is misshapen and cannot make melanin. The rabbit in Figure below is homozygous for temperature-sensitive tyrosinase. The main part of its body, which has a higher temperature, is white because its tyrosinase does not work at high temperatures. The tips of the rabbit’s ears, nose, and feet, however, are black, because the enzyme can work at the slightly lower temperatures of these extremities. What do you think such a rabbit would look like if it developed in the arctic? The tropics?

Figure 13.5
Environment can influence the expression of a gene. California rabbits are bred for a temperature-sensitive allele of the pigment-producing enzyme tyrosinase. Cooler extremities produce melanin, but warmer body parts do not.
In conclusion, heritable variations, which Darwin knew were necessary for natural selection, are determined by the variety of alleles, or different forms of genes, which build proteins. The environment may add to the variety of phenotypes by influencing their expression, but the genes themselves remain unchanged. Only genes and their alleles can be sources of heritable variations in traits.
Sources of Variation
Genetic variation results from the diversity of alleles. How do alleles form?
You may recall that mutations change the sequence of nucleotides in DNA. Mutations can alter single nucleotides or entire chromosomes Figure below, and they are the sole source of new alleles.
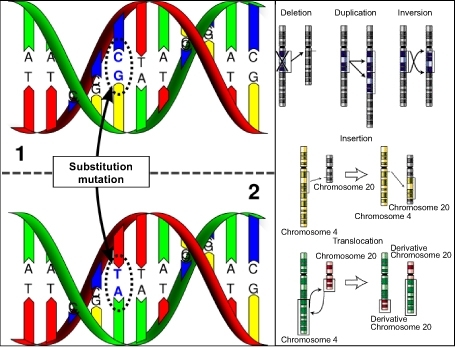
Figure 13.6
The ultimate source of genetic variation, random mutations are changes in nucleotide sequences of DNA. They may involve only a single base pair - as in (A), or many - as in chromosomal mutations (B).
Even a single nucleotide substitution can cause major changes in an organism; Figure below shows the molecular and cellular effects of the well-known substitution mutation which causes sickle-cell anemia in humans.
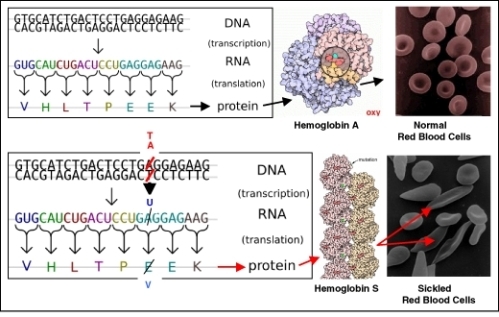
Figure 13.7
Mutations in DNA cause changes in the amino acid sequence of proteins, which in turn cause changes in traits. The disease Sickle Cell Anemia results from a single base-pair substitution in the gene for Hemoglobin. The single amino acid change dramatically alters the shape and function of the protein - and of red blood cells, which each contain 280,000 molecules of hemoglobin.
Caused by ultraviolet or ionizing radiation, certain chemicals, or viruses, mutations occur entirely by chance; they are not in any sense goal-directed. Usually, they reduce an organism’s fitness - its ability to survive and reproduce. However, many mutations are neutral; they have no effect on an organism’s fitness. A few may actually improve fitness. Sickle-cell hemoglobin (Hemoglobin S – see Figure above) prevents malarial infection, so in equatorial environments where malaria is prevalent, one copy of the Hemoglobin-S allele increases survival and reproduction.
New alleles arise only by chance mutations, yet without them, there would be no diversity and no evolution. Although they are rare due to repair mechanisms, mutations provide the creative potential for adaptation to environmental change.
Rates of mutation depend largely on reproductive rate, and are highest in bacteria and viruses. HIV, for example, can produce over one trillion new viruses per day, and each replication of its genome provides an opportunity for mutation. Their high mutation rates explain why viruses and bacteria so often become resistant to drug treatments.
Mutations resulting in heritable variation for multicellular organisms happen constantly, but at a lower rate. In part, this is because mutations in body cells do not affect the DNA in eggs and sperm. While this prevents many damaging mutations from dooming offspring, it also reduces diversity and the potential for adaptation to changing environments. Sexual reproduction, however, compensates at least in part for this loss.

Figure 13.8
Sexual reproduction cannot produce new alleles, but it does shuffle alleles into unique combinations, adding to the raw material for natural selection.
Although sexual reproduction cannot produce new alleles, meiosis and fertilization shuffle alleles from past mutations into new combinations Figure above. As Mendel demonstrated, genes (which he called “factors”) segregate and sort independently during the formation of eggs and sperm (return to the Mendelian Genetics if needed), and fertilization is random. The result is that – by chance – each offspring has a unique combination of alleles – a tremendous source of variation and raw material for natural selection.
Populations and Gene Pools
Individuals do not evolve. Natural selection may affect an individual’s chance to survive and reproduce, but it cannot change the individual’s genes. However, a population – a group of organisms of a single species in a certain area – evolves when natural selection imposes differential survival on individuals within it. Population genetics studies populations at the level of genes and alleles in order to discover how evolution works.
If we consider all the alleles of all the genes of all the individuals within a population, we have defined the gene pool for that population. Gene pools contain all the genetic variation – that raw material for natural selection – within a population. The gene pool for a rabbit population, for example, includes alleles which determine coat color, ear size, whisker length, tail shape, and more (Table below). If a population geneticist wants to focus on the variation in an individual gene, she/he may look at the gene pool of all the alleles for that gene alone.
Consider a population of 100 rabbits, including 10 albinos and 90 brown rabbits. The proportion of each phenotype is a measure of variation in this population, but it does not show the true genetic diversity. The gene pool does. Two populations of 10 white and 90 brown rabbits – with identical phenotypic diversity - could differ, because brown rabbits could be either homozygous or heterozygous.
Rabbits, like humans, are diploid. Therefore, each population’s gene pool for coat color contains 200 alleles. The 10 albinos in each population contribute a total of 20 b alleles to each gene pool. However, if population #1 has 40 heterozygotes and population #2 just 20, their gene pools differ significantly.
In population #1, 50 homozygous dominant individuals contribute 100 B alleles, and the 40 heterozygotes contribute 40 more. The heterozygotes also contribute 40 albino b alleles. Thus, population #1 contains a total of 140 B alleles and 60 b alleles.
Population #2, on the other hand, has 70 homozygous dominant rabbits, which add 140 B alleles to the 20 from the heterozygotes – a total of 160 B alleles. Only 40 b alleles are present in the gene pool, so genetic diversity is lower. The use of gene pools allows population geneticists to see variation and the influence of natural selection in detail.
As Darwin saw, populations are the units of evolution. As Mendel showed but Darwin could not have seen, heritable units, which we now know as alleles, determine the amount of variation within a population. A measure of this variation is the population’s gene pool. Once again, individuals cannot evolve, but it is the individual organism that adapts to its environment. It is the gradual accumulation of a series of adaptations - through genetic change - in a lineage from the population that is evolution.
Allele Frequencies
Our analysis of the rabbit populations (Table above) showed that the relative numbers of various alleles are the best measure of variability in a gene pool (or a population). Population geneticists calculate the proportions, or frequencies, of each allele in order to study changes in populations. In fact, a change in allele frequency is the most precise measure of the process of evolution.
An allele’s frequency is the fraction (expressed as a decimal) of a population’s gene pool made up of that particular allele. In rabbit population #1 above, the gene pool for coat color included 200 alleles, 140 of which were B. The frequency of allele B for population #1 is 140/200 = 0.7. Because frequencies must total 1.0, and the only other allele is b, the frequency of allele b is 0.3. (Alternatively, we can calculate the frequency of allele b by dividing the number of this allele by the total number of alleles: 60/200 is again 0.3.) In population #2, 160 of 200 alleles are B, so the frequency of allele B is 0.8, and the frequency of allele b is 0.2.
If all the members of a rabbit population (#3) are homozygous brown, the frequency of B is 1.0, the frequency of b is 0, and there is no diversity. Allele B is said to be fixed, and until a mutation occurs, no possibility for evolution (with respect to this particular gene) exists. A population with frequencies of 0.5 for B and 0.5 for b would have maximum diversity for this two-gene system.
Any change in those frequencies across generations would reflect evolution of the population – the subject of the next lesson.
Lesson Summary
- Population genetics studies populations at the level of genes and alleles in order to discover how evolution works.
- Genes - segments of DNA - determine traits by coding for enzymes that control chemical reactions.
- Many genes have several different forms known as alleles.
- Alleles are responsible for the variation in traits.
- The environment can affect the expression of some genes, but variations induced by the environment are not heritable.
- Mutations – changes in single nucleotides or entire chromosomes - are the sole source of new alleles.
- Ultraviolet or ionizing radiation, chemicals, or viruses cause mutations - in an entirely random way.
- Rates of mutation are high in rapidly reproducing viruses and bacteria, allowing them to adapt quickly to changing environments. An example is drug resistance.
- Rates of heritable mutations are lower in multicellular organisms because only germ cell mutations are passed on to offspring.
- Sexual reproduction recombines existing alleles through meiosis and random fertilization, so that each offspring is unique.
- Mutations and sexual reproduction provide the variety which is the raw material for natural selection.
- Individuals experience natural selection, but not the genetic changes of evolution.
- Populations evolve through genetic change.
- A population’s gene pool includes all the alleles of all the genes of all the individuals within it.
- A gene pool for a single gene includes all the alleles of that gene present in all individuals.
- Analysis of a gene pool can reveal variation which is not visible in phenotypes.
- Changes in allele frequency measure the process of evolution.
- Allele frequency is the decimal fraction of a population’s gene pool made up of that particular allele.
- A gene pool containing only one type of allele is fixed; it lacks variation and potential for evolution, at least until mutation occurs.
- For a gene with two alleles, allele frequencies of 0.5 indicate maximum variation.
- Any change in allele frequency reflects evolution of a population.
Review Questions
- Describe the relationship between genes and traits.
- Explain the goal of population genetics.
- Differentiate between genes and alleles.
- Distinguish environmental effects on gene expression from allelic variations in genes.
- Describe the relationship between mutations, alleles, variation, natural selection, and chance.
- Compare rates of mutation in microorganisms to those in multicellular organisms.
- Explain why populations, but not individuals, can evolve.
- Distinguish between a population’s gene pool and a gene pool for a single gene.
- Explain how to determine allele frequencies.
- Evaluate phenotype, genotype, and allele frequencies as measures of variation, as raw material for evolution, and as a measure of evolution.
Further Reading / Supplemental Links
Vocabulary
-
allele
-
An alternative form or different version of a gene.
-
allele frequency
-
The fraction (usually expressed as a decimal) of a population’s gene pool made up of a particular allele.
-
evolution
-
A change in the frequency of an allele (or genetic makeup) in a population.
-
fitness
-
The ability of an organism with a certain genotype to survive and reproduce, often measured as the proportion of that organism’s genes in all of the next generation’s genes.
-
gene
-
A segment of DNA which codes for a protein or RNA molecule; a unit of inheritance.
-
gene pool
-
Within a population, the sum of all the alleles of all the genes of all the individuals.
-
genotype
-
The genetic makeup of an organism; specifically, the two alleles present.
-
heterozygous
-
Describes a genotype or individual having two different alleles for a gene.
-
homozygous
-
Describes a genotype or individual having two copies of the same allele for a gene.
-
mutation
-
A change in the nucleotide sequence of DNA or RNA.
-
natural selection
-
The process by which a certain trait becomes more common within a population, including heritable variation, overproduction of offspring, and differential survival and reproduction.
-
phenotype
-
The physical appearance of an organism determined by a particular genotype (and sometimes also by the environment).
-
population
-
A group of organisms of a single species living within a certain area.
-
population genetics
-
The study of the evolution of populations at the level of genes and alleles.
-
sexual reproduction
-
Two-parent gamete based reproduction, involving meiosis and fertilization.
Points to Consider
- Imagine how Darwin felt, knowing that traits were passed on to offspring and that heritable variations “somehow” appeared. What key discoveries now explain these facts?
- As we noted in the last chapter, Theodosius Dobzhansky is famous for his statement, “Nothing in biology makes sense except in the light of evolution.” Do you agree that people cannot understand biology without understanding evolution?
- How does evolutionary theory “make sense of” your similarities to your parents and siblings? Your differences from them? Similarities among all humans? Differences among us?
Lesson 13.2: Genetic Change in Populations
Lesson Objectives
- Compare and relate macroevolution to microevolution.
- Define microevolution in terms of allele frequencies.
- Define genetic equilibrium for a population.
- List the five conditions for genetic equilibrium according to the Hardy-Weinberg model.
- State and explain the generalized equation for Hardy-Weinberg equilibrium.
- Explain how to use the Hardy-Weinberg equation to solve for allele or genotype frequencies.
- Discuss the reasons why the 5 conditions for Hardy-Weinberg equilibrium are rarely met.
- Explain how mutation disrupts genetic equilibrium.
- Predict the possible effects of mutation, and analyze the probability of each type of effect.
- Contrast mutation in microorganisms to mutation in multicellular organisms.
- Define gene flow.
- Describe two possible effects of gene flow on the genetics of a population.
- Define genetic drift.
- Describe three possible effects of genetic drift on populations and/or specific alleles.
- Explain and give an example of the bottleneck effect.
- Clarify and give an example of the concept of “founder effect.”
- Compare and contrast genetic drift and natural selection as causes of evolution.
- Discuss natural selection and evolution in terms of phenotypes and allele frequencies.
- Explain the distribution of phenotypes for a trait whose genetic basis is polygenic.
- Using a normal distribution of phenotypic variation, interpret directional, disruptive, and stabilizing patterns of selection.
- Define fitness as it relates to natural selection.
- Describe how natural selection can sometimes lead to the persistence of harmful or even lethal allele.
- Analyze the logic of kin selection.
Introduction
- How many people in the United States carry the recessive allele for the lethal genetic disease, cystic fibrosis?
- Why must we treat AIDS patients with multi-drug “cocktails?”
- Why do we consider Northern Elephant seals endangered, even though their population has risen to 100,000 individuals?
- Why don’t South African Cheetahs reject skin grafts from unrelated individuals?
- What were the probable effects on human evolution of the six years of “volcanic winter” caused by the “megacollosal” eruption of Mt. Toba 70,000 years ago?
- Why do humans vary in skin color?
- Why doesn’t natural selection eliminate certain lethal genes?
This lesson will introduce you to our current understanding of the causes of evolution and show you how scientists use this understanding to solve problems and answer puzzling questions like those above.
Microevolution
In an earlier chapter, we defined microevolution as evolution within a species or population, and macroevolution as evolution at or above the level of species. The differences between the two are time and scale. Microevolution can refer to changes as small as a shift in allele frequencies for a single gene from one generation to the next. In contrast, macroevolution describes changes in species over geologic time. Many biologists consider evolution to be a single process; they regard macroevolution as the cumulative effects of microevolution. The History of Life chapter discussed patterns and processes of macroevolution; this lesson will focus on genetic changes within populations and species. At this level, we can see how evolution really works – a view that Darwin never had.
A change in allele frequencies within a population from one generation to the next – even if it involves only a single gene - is evolution. Biologists refer to this change in the gene pool as microevolution because it is evolution on the smallest scale. For our rabbit population from the previous lesson, a generation-to-generation increase in the frequency of the albino allele, b, from 0.3 to 0.4 (and the corresponding decrease in the frequency of allele B from 0.7 to 0.6) would be microevolution. The changes in Galapagos finch beak size, documented by Peter and Rosemary Grant, and the color changes of peppered moths will undoubtedly show corresponding changes in allele frequencies once we identify the responsible genes and alleles. Resistance to antibiotics in bacteria and the appearance of new strains of influenza viruses and HIV Figure below also involve changes in allele frequencies. Each of these is an example of microevolution.

