CHAPTER 36
Soft Tissue Augmentation
Antoine J. Panossian1 and Christopher J. Haggerty2
1Panossian Oral and Maxillofacial Surgery, Massapequa, New York, USA
2Private Practice, Lakewood Oral and Maxillofacial Surgery Specialists, Lees Summit; and Department of Oral and Maxillofacial Surgery, University of Missouri–Kansas City, Kansas City, Missouri, USA
Injection of autogenous, synthetic biodegradable and permanent soft tissue fillers for facial aesthetic augmentation and rejuvenation.
- Autogenous fat: A semipermanent filler for all forms of facial soft tissue augmentation, in particular for patients undergoing liposculpting procedures and with previous hypersensitivity to injectable fillers
- Temporary synthetic fillers: Juvederm (Allergan, Irvine, California, USA) (hyaluronic scaid {HA}), Perlane (Medicis Aesthetics Inc, Scottsdale, Arizona, USA) (HA), and Restylane (Medicis Aesthetics Inc, Scottsdale, Arizona, USA) (HA)
- Semipermanent synthetic fillers: Radiesse (Merz Aesthetics Inc, Franksville, Wisconsin, USA) (calcium hydroxylapatite [CaHa]) and Sculptra (Valeant Pharmaceuticals Inc, Laval, Quebec, Canada) (ploy-L-lactic acid [PLLA])
- Permanent fillers: ArteFill (Suneva Medical Inc, Santa Barbara, California, USA) (polymethyl methacrylate microspheres [PMMA]), which is currently the only US Food and Drug Administration–approved permanent injectable filler for facial soft tissue augmentation
Facial Aesthetic Indications
- Lip augmentation
- Effacement of nasolabial folds
- Perioral rhytids and marionette lines
- Cheek enhancement
- Defining lower jaw
- Elevation of lateral brow
- Glabellar lines
- Horizontal forehead lines
- Temporal fossa wasting
- Asymmetrical facial features
- Scars
- HIV lipoatrophy
Contraindications
- Hypersensitivity to injectable material and ingredients
- Infection at the proposed injection site
Anatomy
- Epidermis: Layers, from superficial to deep, are the stratum corneum, stratum granulosum, stratum spinosum, and stratum basale.
- Dermis: Layers, from superficial to deep, are the papillary dermis and reticular dermis.
- Hypodermis: Contents include fat, connective tissue, neural tissue, and blood vessels.
Injection Technique
- Patients are advised to avoid anticoagulant substances or medications prior to facial soft tissue augmentation procedures.
- For lip augmentation, local anesthesia in the form of mental and infraorbital nerve blocks are employed. Care must be taken to minimize local anesthetic distortion of the anatomy of the site to be injected. For areas not amendable to local anesthesia, topical anesthetic (EMLA) cream can be applied 10–30 minutes prior to the procedure.
- Makeup is removed, and injection sites are prepped with an alcohol pad.
- Patients are positioned seated and upright.
- Depth of injection (dermis, mid-dermis, and hypodermis) is dependent on the type of filler used. In general, nonpermanent fillers are injected more superficially, whereas semipermanent and permanent fillers are injected deeper.
- Injection of filler is slow, deliberate, and in a retrograde fashion with aspiration. The filler is injected with the needle bevel facing superiorly.
- The noninjecting hand is used to feel and mold the filler as it is injected into the appropriate tissue plane.
- Patients are allowed to visualize and make recommendations during the procedure. The surgeon always has the final determination of the amount of filler used.
Postoperative Management
- Cold compresses are immediately applied to the injected areas.
- Strenuous activities are avoided for 8–10 hours immediately post injection.
- Makeup can be applied 4–6 hours post injection.
- Vigorous massage of the injected areas is avoided.
Complications
Early Complications (0–7 Days)
- Injection site–related sequelae and hypersensitivity reactions: Redness, pruritis, pain, edema, and ecchymosis at the injection sites. Typically resolves spontaneously after 3–7 days.
- Overcorrection: Overcorrection can be minimized by injecting slowly and allowing the patient to provide feedback during the process. HA overcorrection can be decreased to some extent with massaging the filler into adjacent tissues. HA can be degraded with the use of hyaluronidase.
- Infection: Infections are rare. Infections can be minimized by cleaning the injection sites with alcohol or a similar prep. Abscesses, cellulitis, and skin infections are treated with oral antibiotics and, if necessary, surgical drainage.
- Vascular compromise and necrosis: Occurs with the intravascular injection of a filler, direct needle injury to a vessel, or external compression of a vessel by surrounding filler. Acute symptoms include severe pain, blanching, duskiness, and ecchymosis immediately post injection. Treatment includes immediately massaging the area, applying heat, a course of aspirin therapy, and nitroglycerine paste applied to the affected area. Hyaluronidase may also be used if the patient was injected with HA; however, it is recommended to first perform prick testing for immediate hypersensitivity. Vascular compromise and necrosis can be minimized by injecting slowly and with minimal pressure, aspirating prior to injection, injecting small volumes per pass, and using small-caliber needles. High-risk areas for vascular compromise and necrosis include the glabella, nose, and nasolabial folds.
