CHAPTER 45
Benign Tumors of the Jaws
Christopher M. Harris1 and Robert M. Laughlin2
1Department of Oral and Maxillofacial Surgery, Naval Medical Center Portsmouth, Portsmouth, Virginia, USA
2Department of Oral and Maxillofacial Surgery, Naval Medical Center San Diego, San Diego, California, USA
Non-odontogenic Tumors
Non-odontogenic tumors include connective-tissue tumors, vascular lesions, reactive lesions, and neurogenic tumors. The clinical presentation and treatment of these lesions vary. This section will describe the general classification of common non-odontogenic tumors and highlight standard treatments. All lesions should be treated individually based on their unique histopathology, aggressiveness, and clinical and radiographic presentation. Please refer to Appendix 3 for further treatment guidelines.
Tumors of Connective Tissue
Osteomas
Osteomas are benign tumors of bone. They are commonly found within the skull, jaws, and sinuses. Osteomas are composed of cortical and cancellous bone in varying proportions. These lesions typically present as an asymptomatic mass, which can produce asymmetry of the jaw bones. Radiographically, these lesions appear as dense radiopaque projections of bone. Gardner's syndrome should be suspected in patients presenting with multiple osteomas. Gardner's syndrome is associated with multiple osteomas, polyps of the large intestine, multiple epidermal skin cysts, and multiple impacted teeth. The colon polyps in Gardner's syndrome are considered premalignant, and a referral to a colon and rectal surgeon is recommended due to the certain malignant transformation of these lesions. Treatment is with tumor excision. Recurrence is rare.
Osteoblastomas
Osteoblastomas are rare, benign tumors of bone. They commonly occur in the long bones and spinal column. They have a peak incidence within the third to fourth decades of life and occur more frequently in males. The mandible is the most commonly affected craniofacial bone. Osteoblastomas may be asymptomatic and found on routine exam. However, localized pain and swelling are commonly seen. Pain is unique in that in many cases it is relieved by aspirin. Radiographically, the osteoblastoma can resemble other benign and malignant tumors. Osteoblastomas are typically radiolucent (early) with radiopaque structures within the lesion (later). The lesions can be well defined or poorly defined. The lesions often have a radiolucent rim associated with them. Teeth may be displaced, and/or resorption of roots may be seen.
Histologically, the osteoblastoma is similar to the osteoid osteoma. These are differentiated mainly by clinical features and size. Osteoblastomas demonstrate osteoid tissue and interconnected trabeculae of woven bone. These trabeculae are surrounded by a single layer of osteoblasts referred to as osteoblastic rimming. A fibrovascular stroma and osteoclasts are also seen. Aggressive osteoblastomas and low-grade osteosarcomas can look similar histologically. Treatment of osteoblastomas is with excision or aggressive curettage. Recurrence is uncommon with appropriate treatment. Recurrence is believed to arise from incomplete removal rather than inherent properties of the lesion. Aggressive osteoblastomas, however, have been reported to have higher recurrence rates and often require resection with definitive margins.
Chondromas
Chondromas are benign cartilaginous lesions of the jaws. They may occur centrally or peripherally. They are exceedingly rare in the head and neck. They are thought to arise in areas where embryonic cartilaginous cell rests are present. Therefore, the condyle, coronoid process, base of skull, and anterior maxilla are commonly involved sites. Radiographically, chondromas are well-demarcated radiolucencies. Local bone destruction should raise suspicion for malignancy. Chondromas have a peak incidence within the third and fourth decades of life and demonstrate an equal sex predilection. Histologically, they demonstrate mature hyaline cartilage. Treatment should be directed at total tumor excision due to the similarities of low-grade chondrosarcomas histologically.
Vascular and Reactive Lesions
Central Giant Cell Granulomas (CGCGs)
CGCGs are generally accepted to be nonneoplastic entities that behave like neoplasms. Whether these lesions are inflammatory, reactive, or true neoplasms is still debated. The lesions have been described as nonaggressive and aggressive. Aggressive lesions can cause pain, root resorption, cortical expansion and perforation, mucosal involvement, and higher recurrence rates after treatment. Nonaggressive lesions tend to be asymptomatic and do not have the above features. CGCGs have a peak incidence within the third, fourth, and fifth decades of life and have a higher predilection in females. Radiographically, these lesions can appear as unilocular or multilocular radiolucencies with noncorticated borders. Histologically, CGCGs demonstrate multinucleated giant cells (osteoclasts) scattered within a spindle cell background. Hemosiderin and erythrocytes may be seen. Fibrosis, osteoid, and bone may also be seen. Histological similarities to Brown tumors and aneurysmal bone cysts are noted. Hyperparathyroidism should be ruled out in these patients. Aneurysmal bone cysts may be a cystic variant of CGCGs. Cherubism also has similar histologic findings, but can usually be ruled out with clinical findings.
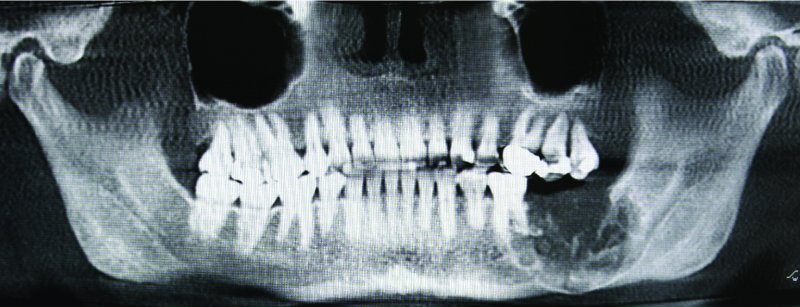
Figure 45.1. Radiograph demonstrating a multiloculated, aggressive, expansile hard tissue lesion located within the left posterior mandible with root resorption, and involvement of the inferior border of the mandible.