Figure 13.9
This portion of the evolutionary tree for Human Immunodeficiency Virus (HIV) shows 8 or more strains of the HIV-1 M (Main) group, and a single strain of the HIV-1 N (Not Main/Not Outlier) group. The complete tree includes strains of SIV (Simian Immunodeficiency Virus). Each strain represents a change in allele frequencies.
What forces cause changes in allele frequencies? What mechanisms determine how microevolution happens? Before we tackle these questions, we will examine a population at equilibrium – a population which is not evolving.
Populations at Equilibrium: The Hardy-Weinberg Model
Like Darwin and Wallace, who independently developed similar ideas about evolution and natural selection, mathematician Godfrey Hardy and physician Wilhelm Weinberg independently developed a model of populations at equilibrium. The Hardy-Weinberg model (sometimes called a law) states that a population will remain at genetic equilibrium - with constant allele and genotype frequencies and no evolution - as long as five conditions are met:
- No mutation
- No migration
- Very large population size
- Random mating
- No natural selection
We will consider the above in more detail in later sections of the lesson, because deviations from these conditions are the causes of evolutionary change. For now, let’s look more closely at the Hardy-Weinberg’s equilibrium model. We’ll use another hypothetical rabbit population to make the model concrete: 9 albino rabbits and 91 brown rabbits (49 homozygous and 42 heterozygous). The gene pool contains 140 B alleles and 60 b alleles – gene frequencies of 0.7 and 0.3, respectively. Figure below summarizes the data for this parent population.
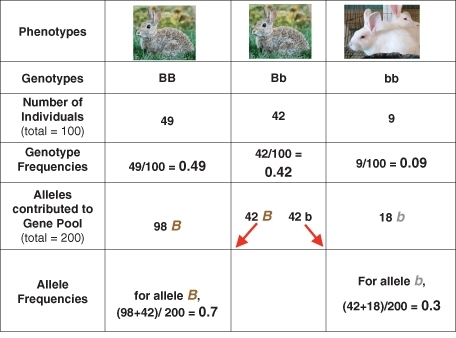
Figure 13.10
The coat color gene pool for a hypothetical rabbit population includes two alleles. Genotype and allele frequencies are calculated, given the number of individuals having each of the three possible genotypes.
If we assume that alleles sort independently and segregate randomly as sperm and eggs form, and that mating and fertilization are also random, the probability that an offspring will receive a particular allele from the gene pool is identical to the frequency of that allele in the population. A Punnett Square (Figure below) using these frequencies predicts the probability of each genotype (and phenotype) in the next generation:

Figure 13.11
A Punnett Square predicts the probability of each genotype and phenotype for the offspring of the population described in . A summarizes the frequencies of each genotype among offspring, and B calculates allele frequencies for the next generation. Comparing these to the parent generation shows that the gene pool remains constant. The population is stable at a Hardy-Weinberg equilibrium.
If we calculate the frequency of genotypes among the offspring predicted by the Punnett square, they are identical to the genotype frequencies of the parents. Allele frequency remains constant as well. The population is stable – at a Hardy-Weinberg genetic equilibrium.
A useful equation generalizes the calculations we’ve just completed. Variables include p = the frequency of one allele (we’ll use allele B here) and q = the frequency of the second allele (b, in this example). We will use only two alleles, but similar, valid equations can be written for more alleles.
Recall that the allele frequency equals the probability of any particular gamete receiving that allele. Therefore, when egg and sperm combine, the probability of any genotype (as in the Punnett square above) is the product of the probabilities of the alleles in that genotype. So:
Probability of genotype BB = p X p = p2 and
Probability of genotype Bb= (p X q) + (q X p) = 2 pq and
Probability of genotype bb = q X q = q2
We have included all possible genotypes, so the probabilities must add to 1.0. Our equation becomes:
|
p2
|
+
|
2 pq
|
+
|
q2
|
=
|
1
|
|
frequency of genotype BB
|
|
frequency of genotype Bb
|
|
frequency of genotype bb
|
|
This is the Hardy-Weinberg equation, which describes the relationship between allele frequencies and genotype frequencies for a population at equilibrium.
The equation can be used to determine genotype frequencies if allele frequencies are known, or allele frequencies if genotype frequencies are known. Let’s use a common human genetic disease as an example. Cystic fibrosis (CF) is caused by a recessive allele (f) which makes a non-functional chloride ion channel, leading to excessive mucus in the lungs, inadequate enzyme secretion by the pancreas, and early death (Figure below).

