Advance Acclaim for
Targeting a Cure for Type 1 Diabetes
“Finding a cure for type 1 diabetes is an enormous challenge, but those of us involved in supporting and promoting diabetes research understand that cures are not discovered in single, “aha!” moments. Rather they emerge through decades of incremental scientific advances in our understanding of what triggers a disease and what can be done to slow, prevent, or reverse it. Targeting a Cure does a most commendable job of explaining the complexities of this scientific journey and the amazing distance we have come already, as well as what it will take to get us to our ultimate destination.”
—Larry Hausner, MBA
Chief Executive Officer, American Diabetes Association
“This book is a compelling and highly readable synthesis of the many efforts to find a cure for type 1 diabetes. The search for the cure, by necessity, is fragmented, sprawling, and fluid, so diaTribe has done a great service by providing a coherent picture of the opportunities and obstacles before us. There is no magic bullet for curing type 1 diabetes, but this book describes the very real ammunition being used to try to achieve that end.”
—Irl B. Hirsch, MD
Professor of Medicine, University of Washington School of Medicine, Seattle, WA
“Targeting a Cure for Type 1 Diabetes is a valuable and thought-provoking overview of research efforts underway to cure type 1 diabetes. Education about the progress being made—and the hurdles to be faced—is crucial if we’re to achieve our ultimate goal of ridding type 1 diabetes from the lives of our loves ones. I thank the diaTribe team for their ongoing efforts to inform and enlighten all those who care about type 1 diabetes.”
—Jeffrey Brewer
President and CEO, Juvenile Diabetes Research Foundation, New York, NY
“Many of us go to bed each night and wonder when the cure for type 1 diabetes will be here, and wonder as well what forms it might take. Targeting a Cure for Type 1 Diabetes brings into focus the long history, the complexity of human physiology, the panoply of options, and the advances that are being made so that we can all be grounded in real-life science and technology when we put our heads on our pillows each night. After reading about Kelly Close and her teams’ incredible journey of discovery, we cannot only continue to dream, but we can open our eyes each morning to a reality that is bringing us closer, inch by inch, discovery by discovery, to a day when glucose control will be automatic and people with type 1 diabetes will be ‘cured.’”
—Francine R. Kaufman, MD
Chief Medical Officer and Vice President, Global Medical, Clinical & Health Affairs, Medtronic Diabetes Emeritus Professor of Pediatrics and Communications at USC and Children’s Hospital Los Angeles, Los Angeles, CA (Former President, ADA)
“Since the start of the DCCT, almost 30 years ago, I have watched the evolution of the diabetes care team dedicated to help individuals with type 1 diabetes who struggle every day to control their blood glucose and live well with their disease. Kelly Close and her colleagues chronicle, in as comprehensive yet engaging a way as I have ever seen, the exciting evolution of the diabetes cure team. The cure team still includes endocrinologists and diabetes educators but now we learn of the need for immunologists, islet biologists, stem cell biologists, materials science experts, animal engineers, device manufacturers, transplant surgeons, research advocates, philanthropists and more. Targeting a Cure for Type 1 Diabetes will give you hope that someday struggling with the management of type 1 diabetes will only be a memory.”
—Richard M. Bergenstal, MD
Executive Director, International Diabetes Center, Minneapolis, MN
“Leave it to Kelly Close and her team to not simply synthesize and define what a cure really means, but demystify the tangled web of cure pursuit and research. I thought I was pretty well informed until I read Targeting a Cure for Type 1 Diabetes. It is impossible not to come away smarter about the biology and complexity of diabetes and indeed reassured that a cure is entirely possible in my lifetime. Targeting a Cure For Type 1 Diabetes is a comprehensive, but easy to read summation of a complex subject. A gift to all with diabetes and those that love and care for them.”
—Howard Steinberg
Founder and CEO, dLife and LifeMed Media, Westport, CT
“In this information age, it is a constant struggle to keep abreast of, well, information itself, especially technical and detailed cutting edge research concepts outside of one’s field of focus. Kelly and her ever-present team of alchemists have once again managed to distill a disparate array of clinical and research pearls into rich, easily digestible nuggets, this time with an overarching theme of curing type 1 diabetes. The book is a vital, unsparing account of those efforts.”