Various treatment modalities have been described in the current literature. Surgical curettage has a nearly 20% recurrence rate. Intralesional steroid injections have been used with mixed results. The protocol typically used includes a 50/50 mixture of lidocaine and triamcinolone, injecting 2 mL per 1 cm of lesion. Intralesional steroid injections are typically performed weekly for a total of 6 weeks. Calcitonin injections (100 μ/day) have been performed for up to 24 months with reported success. Subcutaneous interferon therapy has also been utilized. Nonsurgical treatments modalities frequently do not resolve the CGCG, but may allow for surgical intervention with less morbidity and cosmetic deformity and should be considered for large lesions. CGCGs that exhibit aggressive behavior, are recurrent or are refractory to intralesional steroid injections may be treated with peripheral ostectomy or resection with 5–10 mm margins.
Central Hemangiomas
Hemangiomas are benign proliferations of vascular tissues. It is debated whether hemangiomas are a proliferation of endothelium (true neoplasm) or a hamartomatous proliferation of mesoderm, which undergoes endothelial differentiation. Regardless of the etiology, they are potentially life-threatening entities. They are most commonly identified within the posterior mandible, but are otherwise rare within the jaws. They have a peak incidence within the first and second decades of life and a female predilection. Hemangiomas typically present as painless, firm swellings of the underlying bone. Patients may report a pulsation over the lesion. Teeth may be mobile with bleeding around the gingival margins. A bruit and thrill may be present. However, many hemangiomas are asymptomatic and present with none of the above findings. Radiographically, the lesions may be unilocular or multilocular radiolucencies. The multilocular variant has been described as the classic soap bubble or honeycomb appearance. Root resorption and cortical expansion with thinning may be seen, and phleboliths may be noted. There is no absolute pathognomonic radiographic finding for central hemangiomas; therefore, all lesions within the jaws should be initially treated as if they have a vascular component. Lesions that are suspicious for hemangiomas should undergo needle aspiration prior to biopsy. Aspirations that reveal frank blood are nearly pathognomonic for a vascular lesion. Angiography is required prior to any surgical manipulation of these lesions due to the potential for life-threatening hemorrhage and airway embarrassment. Angiography can demonstrate the borders of the lesion and afferent feeding vessels, and can be utilized for selective embolization prior to surgical removal. Embolization therapy with surgical removal (curettage or resection) the surgical ligation of afferent vessels, followed by surgical removal (curettage or resection), are the preferred therapies. Recurrence rates are low with complete removal of the lesion.
Fibrous Dysplasia (FD)
FD is a benign, non-encapsulated neoplasm characterized by cellular fibrous connective tissue and irregular islands of metaplastic bone replacing normal bone. FD occurs by mutations of the gene GNAS-1 (guanine nucleotide-binding protein, α-stimulating activity polypeptide-1). Postnatal mutations will cause localized monostotic disease. Mutations affecting stem cells in embryonic stages will cause systemic conditions (i.e., Jaffe–Lichenstein or McCune-Albright syndromes) characterized by defects in multiple cell lines resulting in multiple bone and cutaneous lesions, as well endocrine abnormalities.
FD commonly is discovered within the second decade of life. Sex predilection is equal. The lesions are typically nonpainful and expansile. Displacement of adjacent structures is common. Maxillary involvement is more prevalent than mandibular involvement. With maxillary involvement, other adjacent facial bones may be involved. This is described as craniofacial fibrous dysplasia. Growth generally ceases with skeletal maturation. Radiographs demonstrate an expansile “ground-glass” lesion with poorly defined borders. Histologically, the lesion is characterized by a cellular fibrous tissue with woven bone trabeculae that do not connect interspersed throughout this background. The bone trabeculae have been classically described as resembling “Chinese characters.” The edges of the lesion fuse with the normal bone without a capsule. Treatment involves resection of small lesions. Surgical reduction and contouring are performed with larger lesions that cause functional or aesthetic concerns. In up to 50% of cases, repeated debulking procedures are necessary until growth ceases. Long-term surveillance is needed due to the possibility of malignant transformation.
Odontogenic Tumors
Odontogenic tumors arise from structures involved with tooth formation. Benign odontogenic tumors encompass a variety of lesions within the jaws. Odontogenic tumors vary significantly both histologically and with their clinical behavior. Many odontogenic tumors are true benign neoplasms, whereas others are extremely aggressive, locally destructive lesions. Malignant variants of these lesions are also encountered. This section will describe the general classification of these tumors and highlight commonly encountered lesions. All lesions should be treated individually based on their unique histopathology, aggressiveness, and clinical and radiographic presentation. Please refer to Appendix 3A for further treatment guidelines.