Figure 13.12
Cystic fibrosis is an inherited disease of the lungs and pancreas. A recessive allele of a gene for a chloride ion channel located on chromosome 7 (the gene locus is colored red, right) causes the disease. Treatment includes ventilator and antibiotic therapy (above).
Knowing that 1 of every 3,900 children in the United States is born with CF, we can use the Hardy-Weinberg equation to ask what proportion of the population unknowingly carries the allele for cystic fibrosis. An individual who has CF must have the genotype ff, because the allele is recessive. Using the value for the frequency of the homozygous recessive genotype, we can calculate q, the frequency of the recessive allele,f:
1/3900 = 0.0002564 = frequency of ff genotypes = q2
To find q, the frequency of allele f, we must take the square root of the frequency of ff genotypes:
q = √0.0002564 = 0.016 = frequency of the f allele
Because p + q = 1 (the sum of the frequency of allele f and the frequency of allele F must equal 1.0),
p = 1 – q = 1.0 – 0.0160 = 0.9840 = frequency of the F allele
According to the Hardy-Weinberg equation, for a population at equilibrium the frequency of carrier genotypes, Ff, is 2pq = 2 X 0.016 X 0.984 = 0.0315.
In other words, if the population is indeed at equilibrium for this gene, just over 3% of the population carries the recessive allele for cystic fibrosis.
Of course, this calculation holds only true if the US population meets the five conditions we listed at the beginning of this section. In nature, populations seldom satisfy all five criteria. Let’s consider how well each condition describes the US population for the cystic fibrosis gene:
Very Large Population Size
Although the equation ideally describes an infinitely large population – never found in nature, of course – the US population is probably large enough that this condition alone does not significantly disrupt equilibrium.
No Mutation
Mutations happen constantly, if at a low rate, so “no mutation” is a second unrealistic condition. However, mutations affecting any one particular gene are rare, so their effect on an otherwise large, stable population is small.
No Migration
This condition assumes no net additions or losses of either allele to the gene pool through immigration or emigration. For the US population, immigration is probably more significant than emigration. Gene flow, in essence is the flow of alleles into or out of a population, may be the most significant problem for this particular gene, because the frequency of the allele for cystic fibrosis varies greatly according to ancestry. Although 1 in 25 Europeans carry the f allele, the frequency is just 1 in 46 among Hispanics, 1 in 60 among Africans, and 1 in 90 among Asians. Therefore, disproportionate immigration by certain groups changes allele frequencies, destabilizing the Hardy-Weinberg equilibrium.
Random Mating
Here is another assumption which is probably not realistic. Marriage between individuals of similar ancestry and culture is still more common than intermarriage, although both occur. If marriages are not random, Hardy-Weinberg equilibrium does not apply.
No Natural Selection
The final condition is that all genotypes must have an equal chance to survive and reproduce. Victims of cystic fibrosis (genotype ff) have shorter lifespans, which inevitably reduce reproduction compared to individuals without the disease (genotypes FF and Ff). Although medical care is improving, differential survival and reproduction among genotypes means the gene pool is not at equilibrium.
With respect to the cystic fibrosis gene, the U.S. population fails to meet at least three of the criteria for equilibrium. Therefore, the actual frequencies of alleles and genotypes probably deviate somewhat from those we calculated.
In nature, very few populations meet the Hardy-Weinberg requirements for equilibrium. If we look at this fact from a different angle, we see that any of these conditions can destabilize equilibrium, causing a change in allele frequencies. In other words, these five conditions are five major causes of microevolution. The remaining sections of this lesson will explore each cause in detail.
Causes of Microevolution: Mutation and Gene Flow
As discussed in the last lesson, mutation – a random, accidental change in the sequence of nucleotides in DNA – is the original source of genetic variation. Only mutation can create new alleles – new raw material for natural selection. UV or ionizing radiation, chemicals, and viruses constantly generate mutations in a gene pool, destabilizing genetic equilibrium and creating the potential for adaptation to changing environments. However, both rates of mutation and their effects on the fitness of the organism vary.
In multicellular organisms, most mutations occur in body cells and do not affect eggs and sperm; these are lost when the individual dies and usually do not affect evolution. Only mutations in gamete-producing cells can become part of the gene pool. The rate at which mutations enter the gene pool is low, due to DNA “proofreading” and repair enzymes - and the extensive amount of “junk” DNA which does not code for protein. Mutations which do change nucleotide sequences in functional genes may also have no effect, because the Genetic Code is redundant (multiple similar codons for a single amino acid), or very little effect, if the amino acid is not located in a critical part of the protein.
Occasionally, however, a single nucleotide substitution can have a major effect on a protein – as we saw with sickle-cell anemia in the last lesson. Usually, the effect of a mutation on a protein is harmful; rarely is it helpful. In sickle-cell anemia, it is both – in certain environments. Sickled cells carry oxygen much less efficiently, but prevent malaria infections. Overall, the chance that a single mutation will increase the fitness of a multicellular organism is extremely low. If the environment changes, however, the adaptive value of a new allele may change as well. Over time, mutations accumulate, providing the variation needed for natural selection.
For the small genomes of viruses and bacteria, mutations affect genes directly and generation times are short, so rates of mutation are much higher. For an HIV population in one AIDS patient, rates of viral mutation and replication are so high that in a single day, every site in the HIV genome may have experienced mutation (Figure below). This rapid generation of new alleles challenges our best efforts at drug treatment, and explains the evolution of drug antibiotic resistance. Because of the abundance of random, spontaneous mutations, HIV generates a large amount of raw material for natural selection, and readily evolves resistance to new “environments” created by single drugs (Figure below). Drug “cocktails,” which contain multiple anti-viral chemicals, are our effort to change the “environment” and keep up with mutation in the human-HIV evolutionary race. For microorganisms, mutation is a strong force for evolution.
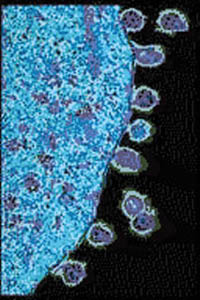
Figure 13.13
HIV daughter particles are shed from an infected human T-cell host. HIV replication and mutation rates are so high that during a single day, the HIV population in one AIDS patient generates mutations at every site in the HIV genome. New alleles provide the potential for extremely rapid evolution, including the development of resistance to drugs.

Figure 13.14
Extremely high HIV mutation rates provide many new alleles raw material for natural selection in AIDS patients. This variation allows at least some mutant viruses to survive and reproduce in the changing environments of new drug therapies. The graph shows the effects of one type of drug: an initial decrease in the HIV population and a temporary rise in the number of human host (CD4) cells. Before long, however, mutants have alleles conferring drug resistance appear and begin to reproduce, and the population recovers. This change in allele frequencies is microevolution, caused by mutation and natural selection.
For all organisms, mutations are the ultimate source of genetic variation. For a population, however, the immigration or emigration of individuals or gametes may also add to or subtract from a gene pool – a process known as gene flow. For example, wind or animals can carry pollen or seeds from one plant population to another. In baboon troops or wolf packs (Figure below), juvenile males may leave the group to find mates and establish separate populations. Human history includes countless migrations; gene flow continues to mix gene pools and cause microevolutionary change.

Figure 13.15
Dominant alpha wolves lead their pack in Yellowstone National Park. The lowest ranking omega individual, in the rear, may eventually leave the pack to find a mate and establish his own territory and pack. He would carry some of the genes from his pack to the new one a form of gene flow which seems built in to wolves social organization.
Gene flow can bring into a population new alleles which occurred by chance and were successful in other populations. In this way, it can accelerate microevolution. However, if exchange between populations is frequent, it reduces differences between populations, in effect increasing population size. In this case, gene flow tends to maintain separate populations as one species, reducing speciation, if not microevolution.
Mutation, together with recombination of existing alleles by sexual reproduction (see lesson 13.1) provides the diversity which is the raw material for natural selection and evolution. Gene flow can accelerate the spread of alleles or reduce the differences between populations. Both can contribute significantly to microevolution. However, many biologists consider the major causes of microevolutionary change to be genetic drift and natural selection.
Causes of Microevolution: Population Size and Genetic Drift
Recall that the third requirement for Hardy-Weinberg equilibrium is a very large population size. This is because chance variations in allele frequencies are minimal in large populations. In small populations, random variations in allele frequencies can significantly influence the “survival” of any allele, regardless of its adaptive value. Random changes in allele frequencies in small populations are known as genetic drift. Many biologists think that genetic drift is a major cause of microevolution.
You see the effects of chance when you flip a coin. If you flipped a penny 4 times, you would not be too surprised if it came up heads 4 times and tails not at all. If you tossed it 100 times, you would be very surprised if the results were 100 heads and no tails. The larger the “population” of coin tosses, the lower the effects of chance, and the closer the results should match the expected 50-50 ratio. The same is true for populations. If we imagine a rabbit population with a very small gene pool of just 2 B alleles and 2 b alleles, it is not difficult to understand that occasionally, chance alone would result in no albino offspring (only genotypes BB or Bb) – or even no brown offspring (only genotype bb). However, a gene pool of 100 B alleles and 100 b alleles would be very unlikely to produce a generation of offspring entirely lacking one allele or the other, despite having identical initial allele frequencies of 0.5.
Because chance governs meiosis and fertilization, random variations can influence allele frequencies, especially for small populations. Note that these chance variations can increase the frequency of alleles which have no adaptive advantages or disadvantages – or decrease the frequency of alleles which do have adaptive value. Genetic drift can result in extinction of an allele or an entire population – or rapid evolution (Figure below). Two sets of circumstances can create small populations for which genetic drift can have major consequences: the bottleneck effect and the founder effect.

Figure 13.16
Computer models show that the effect of small population size on allele frequencies is a significant increase in variation due to chance. Each line depicts a different allele. In the small population (above), most of 20 alleles, beginning at frequencies of 0.5, become either fixed (frequency = 1.0) or extinct (frequency = 0) within 5 25 generations. In the larger population (below), only one pair of alleles shows fixation/extinction and that occurs only after 45 generations. Note that these variations are independent of natural selection; they do not necessary fit the organism to its environment.
The Bottleneck Effect
Natural catastrophes such as earthquakes, floods, fires, or droughts can drastically reduce population size – usually without respect to allele frequencies. As a result of the disaster, some alleles may be lost entirely, and others may be present in frequencies which differ from those of the original population. The smaller population is then subject to genetic drift, which may further reduce diversity within the population. The loss of diversity resulting from a drastic reduction in population size and subsequent genetic drift is the bottleneck effect (Figure below). Much of our concern for endangered species derives from our understanding of the way in which small population size can reduce diversity by increasing genetic drift. We will look at two examples of the bottleneck effect – one caused by humans, and the other probably experienced by our human ancestors.