Late Complications (Greater than 7 Days)
- Subcutaneous nodular formation, lumps, and beading: Occurs from the injection of filler too superficially, injection of too much filler, and poor injection technique. Treatment for HA is time or hyaluronidase. Treatment for semipermanent and permanent fillers is intralesional steroids.
- Granulomatous reaction: All fillers can potentially cause granuloma formation. CaHa is frequently linked to the formation of granulomas. Treatment involves intralesional steroids.
- Filler migration: Minimized by avoiding strenuous activity and vigorous massage immediately after filler placement.
Key Points
- Pre– and postoperative photographs are always taken in order to demonstrate to the patient their change in facial appearance.
- Fillers can be utilized to lessen facial rhytids; however, they should be utilized as an adjunct to Botox and surgery, which treat the underlying dynamic muscle contractions and excessive skin laxity.
- It is the author's recommendation to utilize synthetic biodegradable products (HA) for first-time patients. This will allow patients to evaluate outcomes and develop realistic expectations. It may also avoid complications of unsatisfied patients with permanent fillers.
- Filler selection is extremely important as different fillers are more ideal for certain areas of the facial skeleton and vary in viscosity, length of degradation, and injection depth. The manufacturer's recommendations should be followed with respect to filler site, depth, and frequency.
- Certain fillers are more prone to complications than others (nodules and granulomas with PLLA and CaHa). Hyaluronic acids, when injected properly, have the advantage of temporary augmentation and few side effects.
- It is generally better to err on the side of injecting too deep versus too superficial. Superficial injections may overcorrect or overcontour defects, lead to surface irregularities, and cause the epidermis to become transparent (HA), which may lead to bluish spots (known as th Tyndall effect).
- It is better to undercorrect an area and have the patient return in 2–4 weeks for a minor touchup than to overcorrect the area. This is especially true with permanent fillers.
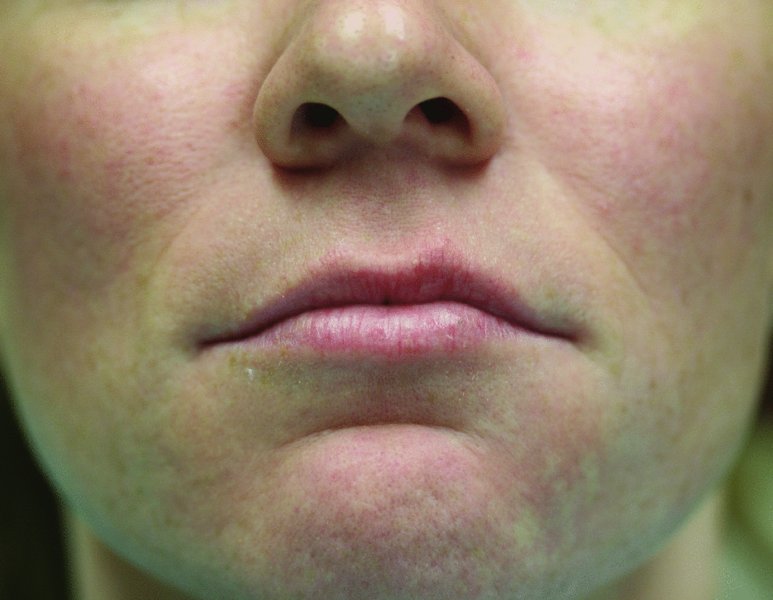
Figure 36.1. Pre-injection photograph demonstrating thin upper and lower lips, upper lip asymmetry, and deepened nasolabial folds.
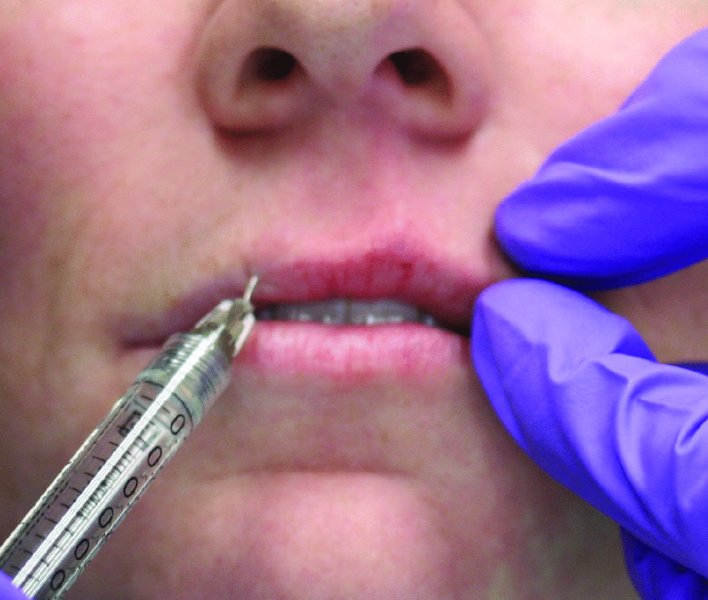
Figure 36.2. Volume is added to the bilateral philtrum columns.