—Howard Zisser, MD
Director of Clinical Research and Diabetes Technology,
Sansum Diabetes Research Institute, Santa Barbara, CA
“Each new advance in type 1 diabetes research carries with it the potential for a cure. Reading a book like Targeting a Cure for Type 1 Diabetes offers much more, however, than an interesting and complicated tale of how we might get there. The book brings together the many strands focusing on different aspects of the disease, helps us to understand what is nearly in reach, and gives us an idea of what may soon come to pass. My congratulations to the authors for a marvelous book.”
—Zachary T. Bloomgarden, MD
Professor of Medicine, Mount Sinai School of Medicine, New York, NY
“I have long followed the efforts to cure type 1 diabetes, but this book is unusual—in the best way—in that it provides a comprehensive assessment of where that research is right now. The document will be accessible to readers with all levels of expertise, and it also provides the most updated analysis of the Artificial Pancreas that I’ve read in a long time.”
—Howard Wolpert, MD
Director of Insulin Pump Program, Joslin Diabetes Center and
Harvard Medical School, Boston, MA
“When will we have a cure? In the 1980s, I remember when parents of a child newly diagnosed with diabetes would ask us that question, our answer was, “By the end of the century, we will have conquered diabetes.” Most of us in the diabetes club believed it. We believed the cure was right around the corner, but we were overly optimistic. Now, I have come to understand that even more than data and projected outcomes, the most important thing that we give our patients is hope…and this book does a wonderful job of providing a compendium of hope.”
—Virginia Valentine, CNS, BC-ADM, CDE, FAADE
CEO, Diabetes Network at Northside Family Medicine, Albuquerque, NM
“I believe that Targeting a Cure for Type 1 Diabetes is a must-read for anyone who really wants to know what a cure might look like and when it might get here. The report makes no promises, but it does convey how researchers are amassing new insights while developing and refining new cure therapeutics that will transform this disease in ways that most of us have not yet imagined.”
—Jane Jeffrie Seley, DrNP, MPH, MSN, BC-ADM, CDE
Diabetes Nurse Practitioner and Certified Diabetes Educator,
New York Presbyterian/Weill-Cornell Medical Center, New York, NY
“This monograph provides a thorough look at the current state of type 1 diabetes research and provides realistic expectations for new therapies and interventions. I know of no other single source that summarizes so well the research to date in immune therapies, transplantation, beta cell regeneration, and artificial pancreas, written at a level that makes it of value for patients, clinicians, and researchers.”
—Roy W. Beck, MD, PhD
Executive Director of the Jaeb Center for Health Research, Tampa, Florida and father of a son with type 1 diabetes
“This is a remarkable piece of work. Lisa, Ben, Mike, Adam, Hannah, and Kelly put in context a very complicated topic and their words make for valuable learning even for those who already follow the field quite closely—as well as for all patients with type 1 diabetes. An excellent review and a conprehensive update. I loved it!”
—Dr. Steven Edelman, MD
Professor Medicine, University of California, San Diego, Veterans Affairs Medical Center,
co-Founder, Taking Control of Your Diabetes
“A cure for type 1 diabetes could come from one of the many areas of research, and this report does an outstanding job in explaining and assessing where the action is. While I would love to see a “real cure” through either prevention and/or islet cell transplantation with minimal immune-modulation, I expect early stages of a “cure” may come from different versions of the “closed-loop” devices that are well-described here. Whichever path may lead to a cure, I hope for a closer partnership with academia, regulatory agencies and pharma.”
—Dr. Satish K. Garg, MD
Editor-in-Chief, Diabetes Tech. & Therap., Professor of Medicine and Pediatrics, Endowed Chairs & Director, Adult Program, Barbara Davis Center for Childhood Diabetes, University of Colorado Denver
“The Close Concerns and diaTribe teams have endeavored to put into perspective the progress that is being made towards a cure for type 1 diabetes. It is an arduous road that takes us down multiple paths, including too many blind alleys. Yet, the progress has been remarkable. Nevertheless, one has to appreciate the long timelines still ahead. We shall have a cure for type 1 diabetes, but the reader must learn that science moves in fits and starts.”