Figure 13.17
A random but major catastrophe which causes a sudden, severe reduction in population can lead to a bottleneck effect. The reduction in gene pool size - and often diversity - leaves populations subject to genetic drift; chance variations can cause extinction or accelerated evolution leading to recovery. Even if recovery occurs, genetic diversity remains low.
During the 19th century, overhunting reduced the worldwide population of Northern Elephant Seals (Figure below) to fewer than 100 individuals. Because an alpha bull typically mates with a “harem” of 30-100 females, it is possible that just a single male fathered all the seals which exist today! After legal protection, their numbers have rebounded to 100,000. However, the effects of the past bottleneck - significant loss of genetic variability – remain. Reduced genetic diversity means that today’s seals are more susceptible to disease and weather. Effects of genetic drift on the gene pool may have contributed to the loss of 80% of pups during the El Nino year of 1997-98.

Figure 13.18
The Northern Elephant Seal population fell to fewer than 100 individuals due to overhunting during the 19 century. Although their numbers have recovered, the bottleneck effect of reduced genetic variation limits their potential to adapt to future environmental changes.
Although the exact cause is unknown, a bottleneck for South African Cheetahs (Figure below) during the last ice age about 10,000 years ago has apparently led to extremely low genetic variability. Genetic variation among cheetahs has been compared to that of highly inbred varieties of laboratory mice; skin grafts between unrelated individuals are not rejected. These animals also suffer from low sperm counts. Like many endangered species, cheetahs are threatened not only by habitat loss, but also by reduced genetic diversity, which reduces their potential to adapt to changing environments.

Figure 13.19
Although the cause is unknown, South African Cheetahs apparently experienced a population bottleneck 10,000 years ago. Their current genetic uniformity is remarkable; skin grafts between even unrelated individuals do not elicit rejection responses.
We humans may have experienced a population bottleneck between 70,000 and 75,000 years ago, when supervolcano Mount Toba exploded with category 8 (“megacolossal!”) force in Sumatra. According to anthropologist Stanley Ambrose’s theory, global temperature dropped as much as 5 degrees Celsius for several years, possibly leading to an ice age. Ambrose believes that the environmental effects (“six years of relentless volcanic winter”) reduced the total human population to less than 10,000, and that isolated individual populations would have experienced genetic drift and rapid evolution or extinction.
The Founder Effect
Whereas a drastic reduction in population size causes the bottleneck effect, a form of population expansion leads to the founder effect. If a small group of individuals (the founders) breaks off from a larger population to colonize a distant area, they will probably carry with them only a limited amount of the genetic diversity of the original population (Figure below). For this reason, the new population they establish may differ significantly in genotype and phenotype. Inevitably, it will also be small and therefore subject to genetic drift.

Figure 13.20
In this diagram, the red squares and blue dots represent individuals with different alleles. Small groups of individuals (see the right three circles) which leave a larger population (left) to colonize a new area carry with them smaller gene pools with allele frequencies which may differ significantly from that of the parent population due to chance. The effects of chance in these new populations become more important; genetic drift may result in extinction or rapid evolution.
On newly formed islands, such as the Galapagos, Hawaii, and more recently Surtsey, Iceland, founder populations are often the only source of life on the island. Many founder populations probably become extinct, but others evolve rapidly, due to genetic drift. Some may diverge rapidly to occupy many available ecological niches – a process known as adaptive radiation. As we have discussed in past chapters, Galapagos finches and Hawaiian honeycreepers probably each evolved from small populations of a single ancestral finch-like species (see the previous chapter on Evolutionary Theory).
Historically and even today, human populations have experienced founder effects. In some cases, migration and colonization are the cause. Quebec was founded by a group of no more than 2,600 people, ancestors of today’s more than 7 million Quebecois, who show remarkable genetic similarity and a number of heritable diseases, well studied by geneticists.
Cultural isolation, as well as colonization, can result in founder effects. Amish populations in the United States have grown from an initial group of about 200 immigrants, dating back to the mid-1700s. Because they have remained culturally and reproductively isolated from non-Amish Americans, they show considerable uniformity. The Amish today are often studied for their genetic uniformity, as well as certain recessive conditions. Geneticists believe that just one or two of the initial 200 Amish carried a recessive allele for Ellis-van Creveld syndrome (short limbs, extra fingers, and heart anomalies), yet through genetic drift, the isolated Amish population now has the highest incidence of this syndrome in the world (Figure below).

Figure 13.21
Polydactyly (extra digits) and short limbs are characteristics of Ellis-van Creveld syndrome, a genetic disease which is rare worldwide but more common in the Amish population. Because the Amish population began with just 200 immigrants and has remained isolated, their high incidence of this syndrome may be a result of the founder effect.
Natural Selection
While genetic drift, including the bottleneck and founder effects, can cause microevolution (generational change in allele frequencies), its effects are mostly random. The results of genetic drift may include enhanced capabilities, but more often, they are neutral or deleterious. Natural selection depends not on chance, but on differential survival determined by an individual’s traits. Even though the variations are due to chance, the products of natural selection are usually organisms well-suited to their environment.
Recall that Hardy-Weinberg equilibrium requires that all individuals in a population are equal in their ability to survive and successfully reproduce. As Darwin noted, however, overproduction of offspring and variation among individuals often lead to differential survival and reproduction – in other words, natural selection (Figure below). We discussed natural selection as a part of Darwin’s theory of evolution in the last chapter, but in this section, we will go deeper than Darwin could. We will explore natural selection at the level of populations – in terms of allele, genotype, and phenotype frequencies.

Figure 13.22
Natural selection involves (1) heritable variation (here, giraffe neck length); (2) overproduction of offspring (3 giraffes born, not all can survive); (3) differential survival and reproduction (not enough food for all giraffes; those with shorter necks starve); and (4) gradual change in traits in the population (long-necked giraffes survive and reproduce, so their genes for long necks increase in frequency in the next generation).
Acting on an Organism's Phenotype
Natural selection acts on an organism’s phenotype (appearance), which is a product of genotype and any environmental influences on gene expression. By selecting for alleles which improve survival and/or reproduction and selecting against harmful alleles, natural selection changes the proportion of alleles from one generation to the next – causing microevolution. Let’s return once more to our rabbit population. If a predator such as a hawk can see white rabbits (genotype bb) more easily than brown rabbits (BB and Bb), brown rabbits are more likely than white rabbits to survive hawk predation. Because more brown rabbits will survive to reproduce, the next generation will probably contain a higher frequency of B alleles. Note, however, that the recessive b alleles are unlikely to disappear completely, because they can “hide” from the hawks in heterozygous brown rabbits. This is a good reminder that natural selection acts on phenotypes, rather than genotypes. The hawk - or natural selection - is unable to distinguish a BB rabbit from a Bb rabbit. Natural selection - and the hawk - is only able to distinguish a brown rabbit from a white rabbit, demonstrating how natural selection acts on the phenotype rather than the genotype of an organism.
Consider a different example, which emphasizes reproduction rather than survival: If both brown and white rabbits preferred to mate with white rabbits, the next generation’s gene pool would probably show an increase in the frequency of the b allele, because white rabbits would be more likely to reproduce successfully.
Although some traits are determined by a single gene, many are influenced by more than one gene (polygenic). The result of polygenic inheritance is a continuum of phenotypic values which often show a bell curve pattern of variation. Figure below shows the effect of three genes, each having two alleles, on human skin color; the result is a normal distribution ranging from very dark to very light, with a peak near the middle. You can demonstrate polygenic inheritance (probably with some environmental influence) for height, ear length, or handspan by measuring your classmates and graphing the data in a similar fashion. Some curves will be flat, and others sharp – but most will resemble the normal “bell” shape.
Given this pattern of phenotypic variation, natural selection can take three forms (Figure below). We will use the theoretical human skin color distribution Figure below to illustrate the three types of selection. Directional selection shifts the frequency curve away from the average by favoring individuals with an extreme form of the variation. The skin of early humans living in sun-rich Africa received high levels of UV radiation, which destroys vitamin B (folate) and leads to severe birth defects such as spina bifida. Selection, then, favored darker-skinned individuals, and the frequency of the darker alleles increased. After several generations, the curve would still be bell-shaped, but it would have shifted to the right, in the direction of the darker alleles. The average individual would have had darker skin as result of this microevolutionary change.

Figure 13.23
Three types of selection can alter allele frequencies, causing microevolution. The effect of stabilizing selection (1) is to reduce variation. Disruptive selection (2) results in two different populations, which may eventually be isolated from one another. Directional selection (3) enhances or reduces a single characteristic, such as trunk or snout length in the above example.
Natural Selection and Human Migration
As humans migrated into the northern hemisphere, excessive UV radiation was no longer a problem, but the relative lack of sunlight led to lower levels of vitamin D3, normally synthesized in the skin and necessary for calcium absorption and bone growth. Thus, selection in the north favored lighter-skinned individuals – by itself another example of directional selection. However, if we consider the human population as a whole at that time, disruptive selection would describe the microevolution taking place. In northern climates, alleles for light skin would be favored, and in southern climates would select for alleles for dark skin, resulting in two distinct peaks in the distribution of skin color phenotypes and their corresponding genotypes. Keep in mind that the “three gene – dark/light” model is an oversimplification of the genetics underlying skin color, but the adaptive values are real, and the model allows us to illustrate how microevolution works. Note that a map of human skin colors supports this type of selection to some extent (Figure below).