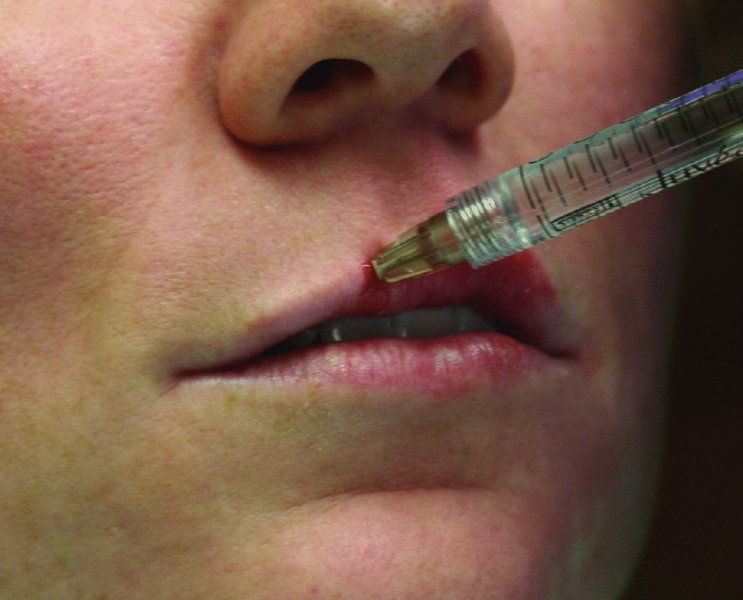
Figure 36.3. The vermillion border of the upper lip is injected in a retrograde fashion to add definition and volume.
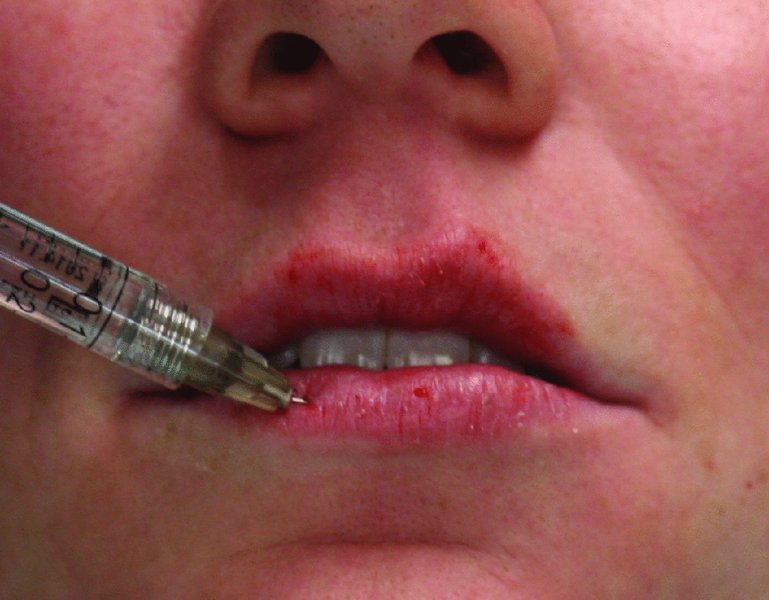
Figure 36.4. Additional volume is added to the body of the upper and lower lips.
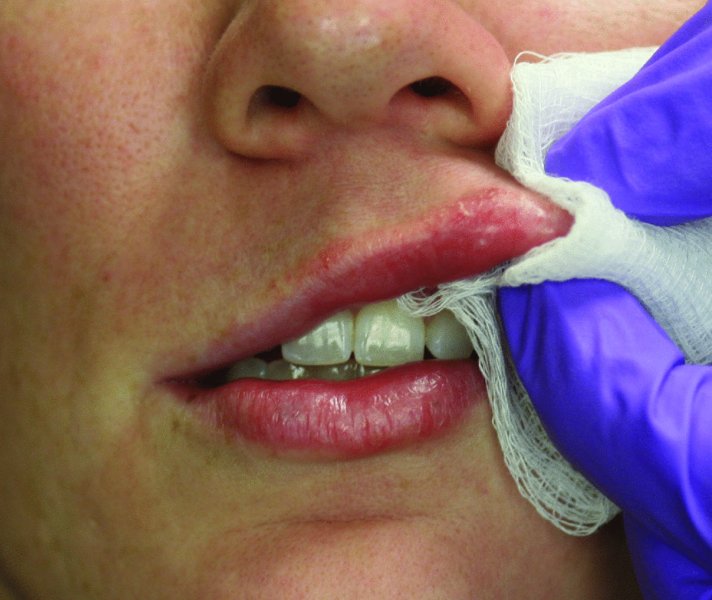
Figure 36.5. Gauze is utilized to smooth the Juvederm Ultra Plus and to minimize filler beading.