Benign odontogenic tumors can be characterized by the embryonic tissue of origin. Classifications include (i) odontogenic epithelium, (ii) odontogenic ectomesenchyme (primarily), and (iii) mixed odontogenic epithelium and ectomesenchyme. Ectomesenchymal tumors may have odontogenic epithelium histologically, but they are not believed to play a significant role in the pathogenesis. The classic odontogenic epithelial tumors are the ameloblastoma and the calcifying epithelial odontogenic tumor (CEOT). Ectomesenchymal tumors include the odontogenic myxoma and cementoblastoma. Mixed tumors include the ameloblastic fibroma and fibro-odontoma or odontoma lesions.
Odontogenic Epithelial Tumors
Ameloblastomas
Ameloblastomas are one of the most commonly encountered benign odontogenic tumors. There are several histological variants and three major clinical variants. The major clinical variants include the multicystic or solid variant, the unicystic variant, and the peripheral ameloblastoma.
The multicystic or solid variant can be locally invasive and destructive. These lesions are typically slow growing and show little tendency to become aggressive (i.e., malignant). The most common location is within the posterior mandible, but they can occur in other locations of both jaws. One exception is the desmoplastic variant, which tends to occur within the anterior maxilla. Most cases occur in adulthood and are typically asymptomatic, exhibiting no neurosensory changes. Radiographically, these lesions are typically multilocular and expansile in nature. Radiopacities are not seen with ameloblastomas. The standard treatment is resection with 1 to 1.5 cm bony margins. For lesions that have perforated through the cortical plate and/or extended into adjacent areas, an intact anatomical boundary (i.e., periosteum) is included with the specimen. Recurrence is rare with definitive negative margins. Long-term follow-up is required.
The unicystic ameloblastoma is less common than the solid variant. The tumor's origin and whether it arises de novo or from neoplastic cystic transformation are still debated. Typically, these lesions occur in young adults within the posterior mandible. They are frequently associated with impacted third molars and can be mistaken for a cystic lesion (i.e., a dentigerous cyst or odontogenic keratocyst). Clinically, they may be asymptomatic or demonstrate a painless jaw swelling. Pain and neurosensory disturbance are not typical. Radiographically, unicystic ameloblastomas present as a unilocular radiolucency with or without expansion. The unicystic ameloblastoma varies in treatment depending on the histological findings of the tumor.
Three histological types are seen with unicystic ameloblastomas. These are the luminal, intraluminal, and mural ameloblastoma. These ameloblastic changes tend to support the concept of an evolving spectrum of neoplastic transformation of odontogenic cysts. Luminal unicystic ameloblastomas have ameloblastoma tumor confined within the cystic lining itself. Intraluminal variants have projections of ameloblastoma into the cystic lumen. Mural ameloblastomas have tumor that is within the cystic lining and invades the surrounding noncystic regions of the surrounding tissue. Treatment of the luminal and intraluminal unicystic ameloblastoma is typically complete with enucleation of the cyst. Marginal or segmental resections may be warranted for mural unicystic ameloblastomas due to the invasion of surrounding tissue. Long-term follow-up is recommended for all variants.
The peripheral ameloblastoma is histologically identical to the intraosseous ameloblastomas; however, they are alveolar mucosal lesions. They are typically small (<2.0 cm), non-ulcerated, nonpainful, and located within the posterior mandibular alveolar mucosa. Treatment consists of local excision with long-term follow-up as the recurrence rate is approximately 15%.
Calcifying Epithelial Odontogenic Tumors (Pindborg Tumors)
Calcifying epithelial odontogenic tumors (CEOT) behave as aggressive, benign lesions arising from odontogenic epithelium. Like the ameloblastoma, these lesions can be locally destructive and invasive. They are commonly identified within the posterior mandible, but can be found in other regions. Patients typically present with a painless expansion of the posterior mandible. Radiographically, CEOTs are typically multilocular and expansile, and demonstrate calcifications. Histologically, amyloid deposits with calcified ringlike structures called Liesgang rings are noted. Treatment of the CEOT is similar to the ameloblastoma with marginal or segmental resection with 1.0 cm margins. Recurrence rates have been reported at approximately 15%. Enucleation and curettage procedures are associated with higher recurrence rates.
Odontogenic Adenomatoid Tumors (OATs)
OATs are non-aggressive benign neoplasms arising from odontogenic epithelium. OATs occur more frequently in females with a peak incidence within the second decade of life, and they frequently involve an unerupted canine. OATs have been coined the two-thirds tumor: two-thirds occur in females, two-thirds occur in the maxilla, two-thirds are associated with unerupted teeth, and two-thirds are associated with canines. OATs can produce swelling if they are large; however, most are small, asymptomatic, and incidentally found on routine imaging. Radiographically, these lesions can be radiopaque or have small radiopaque structures within the lesion. The entire structure of an unerupted tooth is frequently involved. This differs from the dentigerous cyst, which tends to involve only the crown of the involved tooth. Large lesions may cause tooth displacement. Histologically, odontogenic epithelium is seen with ductlike structures possessing a lumen lined with a single row of low columnar cells with reverse polarization. Intraluminal eosinophilic material and scattered calcifications may also be seen. Clinically, OATs have a well-formed capsule with excision. Treatment is enucleation with the removal of the involved tooth. Recurrence rates are low.