Figure 13.24
The distribution of skin colors at least partially supports disruptive selection for lighter skin in the north, to allow sunlight to form vitamin D in the skin, and for darker skin toward the equator to prevent UV radiation from breaking down vitamin B-folate.
Today, extensive migration, mobility, and intermarriage in the human population may be changing selective pressures on skin color once again. For the sake of argument, let’s make the somewhat unrealistic assumption that mixing becomes complete and that all people will be sufficiently mobile that they experience intermediate levels of sunlight. These conditions would select against both extremely dark skin (too little vitamin D3) and extremely light skin (too little vitamin B-folate). The result would be a taller, narrower distribution – less diversity - about the same mean, a phenomenon known as stabilizing selection. Although our example is perhaps unrealistic, stabilizing selection is probably the most common form of natural selection, preventing form and function from straying away from a “proven” norm.
Stabilizing Selection
Stabilizing selection can lead to the preservation of harmful alleles. A famous example, which we considered in earlier lessons, is sickle-cell anemia. The gene for Beta-hemoglobin - half of the oxygen-carrying protein in our blood - has two alleles, which we will call Hgb-A and Hgb-S. Individuals having two copies of the Hgb-S allele suffer from sickle-cell anemia, a potentially lethal disease in which sickled cells clog capillaries and cannot carry oxygen efficiently. In equatorial regions, individuals with two copies of Hgb-A become infected with Plasmodium parasites and often die from malaria. However, individuals with one copy of each allele (the heterozygous genotype) escape both causes of death; although they may experience slight sickling at high altitudes, they do not suffer from full-blown anemia, and malaria parasites cannot infect their red blood cells. Stabilizing selection has maintained the frequencies of both alleles, even though each is potentially lethal in the homozygous state.
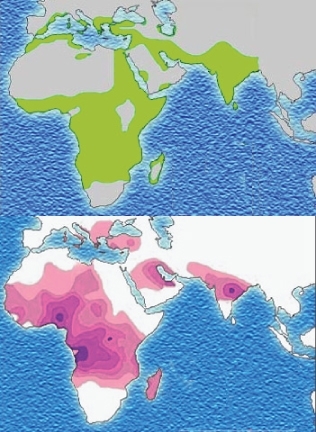
Figure 13.25
The distribution of malaria (top) correlates closely with the distribution of the sickle-cell allele (bottom). Because the heterozygous genotype confers immunity to malaria, this allele which is lethal in the homozygous condition persists in environments where malaria is common. Thus, natural selection can occasionally result in persistence of harmful alleles.
Selection for a particular trait may also select for other traits which do not directly affect fitness – if, for example, genes are linked, or if a single gene influences several different traits.
Fitness
Another way to look at natural selection is in terms of fitness - the ability of an organism with a certain genotype to reproduce. Fitness can be measured as the proportion of that organism’s genes in all of the next generation’s genes. When differences in individual genotypes affect fitness, the genotypes with higher fitness become more common. This change in genotype frequencies is natural selection.
Kin Selection
An intriguing corollary of genotype selection is kin selection. Behaviors which sacrifice reproductive success or even survival can actually increase fitness if they promote the survival and reproduction of close relatives who share a significant proportion of the same genes. Examples include subordinate male turkeys, who help their dominant brothers display to potential mates (Figure below) and honeybee workers, who spend their lives collecting pollen and raising young to ensure that their mother, the queen, reproduces successfully (Figure below).

Figure 13.26
Wild turkeys display in groups of closely related individuals, but only alpha males eventually mate. Subordinate males sacrifice their chance to reproduce, even chasing away other males to promote their dominant brothers success, because this behavior increases the chance that the genes they share will be represented in the next generation. This means of increasing gene frequency is kin selection.

Figure 13.27
Many social insects also illustrate kin selection. These honeybee workers are sterile. They spend their lives collecting pollen, feeding larvae, and cleaning and defending the hive. With no chance of reproductive success of their own, they dedicate their lives to the reproductive success of the hives queen their mother who shares 50% of her genes with each of them.
We have looked carefully at equilibrium populations and at possible disruptions of equilibrium which cause microevolution – a generational change in a population’s allele frequencies:
- Mutation, which together with sexual reproduction is the ultimate source of variation, and is an important cause of microevolution in microorganisms
- Gene flow, which can accelerate microevolution by importing new, already successful alleles
- Genetic drift, which can increase the effect of chance variations in small populations
- Natural selection, which can be directional, disruptive, or stabilizing
- Specialized types of selection, such as mate selection and kin selection
Evolutionary biologists are not yet in agreement regarding the relative importance of each type of selection to the history of life, although most would agree that natural selection is the primary force in microevolution. In the next lesson, we will apply our understanding of microevolutionary processes to that “mystery of mysteries,” as Darwin and Herschel called it: the origin of species.
Lesson Summary
- Macroevolution – change in species over geologic time – is the cumulative effect of microevolution.
- Microevolution – evolution within species or populations – can be measured as a generation-to-generation change in allele frequencies.
- Non-evolving populations have constant frequencies of alleles and genotypes – genetic equilibrium.
- The Hardy-Weinberg model holds for equilibrium populations under 5 conditions:
- no mutation
- no migration
- very large population size
- random mating
- no natural selection
- A generalized form of the Hardy-Weinberg equation for a gene pool of two alleles at equilibrium is:
|
p2
|
+
|
2 pq
|
+
|
q2
|
=
|
1
|
|
frequency of genotype BB
|
|
frequency of genotype Bb
|
|
frequency of genotype bb
|
|
where p is the frequency of one allele and q the frequency of the other allele.
- In nature very few populations meet the Hardy-Weinberg requirements for equilibrium, due to constant mutation, gene flow, small populations, nonrandom mating, and environmental change.
- Random, spontaneous mutations constantly generate new alleles in a gene pool, destabilizing genetic equilibrium and creating the potential for adaptation to changing environments.
- In multicellular organisms, only mutations that affect germ cells become part of the gene pool.
- Because of extensive “junk” DNA, repair enzymes, and multicellularity, many mutations do not reach the gene pool.
- Many mutations are harmful, disrupting protein function.
- Because the genetic code is redundant and some amino-acid substitutions may not change protein function, many mutations have no effect on an organism’s fitness.
- Even neutral mutations hold potential for future selection if the environment changes.
- A few mutations may be advantageous, improving or changing protein function.
- In microorganisms mutation rates are much higher due to rapid reproduction and small genomes.
- Mutation, together with recombination of existing alleles provides the diversity which is the raw material for natural selection and evolution.
- The movement of genes from one population to another (gene flow) can change allele frequencies.
- Gene flow can accelerate the spread of successful alleles or reduce differences between populations.
- In small populations random variations in allele frequencies can significantly influence the “survival” of any allele, regardless of its adaptive value; this phenomenon is genetic drift.
- Genetic drift can result in extinction of an allele or an entire population.
- Alternatively, genetic drift can lead to rapid evolution of a population.
- In the bottleneck effect, a catastrophe or disease or overhunting dramatically reduces a population’s size and genetic variation, increasing its susceptibility to the effects of genetic drift.
- In the founder effect, a small group leaves a larger population to colonize a new area. Again, genetic drift may lead to loss of genetic diversity, extinction, or rapid evolution.
- Genetic drift leads to evolution in populations of small size, but results are mostly due to chance.
- Natural selection occurs in populations of any size, and results are more likely to adapt a population to its environment.
- Natural selection acts on phenotype variations, so may include environmental effects which are not heritable.
- Evolution is a result of natural selection, and is measured as a change in allele frequencies.
- For a trait whose genetic basis is polygenic, the pattern of phenotypic variation usually forms a bell curve about an average value.
- Directional selection results in a shift of allele frequencies toward one extreme.
- Disruptive selection favors both extremes over the average phenotypic value.
- Stabilizing selection maintains or narrows existing variation in phenotype.
-
Fitness is the ability of an organism with a certain genotype to reproduce successfully.
- Because alleles often affect more than one trait in different ways, or have different effects in different environments, natural selection can sometimes lead to the persistence of harmful or even lethal allele.
- Kin selection involves the sacrifice by an individual of his/her reproductive potential in order to help a close relative reproduce successfully.
Review Questions
- Define microevolution in terms of allele frequencies.
- Describe genetic equilibrium, including the Hardy-Weinberg conditions.
- In the United States, about 1 in every 12 black people of African descent carry one copy of the allele for sickle-cell anemia. Assuming the five conditions of Hardy-Weinberg equilibrium hold (we’ll consider why they probably don’t in question #4), calculate the probability that a member of the next generation will suffer from sickle-cell anemia (have the homozygous Hemoglobin S genotype).
- Discuss the reasons why the 5 conditions for Hardy-Weinberg equilibrium are probably not met by the black population for the Hemoglobin alleles.
- Analyze the possible effects of mutation, and the probability and importance of each.
- Describe two possible effects of gene flow on the genetics of a population.
- Describe three possible effects of genetic drift on populations and/or specific alleles.
- Why do we consider Northern Elephant seals endangered, even though their population has risen to 100,000 individuals?
- Use the distribution of phenotypes for a trait whose genetic basis is polygenic to interpret directional, disruptive, and stabilizing patterns of selection.
- Describe how natural selection can sometimes lead to the persistence of harmful or even lethal allele. Include an example.
Further Reading / Supplemental Links
Vocabulary
-
adaptive radiation
-
Relatively rapid evolution of several species from a single founder population to several to fill a diversity of available ecological niches.
-
allele frequency
-
The fraction (usually expressed as a decimal) of a population’s gene pool made up of a particular allele.
-
bottleneck effect
-
The loss of diversity resulting from a drastic reduction in population size and subsequent genetic drift.
-
directional selection
-
Selection which favors one side of a phenotypic distribution – one allele or one extreme of a normal distribution.
-
disruptive selection
-
Selection which favors the two extremes of a phenotypic distribution – the ends of a bell curve, or the homozygous phenotypes, as opposed to the average, or heterozygous phenotype.
-
fitness
-
The ability of an organism with a certain genotype to survive and reproduce, often measured as the proportion of that organism’s genes in all of the next generation’s genes.
-
founder effect
-
The loss of genetic diversity resulting from colonization of a new area by a small group of individuals which have broken off from a larger population.
-
gene flow
-
The net movement of genes into or out of a population through immigration or emigration.
-
genetic drift
-
Random changes in allele frequencies in small populations.
-
genetic equilibrium
-
State of a population in which allele and genotype frequencies remain constant from one generation to the next – a non-evolving population.
-
Hardy-Weinberg model
-
Describes a population at genetic equilibrium, meeting five conditions: no mutation, no migration, very large population size, random mating, and no natural selection.
-
kin selection
-
Behaviors which sacrifice reproductive success or even survival to promote the survival and reproduction of close relatives who share a significant proportion of the same genes.
-
macroevolution
-
Evolution at or above the species level.
-
microevolution
-
Evolution within a species or population, also defined as a generation-to-generation change in allele frequencies for a population.
-
mutation
-
A change in the nucleotide sequence of DNA or RNA.
-
natural selection
-
The process by which a certain trait becomes more common within a population, including heritable variation, overproduction of offspring, and differential survival and reproduction.
-
polygenic trait
-
Traits that are influenced by more than one gene.
-
stabilizing selection
-
Selection which favors the average or heterozygous phenotype, resulting in no change or in a narrowing of the distribution of phenotypes.
Points to Consider
- This lesson discussed probable past microevolution of alleles for genes for human skin color and hemoglobin. For what other genes (or heritable traits) can you suggest past selective pressures? Do you think certain human genes (or heritable traits) may be at genetic equilibrium? Give some examples, and explain your reasoning.
- Some people suggest that we humans have removed ourselves from natural selection. Do you agree?
- What are some consequences of understanding that chance variations and natural selection can result in the persistence of lethal alleles, such as the alleles for sickle-cell anemia and cystic fibrosis?
- Do you think it is important that people understand the biological basis of skin color? Explain
Lesson 13.3: The Origin of Species
Lesson Objectives
- Recognize that new discoveries since Darwin have added an understanding of speciation to evolutionary theory.
- Explain the concept of a species.
- Define the biological species concept and analyze its usefulness.
- Compare the biological species concept to morphological, genealogical, and ecological concepts.
- Analyze the reasons why biologists consider all humans to be members of the same species.
- Define speciation.
- Describe two conditions that can lead to speciation.
- Explain the results of speciation in terms of adaptation, chance, and changes in the environment.
- Distinguish allopatric from sympatric speciation.
- Describe an experiment which demonstrated allopatric speciation.
- Describe two general types of reproductive isolation.
- Explain how polyploidy can result in sympatric speciation.
- Discuss the use of hybridization to form new crop species.
- Analyze the importance of environmental complexity to sympatric speciation for animals.
- Compare and contrast the gradualist and punctuated equilibrium models of evolutionary change.
- Describe conditions that could increase the rate of speciation.
- Describe circumstances that could lower the rate of speciation.
Species Concept
Darwin avoided the use of the word evolution in his major work about evolution. The word “evolve” appears once, at the very end. He titled his work The Origin of Species – a process or group of processes now called speciation. What exactly is a species? How did the millions of species which exist on earth today arise? Will they (we!) continue to change? How quickly? These are questions Darwin sought to answer – and the questions we will explore in this lesson on evolution. Darwin’s work opened the door to life’s history. This lesson will look at the details and modifications of his ideas which research has made clear in the 150 years since he published The Origin.
What is a Species?
A toddler readily recognizes that she/he is surrounded by distinct groups of individuals which we call “kinds” of plants and animals. Biologists refer to kinds of living things as a species - groups of individual organisms which are very similar. If we look closely, however, variations make it quite difficult to decide where to draw the lines between species. For example, are a St. Bernard and a Chihuahua similar enough to group within the same species? Just how similar must two organisms be for biologists to decide they are members of the same species? The answer to this question is not clear, even to biologists who specialize in classification. We will explore several, because how we define a species can help to clarify how species develop.
The Biological Species Concept
A widely used definition of species is the biological species concept (Figure below), first proposed by evolutionary biologist Ernst Mayr in 1942. Mayr’s concept begins with the idea that members of a species must be similar enough that they could interbreed. Because all dogs - from a St. Bernard to a Chihuahua – are capable of interbreeding, biologists consider all dogs to be members of the same species. If you are familiar with mules, however, you know that this definition needs clarification. If horses and donkeys mate, they produce mules, but mules are sterile and cannot continue to interbreed. Therefore, the biological species concept becomes organisms similar enough to interbreed and produce fertile offspring. Horses and donkeys, therefore, are not members of the same species. As you may know, wolves and dogs can interbreed to produce viable hybrids with fertile offspring; surely, wolves and dogs are not members of the same species? The last part of the definition addresses this problem, and the complete definition becomes:
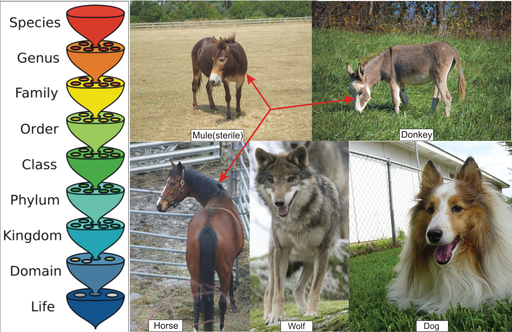
Figure 13.28
The species is the smallest group or level used to classify living things. As for all levels, the goal of classification is to show evolutionary relationships. A biological species is defined as a group of organisms similar enough to reproduce and have fertile offspring under natural conditions. A mating between a horse and a donkey produces a mule, but the mule is sterile, so the horse and donkey are not considered members of the same species. On the other hand, dogs and wolves can interbreed successfully, but because they do not do so in nature, they are not classified as members of the same species.
A biological species is a group of organisms similar enough that they could interbreed and produce fertile offspring under natural conditions.
This definition serves the goal of defining members of a species as individuals which are still undergoing evolution – they form a distinct yet potentially common gene pool. You will learn much more about classification in the next chapter, but here it is important to realize that one of the primary goals of classification is to show evolutionary history – patterns of common ancestry. This makes the biological species – a functionally reproducing unit – an important foundation of classification. Closely related species descended from relatively recent common ancestors, and distantly related species descended from more distant common ancestors. The emphasis the biological species concept places on successful reproduction fits this goal of classification quite well.
Another advantage of the biological concept is that it has the potential to explain how speciation occurred – that is, how two closely related groups of organisms became different species through reproductive isolation. Members of two species may appear very similar, yet fail to interbreed because of reproductive barriers. Barriers to reproduction may either prevent mating, or prevent development of a fertilized egg after mating. Elaborate courtship behaviors, including the blinking pattern of fireflies or the songs of birds, are often required to elicit mating behavior – and limit mating to members of a species (Figure below). Different breeding seasons, such as flowering dates, can also prevent interbreeding. Molecular differences between even closely related species may prevent sperm or pollen from actually fertilizing eggs. Once the eggs are fertilized, chromosomes may be so incompatible that mitosis and meiosis cannot proceed normally; if the zygote cannot develop, offspring do not survive. All of these barriers between species work to ensure successful reproduction within species, keeping specific, useful adaptations “in the family,” so they are a logical way for us, too, to distinguish members of one species from members of another.