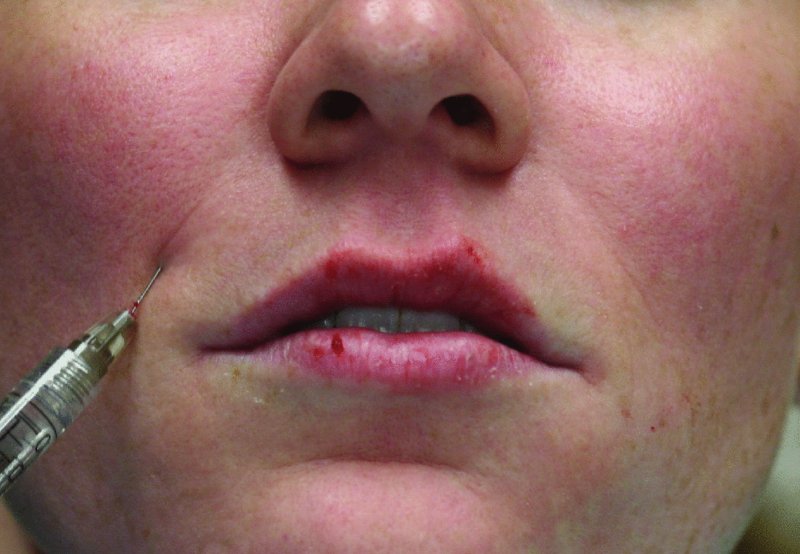
Figure 36.6. Volume is added to the nasolabial folds. Injections should be kept medial to the nasolabial folds and within the dermis. Superficial injections may result in unaesthetic beading of the soft tissue filler.
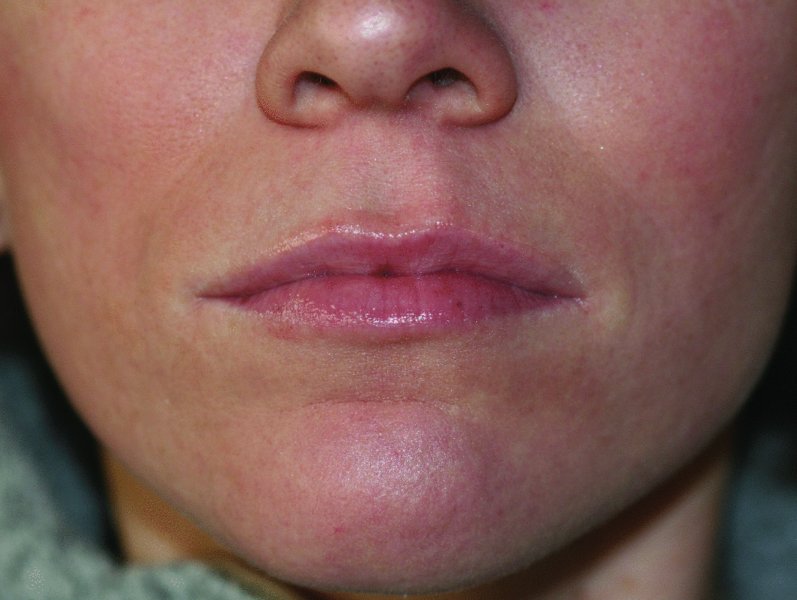
Figure 36.7. Two-week postoperative result. Note the added definition to the vermillion border and philtrum, added volume to the lower lip and nasolabial folds, and correction of minor upper lip asymmetry.
References
- Daines, S.M. and Williams, E.F., 2013. Complications associated with injectable soft-tissue fillers: a 5-year retrospective review. JAMA Facial Plastic Surgery, 15, 226–31.
- Narins, R.S., Michales, J. and Cohen, J.L., 2012. Hylans and soft tissue augmentation. In: J. Carruthers and A. Carruthers, eds. Soft tissue augmentation. 3rd ed. Amsterdam: Elsevier.
- Ozturk, C.N., Tung, R., Parker, L., Piliang, M.P. and Zins, J.E., 2013. Complications following injection of soft-tissue fillers. Aesthetic Surgery Journal, 33, 862–77.
- Wilson, Y.L. and Ellis, D.A., 2011. Permanent soft tissue fillers. Facial Plastic Surgery, 27, 540–46.