Ectomesenchymal Tumors
Odontogenic Myxomas
Odontogenic myxomas are aggressive lesions that can arise in both jaws; however, mandibular involvement is more common. Patients may be asymptomatic or have a painless jaw swelling depending on the size of the lesion. Odontogenic myxomas have a peak incidence within the second and third decades of life and appear to have no sexual predilection. Radiographically, the lesions are typically multilocular with thin bone trabeculae noted. Expansion, cortical thinning, and tooth resorption or displacement are common. Impacted teeth are not necessarily seen with the lesion. Histologically, the lesion resembles dental pulp due to the mesenchymal tissue origin. Randomly arranged spindle cells within a loose myxoid stroma with minimal collagen fibrils scattered within are typical. Odontogenic epithelial rests and bone trabeculae are also noted. The lesions are not encapsulated and tend to penetrate bone further than their radiographic depiction. Due to this, the recommended treatment is marginal or segmental resection with 1.5 cm margins and an uninvolved anatomic boundary. Enucleation or curettage is reserved for only easily accessible and small lesions. Routine follow-up is recommended for enucleated lesions due to their propensity to recur.
Cementoblastomas
The cementoblastoma is a benign tumor of hamartomatous cementoblasts, which form a tumor of disorganized cementum. The lesion develops around the apical half of premolar and molar teeth, typically mandibular. Pain may be associated with the lesions. Radiographically, a radiopaque mass involving the roots of the teeth with a radiolucent border is seen. The involved teeth will typically test as vital with pulp testing. Areas of noncalcified tissue in the area of the radiolucent rimming are also seen. Histologically, cementum-like proliferation continuous with the tooth root is noted. Root resorption may be seen. Treatment consists of excision of the lesion and removal of the tooth. Recurrence is not expected.
Ossifying Fibromas (Cemento Ossifying Fibroma)
Ossifying fibromas are a type of benign fibro-osseous tumor. These lesions can arise in both jaws, but they have a predilection for the mandible. These lesions are thought to arise from the periodontal ligament. The premolar and molar regions are commonly affected. The lesions are slow growing, expansile, and typically not painful. Facial asymmetry is noted with larger lesions. Women are affected more frequently than men. Early lesions may appear completely radiolucent. With maturation, radiopacities appear, and the lesion will eventually become completely radiopaque with a radiolucent rim. The lesions are expansile in all directions and are therefore typically spherical in shape. Treatment is with enucleation and curettage for most lesions. Clinically, these lesions easily shell out of the affected bone. Larger lesions, which have significant expansion or aggressive growth features, may require resection. Recurrence is uncommon, but lifelong surveillance is recommended.
Mixed Odontogenic Tumors
Ameloblastic Fibromas
Ameloblastic fibromas can be classified as either a neoplasm or a hamartoma. The lesions typically arise within the posterior mandible, may be expansile, and are typically nonpainful. Radiographically, the lesion presents as a unilocular or multilocular radiolucent mass. Tooth displacement and/or root resorption may be seen. Patients are typically children between the ages of 6 and 10; however, lesions may be seen in older children and young adults. Histologically, the tumors demonstrate mesenchymal and epithelial components. Strands or islands of odontogenic, “ameloblastic-appearing” epithelium within a background connective tissue and myxoid stroma and a fibrous capsule are present. Treatment involves enucleation and curettage for all but very large lesions, which may require resection. Recurrence is rare with proper treatment. If recurrence occurs, ameloblastic fibrosarcoma should be considered within the differential diagnosis.
Odontomas
Odontomas are hamartomas of tissues of the developing tooth. Two types exist: compound and complex. Compound odontomas appear to be small, tooth-like structures. Complex odontomas present as a calcified mass not resembling teeth. These lesions are generally asymptomatic and found on routine radiographic examinations. Compound odontomas have a predilection for occurring in the anterior jaws, and complex odontomas in the posterior jaws. The lesions are typically found in patients younger than 25 years of age. Radiographically, they most often present as radiopacities, but they may be radiolucent or mixed depending on the progression of calcification of the lesions with time. Histologically, enamel, dentin, cementum, and pulpal tissue are seen. Treatment involves enucleation and curettage, and recurrence is rare.
Technique: Mandibular Resection
- One must determine first if a transcutaneous (larger mandibular lesions with resultant continuity defects) or transoral (smaller mandibular lesions not resulting in continuity defects, and maxillary lesions) approach will be utilized.
- The patient is placed in a supine position. After endotracheal intubation, the patient is prepped and draped in a sterile fashion.
- Local anesthetic containing a vasoconstrictor is infiltrated within the area of the lesion, and regional blocks may also be utilized.
- If performing a transcutaneous approach, the proposed incision should be placed within a natural skinfold, 1.5 to 2.0 cm below the inferior border, to minimize damage to the marginal mandibular branch of the facial nerve.
- If performing a transoral approach, a sulcular incision with or without vertical releasing incisions is performed.
- Transcutaneous incisions will follow sharp dissection through the skin, subcutaneous fat, and platysma muscle to the superficial layer of the deep cervical fascia (SLDCF). Within the region of the premasseteric notch, the marginal mandibular branch of the facial nerve and the facial artery and vein will be encountered deep to the SLDCF. Care is taken to isolate the marginal mandibular branch of the facial nerve. Once isolated, dissection can proceed either superior or inferior to the nerve.
- Once the nerve is protected within the flap, dissection continues to the inferior border of the mandible. The facial vein and artery will be encountered. These vessels may be preserved depending upon the location of the lesion. In the event the vessels do not allow adequate surgical access, the vein and artery should be ligated and divided.