Figure 13.29
The Western Meadowlark (left) and the Eastern Meadowlark (right) appear morphologically identical. However, geography and songs serve as reproductive barriers to interbreeding, so the two are considered to be separate species.
Although the biological species concept is extremely helpful in evolutionary thinking it has serious limitations for practical use. For organisms that reproduce asexually – including all bacteria and viruses - the definition is entirely unworkable. Nor can we detect whether or not fossil organisms would have been able to interbreed – whether or not they coexisted. Biologists must rely on structure and (if available) biochemical similarities to classify fossils and most microorganisms.
The Morphological Species Concept
Alternatives to the biological species concept emphasize the characteristics and processes which unite, rather than divide (reproductive barriers), species. We will look at just a few in order to gain insight into evolutionary thought. A much more practical definition is the morphological species concept, which groups organisms based on structural and biochemical similarities. Recent advances in molecular biology, such as DNA comparison, have strengthened this means of clarifying evolutionary relationships. Biologists probably use this method more than any other to differentiate species in nature, despite its limitations in confirming the potential for interbreeding.
The Ecological Species Concept
The ecological species concept focuses on a group’s common ecological niche – the set of environmental conditions and resources used or required by the group. This concept is based on the idea that ecological and evolutionary processes divide resources in such a way that individuals can most efficiently adapt to use those resources as a group. All members of a species, then, have a unique set of adaptations to a particular set of environmental conditions. Note that both the morphological and ecological definitions “work” for asexually reproducing organisms, and many fossils, as well. However, they do not help to explain how two closely related groups became different species, as does the biological definition.
The Evolutionary Species Concept
The last concept we will consider has the potential to clarify the path of speciation, or evolutionary history – that primary goal of classification. The genealogical or evolutionary species concept defines a species as a group of organisms with a unique genetic history – a group which shares common ancestry without divergence. A species, according to this concept, is a group which forms one tip on the branching tree of life’s history. Modern technology which compares DNA and protein sequences makes this definition workable, but still, the mechanism of speciation is not defined.
Ideally, all of these ways of determining exactly which individuals make up a species would merge; all make valid points about the idea of a species, and all seek the common goal of defining a new, unique unit of life in space and time. Practically, however, each has its own usefulness for different purposes. If we were working in the field, we would undoubtedly use the morphological concept. However, as we explore the idea of speciation mentally and through lab models, we will adopt the biological species concept as our working definition.

Figure 13.30
All humans are members of the same biological species. All races and cultures can and do intermarry, and our DNA is 99.9% identical.
Humans, One Species?
What about humans? Are we all members of the same species? According to the biological species concept, the answer is a resounding yes. Despite our differences in appearance, culture, and location on planet Earth, any human female is capable of producing fertile offspring with any human male. Therefore, all humans are members of the same biological species (Figure above). In contrast, humans and chimpanzees do not interbreed even when they share the same territory, so biologists consider chimpanzees to be a distinct species. Before we leave the “species problem” and Homo sapiens, it is worth noting that all of the various definitions of species confirm the unity of the human species. DNA sequences, cell chemistry, anatomy, ecological resources, and reproductive ability all reveal similarities which unite us as a single species with common ancestry. In fact, the most recent and precise method of comparison – DNA sequences – shows that somewhere between 1 in 100 and 1 in 1,000 base pairs differ, on average, between one human and the next. Based on this analysis, we humans are 99.9% identical. The differences which seem so great to us (skin color, facial features, build, personality) are important for recognition of individuals, and a few may adapt us to particular regions of the Earth, but overall, we are vastly more similar to than different from each other; we are one species.
Although we may not learn exactly how we came to be a separate species, we can gain some insight by looking closely at what is known of the process of speciation. What do we know now – that Darwin did not know - about “the origin of species”?
An Overview of Speciation
Speciation occurs at the boundary between microevolution and macroevolution. At what point do the allele frequency changes of microevolution add up to define a new and separate species?
Ernst Mayr, who developed the biological species concept mentioned above, also identified two essential components of speciation. For two populations to evolve into separate species, they must experience:
- Isolation
- Genetic divergence
Isolation limits gene flow which would tend to maintain uniformity between the two populations. Physical separation by a barrier limits migration and prevents interbreeding, but differing environmental conditions may also provide a form of isolation. Isolation increases the chance that two populations will take separate microevolutionary pathways. Returning to our white and brown rabbits, even a difference in altitude and thus temperature might separate two parts of a population so that albino individuals will be favored higher on a mountain where snow frequently accumulates, but brown individuals would be favored down toward the valleys, where snow rarely covers the ground.
Although the two rabbit populations might be isolated by environmental differences, they would not become separate species unless enough genetic differences accumulate to prevent successful interbreeding. Genetic divergence is more likely if populations are small, increasing the chance of genetic drift. Even in large populations, however, environmental differences may result in large genetic differences which prevent interbreeding. In our rabbit populations, for example, selection for white rabbits might be accompanied by mate selection based on white coat color. If mate preferences were heritable and sufficiently strong, interbreeding between the brown population and the white population would cease, and the two populations would become two species.
The result of speciation is two groups of individuals, each more closely adapted to local environmental conditions than the larger parent population. Although speciation may not always result in a better fit between a species and its environment (remember that genetic drift increases the influence of chance), over time, natural selection tends to increase fitness. A species’ adaptations to both physical and living environments increase the chance that the species will survive. The millions of species which exist today are each carefully attuned to a particular niche. And, just as variation within a species ensures that some individuals will survive environmental change, a great diversity of species increases the chance that at least some organisms will survive major changes in the environment.
Isolation and genetic divergence are both prerequisites for speciation, but they are so closely related that sometimes it is difficult to distinguish one from the other. Physical isolation may encourage genetic divergence, but many evolutionary biologists believe that reproductive isolation can result from genetic divergence alone. That leads us to two major categories of speciation – one based on geographic isolation, and the other based on reproductive isolation alone. These two categories, and examples of speciation from each, are the topic of the next section.
Isolating Mechanisms
Biologists today divide isolating mechanisms into two major categories based on whether they happen in different locations (allopatric = “other fatherland”) or a single location (sympatric = “same fatherland”) (Figure below).
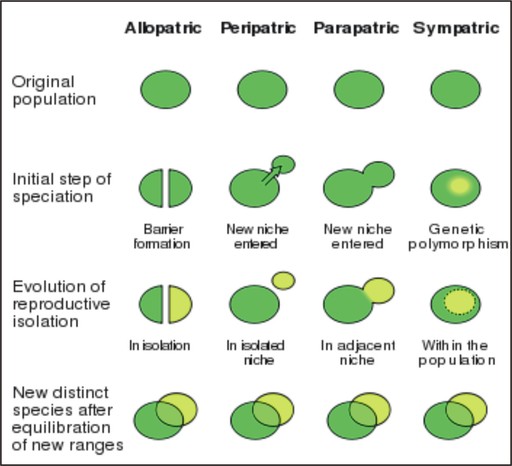
Figure 13.31
Two mechanisms of speciation are allopatric (other fatherland) and sympatric (together in the fatherland) forms. In both cases, a single population divides into two, and heritable differences eventually prevent gene flow between the two through reproductive isolation.
Allopatric Speciation
Allopatric speciation involves geographic barriers, which physically isolate populations. Formation of a land bridge such as the Isthmus of Panama, for example, could separate members of a marine population into two groups. Emergence of a mountain range could separate members of a lowland species. According to the fossil record, the rifting of continents divided populations of terrestrial animals and plants. In these cases, dramatic changes in landforms lead to geographic isolation; however, movements of animals and plants can also result in physical isolation. Single individuals or small groups of individuals may move away from a parent population to colonize a new area, and the new colony could be isolated from its parent population. Such movements are not always intentional; a storm apparently carried a small flock of finches from the coast of South America to the Galapagos Islands. In each case, geographic barriers (the land bridge, the ocean) isolate the two populations so that gene flow stops and genetic divergence can proceed. Natural selection may lead each population to adapt to its own unique environment, or genetic drift may lead to chance differences in gene pools. If genetic divergence results in reproductive incompatibility, the two populations have become two separate species.
Diane Dodd, working with a laboratory population of fruit flies (Figure below), experimentally verified the idea that physical isolation can lead to reproductive isolation and speciation. Dodd split the population into two groups, and fed maltose to one group and starch to the other. She observed that each new environment selected for improved efficiency in digesting the available food molecule. After eight or more generations of isolation, the two populations were recombined. After isolation, “starch” flies preferred to mate with other “starch” flies, and “maltose” flies preferred “maltose” mates. Although the preferences were not absolute, they did demonstrate at least initial formation of a reproductive barrier.

Figure 13.32
If a single population of fruit flies is divided, and the two subpopulations are separated for at least eight generations and fed different foods, members of the subgroups prefer to mate with individuals from their own feeding group. Although this behavioral reproductive barrier was not complete, Diane Dodds data supports the hypothesis that geographic isolation can lead to heritable reproductive isolation.
Sympatric Speciation
Sympatric speciation involves the emergence of a new species within the geographic range of the parent population. In the absence of geographic isolation, reproductive barriers must arise in different ways in order for new species to form. Some biologists doubt the relative importance of sympatric speciation to evolutionary change, but several examples demonstrate the potential for this mechanism of evolution.
Polyploidy
In plants, new species occasionally arise by duplication of chromosomes – a condition known as polyploidy (Figure below). Recall that our human body cells are diploid, and that egg and sperm cells are haploid. If meiosis fails to reduce the number of chromosomes, diploid sex cells result. In plants, which can self-pollinate, diploid pollen may fertilize a diploid egg, resulting in a tetraploid offspring. Although tetraploids may self-pollinate or interbreed with other tetraploids, they cannot successfully reproduce with their parents, because three sets of chromosomes (two from the tetraploid parent and one from the “normal” diploid parent) cannot successfully perform the intricate dance of meiosis. Peanuts, potatoes, and cotton are familiar crops that are tetraploid. Strawberries and sugarcane are octaploid (eight sets of chromosomes in each cell, compared to our two)! Polyploidy is a common form of speciation in plants in nature, as well as in agriculture.

Figure 13.33
One way in which polyploidy can arise is for meiosis to produce diploid (2n), rather than haploid (1n) gametes by nondisjunction. Self-fertilization results in tetraploid (4n) offspring. Strawberries (right) probably experienced two episodes of polyploidy; they are octaploid!
Two species of plants may hybridize – often forming unusually vigorous hybrid offspring. Unfortunately, despite their vigor, these offspring are often infertile due to chromosome incompatibility. However, if polyploidy occurs within the offspring, each of the previously unmatched chromosomes has a partner, offspring fertility is restored, and a new species is formed. Triticale (Figure below), a hexaploid hybrid of wheat and rye, was produced in the laboratory in this manner. Combining the high yield and quality of wheat with the disease-resistance of rye, triticale is now a successful grain crop in Europe, China, and Australia.

Figure 13.34
Triticale (large photo and grains inset, right) is a new crop species formed by hybridizing wheat (inset, left) and rye (insert, center). Reproduction of offspring for the new species was not possible until after polyploidy. The final species is hexaploid.
Polyploidy is less common in animals, perhaps because they less frequently self-fertilize. Some salamanders are polyploid, but offspring are usually sterile and “species” reproduce by parthenogenesis (development from unfertilized eggs). Human and other mammalian livers often contain many polyploid cells.
Although polyploidy is rare among animals, other zoological modes of sympatric speciation exist. For example, environmental change within a population’s range may lead to two habitats in which genetic divergence may take place. Our hypothetical rabbit example in the last section is an example of this type of sympatric speciation. A field example involves the hawthorn fly (Figure below), whose larval maggot stage feeds on hawthorn fruit. Apple trees, introduced to the US, often grow near hawthorns, and some hawthorn flies have begun to feed on apples. The two populations, although sympatric, have developed significant genetic differences, and at least in the wild, no longer tend to interbreed. This apparent reproductive isolation involves both temporal divergence (apples and hawthorns and their respective fly populations mature in slightly different seasons) and mating and egg-laying preferences (which link larval fruit to adult behavior). Many biologists consider these flies to be an example of sympatric speciation in progress.

Figure 13.35
The hawthorn fly (A) appears to be undergoing sympatric speciation. Traditionally, the species laid eggs on hawthorn (lower right), and the larvae (E) fed on the fruits. After the introduction of apples (upper right) to many of the same habitats, some hawthorn flies have begun laying eggs (B) on this species, and the maggots develop normally in the fruit. The two populations have diverged genetically, and no longer interbreed, at least in the wild.
Cichlid fish in East Africa’s Lake Victoria illustrate both allopatric and sympatric speciation (Figure below). Less than a million years old, the Lake harbors nearly 200 closely related cichlid species. Biologists conclude that adaptive radiation by a small group of colonizers into available niches explains the diversity of feeding specializations among these closely related species, much like Darwin’s finches in the Galapagos. Because the various habitats in the huge Lake (or islands in the oceans) isolate populations before speciation, adaptive radiation is often considered a type of allopatric speciation. However, nonrandom mating may have led to at least one more recent sympatric speciation. Females of two closely related species appear to select mates based on differing, brightly colored backs. Although the two color variations can still interbreed in the lab, they do not appear to do so in nature.