-
Wide exposure of the entire lesion (Figures 45.9, 45.10, and 45.11) through subperiosteal or supraperiosteal dissection is necessary. Depending on the type of the lesion and if cortical perforation has occurred, a supraperiosteal dissection may be required to allow for resection with an intact anatomical barrier.
- A reconstruction plate may be pre-bent using a stereolithic model prior to surgical resection (Figures 45.6 and 45.8).
-
Osteotomies are performed using a reciprocating, oscillating, or giggly saw at the predetermined proximal and distal margins of the lesion (Figures 45.3, 45.10, and 45.11). The lesion is removed in its entirety and sent for final pathology (Figures 45.12 and 45.13). A small amount of cancellous bone may be harvested from the distal and proximal ends of the native bone and sent to pathology as frozen sections to identify atypical cells within the marrow.
-
Primary or secondary reconstruction (Figures 45.16 and 45.17) is performed based upon the surgeon's preference and the anticipated recurrence rate of the lesion.
- The four-layer closure is performed to include the ptyergomasseteric sling–periosteum, platysma, subcutaneous tissues, and skin. A suction or passive drain may be inserted and secured to the overlying skin.
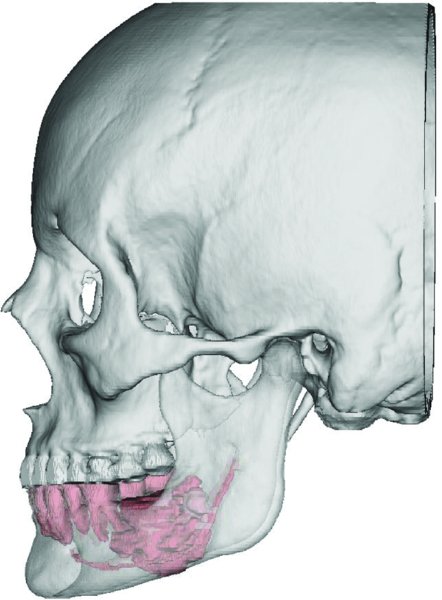
Figure 45.2. Virtual Surgical Planning workup demonstrating the anatomy of the craniofacial bones and teeth. The tumor and the inferior alveolar nerve are highlighted in red. Five-millimeter resection margins are measured from the tumor periphery.
Figure 45.3. Based on the location of the mass, a vertical ramus osteotomy was outlined to resect the posterior margin.
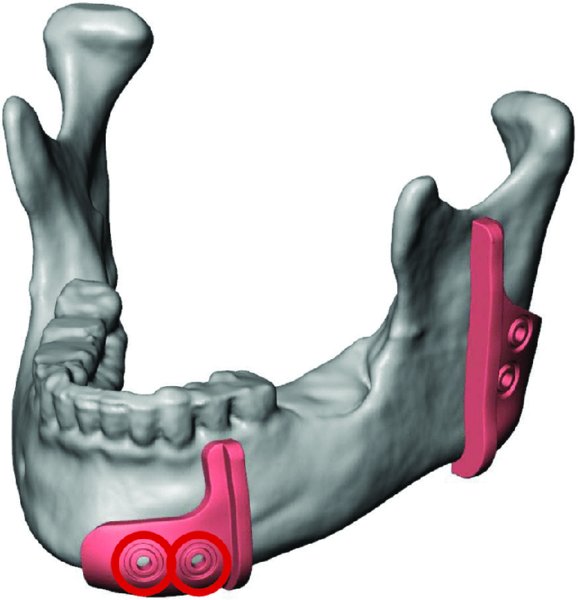
Figure 45.4. An intraoral cut guide is virtually designed. The dental occlusion will stabilize the anterior cut guide during the osteotomy. Predictive holes are marked in red and represent fixation points for both the guide and the pre-bent reconstruction plate.
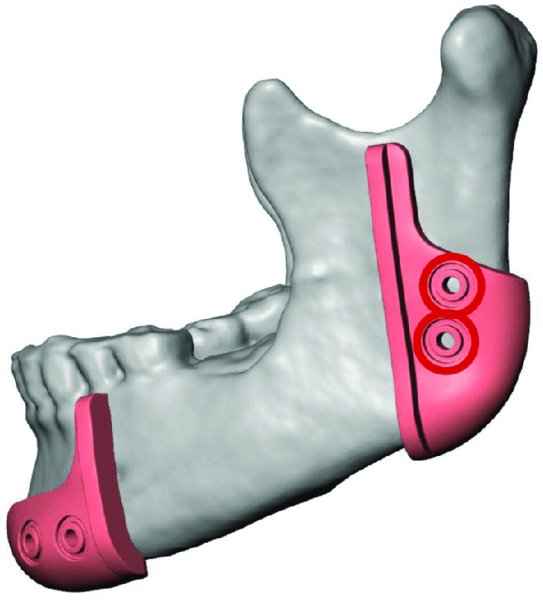
Figure 45.5. The posterior cut guide will articulate with the angle of the posterior mandible. Predictive holes are marked in red.
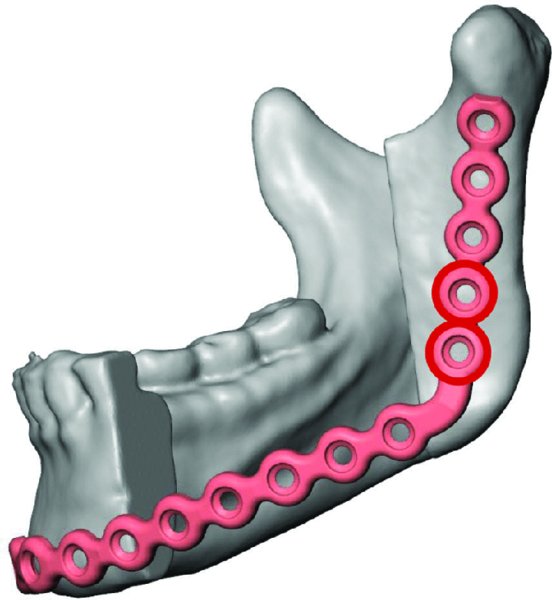
Figure 45.6. A reconstruction plate is virtual adapted to the virtual postoperative mandibular anatomy.
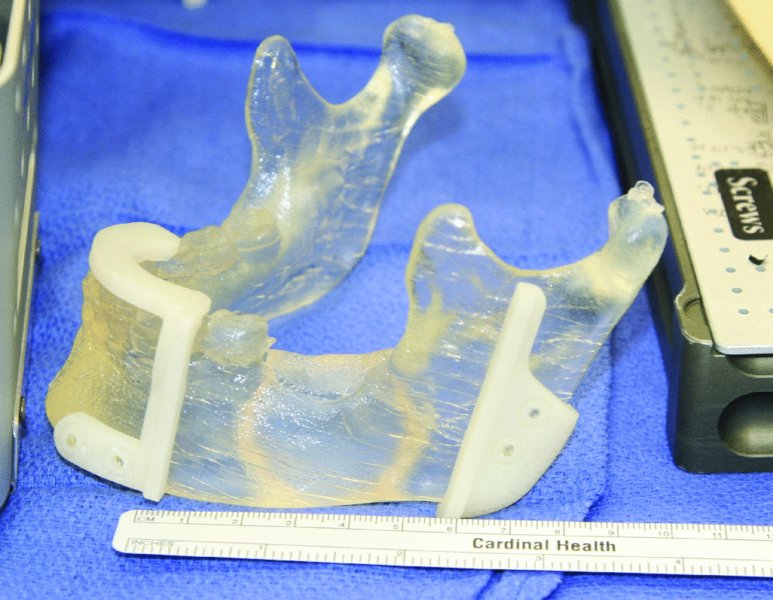
Figure 45.7. Sterile stereolithic model with cut guides in place.
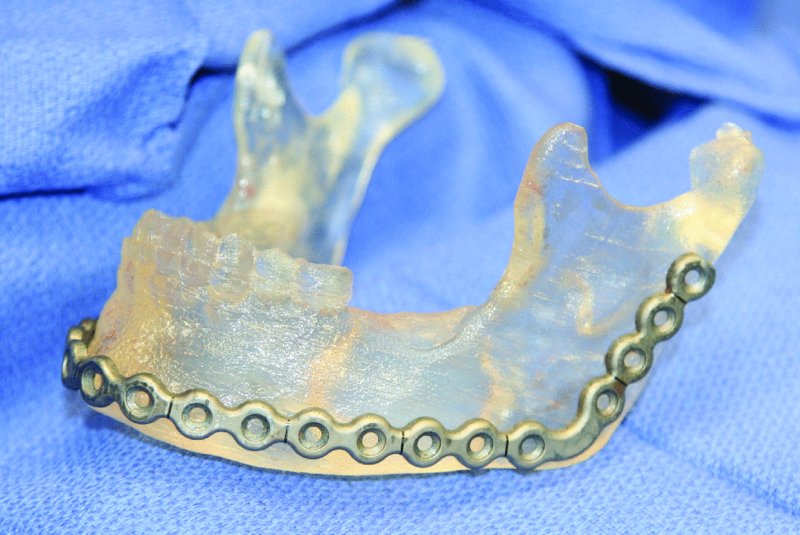
Figure 45.8. Sterile pre-bent reconstruction plate.
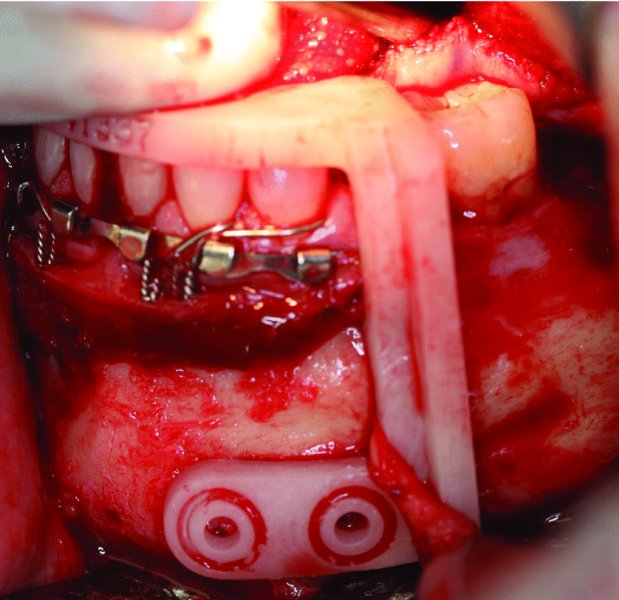
Figure 45.9. Occlusal-based anterior cut guide in place. The mental nerve is retracted anteriorly with the cut guide.