Figure 13.36
Cichlids appear to have undergone tremendous adaptive radiation in the relatively new Lake Victoria in Eastern Africa. Adaptive radiation is a form of allopatric speciation. At least one pair of closely related species may also show sympatric speciation.
The Tempo of Speciation
Speciation and extinction characterize all life on earth; the fossil record clearly documents both. Two startling facts emerge from careful study of the fossil record: First, the average successful species lives for “just” a few million years. Second, over 99.9% of all species that have ever lived have become extinct. The last aspects of speciation that we will consider are the tempo and pattern of species formation.
Over time, geographic changes isolate populations. Small populations experience genetic drift. Mutations alter individual genotypes and gene pools. New habitats form, and small groups colonize them. It is clear that evolution continues to change life. However, there is considerable debate about the rate at which speciation occurs over geologic time. Most biologists agree that single mutations seldom if ever cause new species in single evolutionary “leaps.” Mutations in regulatory genes, which have major effects during development, may be an exception, but in general, mutations are more likely to be harmful, and selected against. Except for the special case of polyploidy, discussed above, speciation cannot occur within a single generation. So, what do we know about the rate and pattern of speciation?
Some evolutionary biologists consider the rate of evolution to be slow and constant, with small changes accumulating to form big changes – the idea of gradualism. Others (Niles Eldridge and Stephen Jay Gould), in response to the apparently “sudden” appearance of new forms in the fossil record, suggest that species diverge in bursts of relatively rapid change, and then remain stable for relatively long periods – a model known as punctuated equilibrium. Gould maintains that speciation and evolution occur rapidly in small, peripheral populations, whereas large, central populations remain stabilized for long periods of time. It is the large, central, stable populations which are represented in our fossil record, he argues – not the small, peripheral, evolving ones. The two models are compared in Figure below.
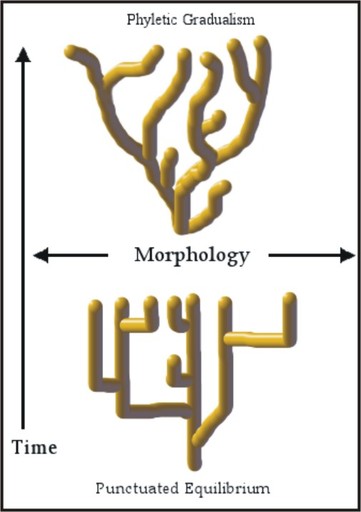
Figure 13.37
Two views of the rate at which speciation occurs: Gradualism (top) holds that small changes accumulate gradually to form big changes. Punctuated Equilibrium (bottom) suggests that rates of change accelerate over short periods (the horizontal changes in morphology which punctuate the tree of life) and then stabilize for relatively long periods (the vertical lines, indicating morphology is at equilibrium). To read such an evolutionary tree, keep in mind that the top represents the present: the tips of all branches which reach the top are todays species, and any branches that fail to reach the top represent extinctions.
In either case, species require several thousand generations – which may require several thousand years – to form. Relative to the species’ “lifespan” and the modest precision of our ability to pinpoint time within the geologic record, the differences between the two models may be minimal. Major characteristics of species could have evolved gradually over thousands of years, and still we would not be able to detect those changes on the geologic time scale. What we do detect are long periods of relative stability, when stabilizing selection tends to maintain equilibrium. Changes in physiology and internal anatomy could have continued during the long periods of apparent equilibrium without our seeing them; the fossil record cannot reveal these types of changes. The debate is not about the mechanism of evolution – only its tempo.
Proponents of either model admit that the pace of evolution probably changes from one set of conditions to another. Lake Victoria in East Africa, for example, is just a million years old, and yet home to more than 300 species of cichlid fish found nowhere else (return to Figure above). Genetic analysis shows that all of them descended from a single ancestral “founder” species – relatively rapid evolution. Without competition, that species expanded across the huge Lake, and natural selection suited individual populations to exploit the many different food sources and habitats present in the Lake. Cichlid evolution is an example adaptive radiation similar to that of Darwin’s finches and Hawaiian honeycreepers, discussed in the Evolutionary Theory chapter. The fossil record shows similar opportunities for accelerated evolution after mass extinctions. The extinction of the dinosaurs opened an abundance of ecological niches, soon occupied by radiating birds and mammals. Speciation may accelerate not only with a major increase in available niches, but also with the appearance of new adaptation, such as legs or wings, which allows a group to enter a variety of new niches. In contrast, long periods of environmental stability may slow the pace of speciation.
If you look back at the opening questions for this lesson, you can see that both evolution and our investigations into how evolution works are ongoing - works in progress. We have come a long way in understanding “the origin of species” since Darwin, but questions remain. Exactly what is selected - genes, genotypes, phenotypes, or individuals – is still debated; some, such as Stephen Jay Gould, accept that selection acts at multiple levels. Exactly how fast speciation occurs also remains under discussion; both gradualism and punctuated equilibrium models have merit. Several definitions of a species today serve different purposes; eventually, they should merge into a single clear picture of this basic unit of evolution.
Behind these disagreements, however, lies a powerfully reinforced theory of evolution and an increasingly detailed story of life on Earth. By any definition, humans form a single species, increasingly united by mobility, common genes, and common resources. We and the millions of species with whom we share the Earth today all arose from a single common ancestor billions of years ago – through deep time and myriad instances of allopatric and sympatric speciation. Amazing adaptive logic was most often a result of: directional, stabilizing, and disruptive environmental forces selected for favorable variations and against unfavorable ones. Chance played a major role – through mutation, gene flow, genetic drift, and much environmental change. The pace of speciation probably varied – from gradual change, to rapid change, to long periods of stability. Both speciation and extinction continue today – and will continue into the future, although we cannot predict the direction, because chance determines so much of the direction evolution takes. As a unique species, we are significantly influencing the amount of extinction – and may consequently affect the rate of speciation, as well. That, however, is a topic for a later chapter.
Lesson Summary
- Ever since Darwin’s publication of The Origin of Species, biologists have continued to work out the details of how species originate – the foundation of evolutionary process.
- A species is the smallest group of organisms into which biologists classify living things.
- A biological species is a group of organisms that can interbreed to produce fertile offspring under natural conditions. This is probably the most widely accepted definition of a species.
- A morphological species groups organisms based on extensive structural and biochemical similarities. This is probably the most practical definition for use in the field.
- A genealogical or evolutionary species includes organisms which share a recent, unique common ancestor. This is the goal of all classification; the only question is how to recognize members.
- An ecological species groups organisms together if they share a unique set of adaptations to a particular set of environmental conditions.
- Ideally, all four definitions would merge to recognize a true species.
- All humans are members of the same biological species, because all races and cultures can and do intermarry, and our DNA is 99.9% identical.
- For populations to become separate species, they must experience isolation and genetic divergence.
- Isolation can be physical or environmental, but it must be accompanied by heritable reproductive isolation, which requires genetic divergence.
- The result of speciation is usually two groups of individuals, each more closely adapted to local environmental conditions than the larger parent population.
- Because genetic drift and environmental change increase the influence of chance, adaptation is seldom “perfect” – but over time, natural selection tends to increase fitness.
- Allopatric speciation involves geographic barriers, which physically isolate populations.
- Barriers to mating and barriers to development of zygotes can both cause reproductive isolation.
- Experiments with fruit flies support the possibility that geographic isolation can lead to reproductive isolation.
- Sympatric speciation involves the emergence of a new species within the geographic range of the parent population.
- In plants, polyploidy is a common sympatric form of “instant speciation.”
- Hybridization together with polyploid has formed many vigorous crop species.
- In animals, environmental complexity may lead to sympatric speciation.
- Cichlid fish in Lake Victoria illustrate both adaptive radiation – a form of allopatric speciation – and more recent sympatric speciation due to heritable changes in mating preference.
- The Gradualism model of speciation holds that small changes accumulate to form big changes.
- The Punctuated Equilibrium model suggests that rates of change accelerate over short periods in small peripheral populations, and then stabilize for long periods in large, central populations.
- Differences between the two may be minimal, because gradual change can occur over thousands of years without our ability to detect it in the fossil record.
- Conditions that may accelerate speciation are mass extinctions and the formation of new habitat (e.g. volcanic islands) because both create a sudden availability of many “empty” niches.
- Evolution of a major new adaptation, such as legs or wings, may also accelerate speciation by suiting a species to multiple new habitats.
- Long periods of environmental stability may slow the rate of speciation.
Review Questions
- Define the biological species concept and analyze its usefulness.
- Compare the biological species concept to morphological, genealogical, and ecological concepts.
- Analyze the reasons why biologists consider all humans to be members of the same species.
- Describe two conditions that can lead to speciation.
- Explain the results of speciation in terms of adaptation, chance, and changes in the environment.
- Distinguish allopatric from sympatric speciation.
- Describe two general types of reproductive isolation.
- Describe the use of hybridization and polyploidy to form new crop species.
- Analyze the importance of environmental complexity to sympatric speciation for animals.
- Compare and contrast the gradualist and punctuated equilibrium models of evolutionary change. Give examples which support each.
Further Reading / Supplemental Links
Dawkins, Richard. The Ancestor’s Tale: A Pilgrimage to the Dawn of Life. 2004. Boston: Houghton-Mifflin, ISBN 0618005838 Punctuated Equilibrium
Phylogenetic Tree
Eastern and Western Meadowlark information, with songs - Cornell Lab of Ornithology
Adaptive Radiation of Cichlids
Vocabulary
-
allopatric speciation
-
The evolution of a new species from a closely related population isolated by geographic barriers.
-
biological species concept
-
A group of organisms similar enough to interbreed and produce fertile offspring under natural conditions.
-
ecological niche
-
The set of environmental conditions and resources used or required by a species; the role a species plays in its ecosystem.
-
ecological species concept
-
A group of organisms which share a unique set of adaptations to a particular set of environmental conditions.
-
evolutionary species concept
-
See genealogical species concept.
-
genealogical (evolutionary) species concept
-
A group of organisms which share a recent, unique common ancestor – common ancestry without divergence.
-
gradualism
-
The idea that the tempo of evolution is slow and constant, with small changes accumulating to form big changes.
-
hybrid
-
Offspring of cross-breeding between two different but closely related species.
-
morphological species concept
-
A group of organisms which share extensive structural and biochemical similarities.
-
polyploidy
-
The duplication of chromosome sets, often resulting in “instant speciation.”
-
punctuated equilibrium
-
The idea that species diverge in bursts of relatively rapid change and then remain stable for relatively long periods.
-
reproductive barrier
-
A condition which prevents mating or prevents the development of offspring.
-
reproductive isolation
-
The separation of closely related populations by barriers to producing viable offspring.
-
speciation
-
The process which results in new, separate and genetically distinct groups of organisms (species).
-
species
-
See biological, ecological, genealogical, and morphological species concepts.
-
sympatric speciation
-
The evolution of new species from closely related populations located in the same area.
Points to Consider
- Which definition of species – biological, morphological, ecological, or genealogical – do you prefer?
- To what extent do you think stabilizing, directional, and disruptive selection affect humans today?
- What effects might genetic engineering have on speciation?
- Do you find the evidence for sympatric speciation (the more disputed of the two forms) convincing?
- Are gradualist and punctuated equilibrium models mutually exclusive?
- Why don’t disagreements about speciation threaten the theory of evolution by natural selection?