Figure 45.10. The anterior resection is performed with a reciprocating saw utilizing the custom anterior cut guide. The premolar tooth anterior to the osteotomy is extracted in anticipation of 3–5 mm of bone die back post-osteotomy.
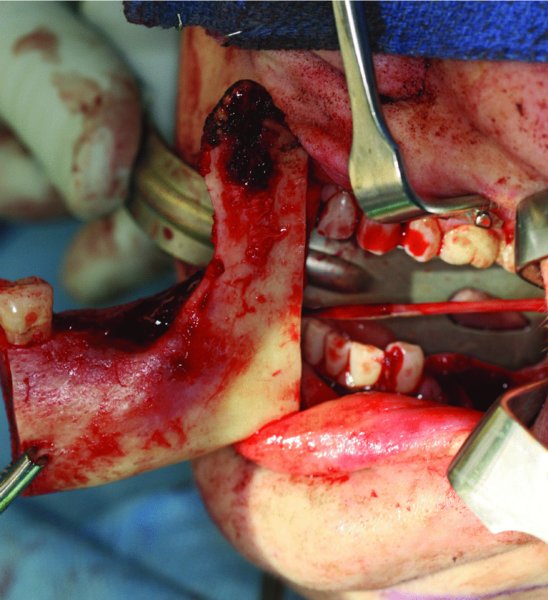
Figure 45.11. An intraoral vertical ramus osteotomy is performed based on the Virtual Surgical Planning workup and utilizing the posterior cut guide. An inferior alveolar nerve pull-through was performed for this benign tumor.
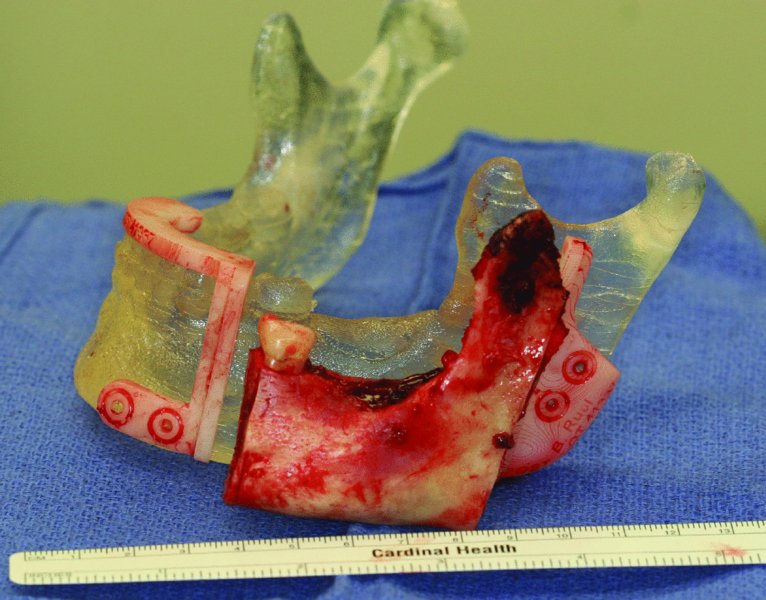
Figure 45.12. En bloc resection compared to stereolithic model and cut guides.
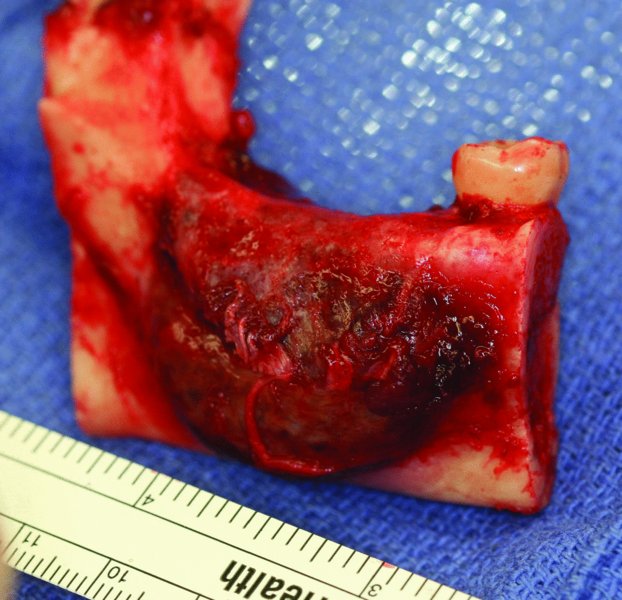
Figure 45.13. Due to the lingual cortical expansion of the mass, a cuff of periosteum was removed to serve as an anatomical barrier.
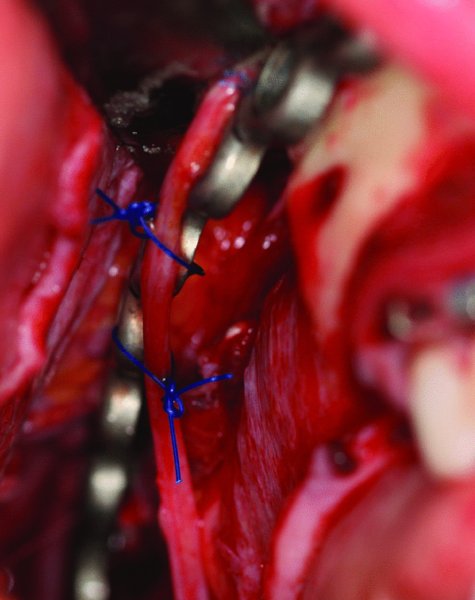
Figure 45.14. The transected inferior alveolar nerve was reapproximated with 7-0 Nylon sutures and secured to the reconstruction plate with 2-0 Prolene sutures.
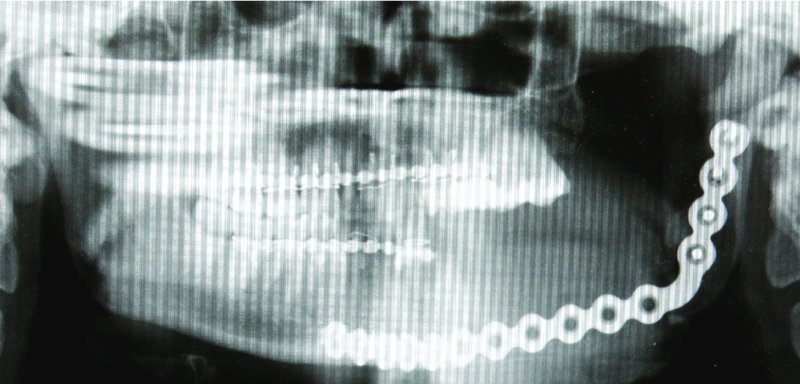
Figure 45.15. Immediate postoperative film depicting a well-positioned reconstruction plate with the condyle ideally seated within the glenoid fossa. The postoperative film correlated well with the Virtual Surgical Planning workup.
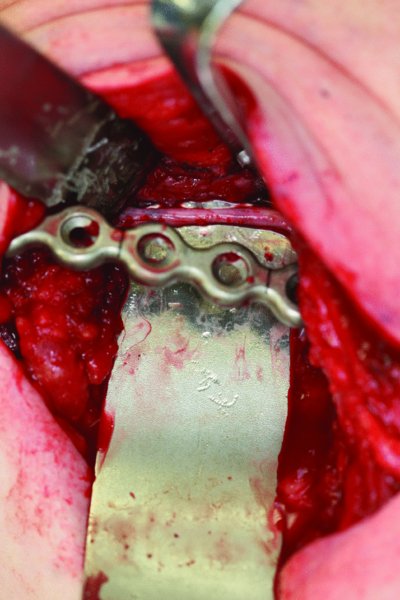
Figure 45.16. Four months after en bloc resection, an extraoral approach was utilized to expose the 4 cm mandibular continuity defect. The inferior alveolar nerve is identified and freed from surrounding scar tissue.
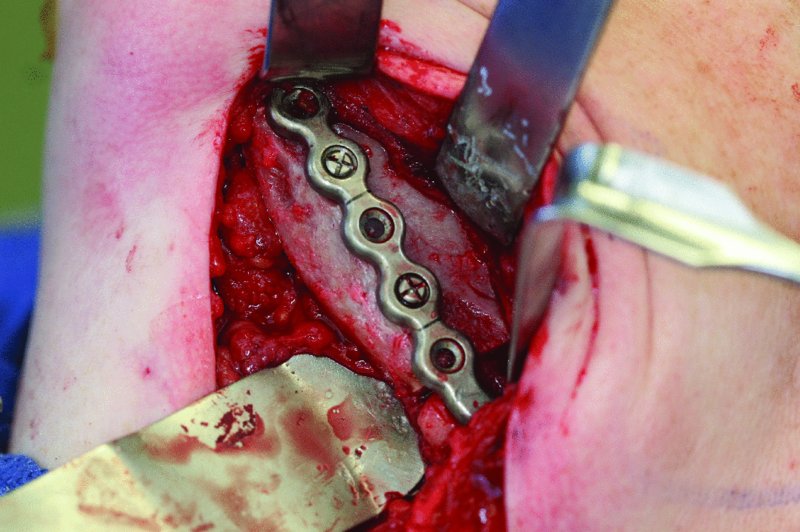
Figure 45.17. The defect was reconstructed with an anterior iliac crest corticocancellous graft secured to the reconstruction plate with fixation screws.
Key Points
- A definitive diagnosis must be made prior to any definitive surgical procedure as lesions have different levels of aggressiveness and recurrence rates.
- Adequate surgical exposure is necessary for both the resection and the reconstruction of pathological lesions.
- Plain films may be utilized in the operating room for en bloc resection of bony lesions to evaluate peripheral margins.
- Margins of the final specimen need to be evaluated on a microscopic level to ensure complete resection.
- En bloc resections require a passively adapted reconstruction plate with at least three (preferably four or more) fixation screws on each side of the defect (Figure 45.15).
- Teeth within or near the line of the planned osteotomy should be extracted in anticipation of 3–5 mm of bone dieback from the osteotomy site.
- All pathologic resections should be planned with the final reconstruction (soft tissue, hard tissue, and prosthetic rehabilitation) in mind.
References
- Ellis, E. and Zide, M.F., 2006. Surgical approaches to the facial skeleton. 2nd ed. Philadelphia: Lippincott, Williams & Wilkins.
- Marx, R.E. and Stern, D., 2003. Oral and maxillofacial pathology a rationale for diagnosis and treatment. 2nd ed. Hnbover Park, IL: Quintessence.
- Neville, B., Damm, D.D., Allen, C. and Bouquot, J., 2009. Oral and maxillofacial pathology. 3rd ed. Philadelphia: W.B. Saunders.