—Dr. Jay S. Skyler, MD, MACP
Professor Medicine, Pediatrics, & Psychology, Deputy Director,
Diabetes Research Institute, University of Miami Miller School of Medicine,
Chairman, Type 1 Diabetes TrialNet
“Ever since my diagnosis, I have been reading up on possible cures to type 1 diabetes. With all of the information available, it is easy to get confused about what all of the research actually means. Targeting a Cure for Type 1 Diabetes puts all of that information at your fingertips so you can really see, and more importantly understand, what is happening. Even the most cynical person out there can get the smallest twinge of hope that this disease will be cured. Thanks Kelly, and all the other authors for putting together this comprehensive book and showing us possible paths to a diabetes-free future.”
—Elizabeth Edelman
Co-Founder and CEO, DiabetesDaily.com
“Targeting a Cure for T1 Diabetes is a MUST-READ for all families touched by this condition. This great work will help you better understand how complex a disease type 1 diabetes is while showing a light at the end of a tunnel, explaining in easy-to-understand terms the paths out of type 1 diabetes, currently being explored by some of the world’s leading scientific minds.”
—Manny Hernandez
President, diabetes hands foundation

eISBN: 9781580405041
Copyright © 2013 American Diabetes Association
This report is dedicated to all the scientists and researchers who are working to cure type 1 diabetes, to the late Dr. George Eisenbarth, who led the way with his vision, drive, and spirit, and to Lilly Varon and the ten million people like her with type 1 diabetes across the planet.
Contents
1.3 The Course of Type 1 Diabetes
Islet and Pancreas Transplantation
Beta Cell Regeneration and Survival
1.6 The Drug Development Process
Chapter 2: Immune Therapeutics
2.2 The Immune System and Type 1 Diabetes
Glutamic Acid Decarboxylase (GAD65) Immunization
2.4 Other ‘Immune Modulating’ Strategies
2.5 The Interface of Vaccines and Immune Modulation
Chapter 3: Islet and Pancreas Transplantation
3.2 Immune Suppression in Transplantation
3.3 The Intended Population for Islet Transplantation
Creating Beta Cells From Stem Cells
3.5 The Bioartificial Pancreas
Chapter 4: Beta Cell Regeneration and Survival Agents
4.2 Beta Cell Regeneration and Survival Factors
Chapter 5: The Artificial Pancreas
5.2 Current State of Closed Loop Components
Better and More Accurate CGM Technology
A Cautious Regulatory Environment
Increasing Use of Other Hormones
Appendix A: Targeting a Cure: The Takeaways
Appendix B: The Late Breaker Website
Appendix C: Adam Brown’s Testimonial at the 2010 Public Workshop on the Artificial Pancreas
Foreword
I became aware of type 1 diabetes in the early 1960s, when a classmate in elementary school was taking cookie breaks in the mornings and afternoons to avoid hypoglycemia. Jim and I became good friends, and my familiarity of the disease grew as he and I travelled together on multiple school activities. I watched him sterilizing his glass syringes and administering insulin four to six times daily. Of course, self-monitoring of blood glucose (SMBG) was almost two decades away and he could only use Clinitest to check for high urine sugars and depended on recognizing symptoms for any low blood sugars.
Twenty years later, when I was an endocrinology fellow at the Joslin Diabetes Center, people with type 1 diabetes were treated with SMBG together with continuous subcutaneous insulin infusions (insulin pumps). Stuart Soeldner, a Joslin researcher at the time, told us, “A cure for diabetes is right around the corner!” It has been a very long street with many false exits and blind alleys. Despite the frustration and disappointment, we have learned a great deal, and the quality of care for those with type 1 diabetes has improved dramatically. Some have had a successful cure, like my friend Sara who, after over 25 years of type 1 diabetes underwent a combined kidney-pancreas transplant 23 years ago and has had normal glucose levels without insulin ever since.
Whole organ pancreas transplants continue to have the greatest success in “curing” diabetes, but organ availability and the toxicity of the necessary anti-rejection medications limit their applicability. Embryonic stem cells, induced pluripotential stem cells, islet transplants, and beta-cell regeneration through growth factor stimulus have had their ups and downs but remain viable, if distant, targets for therapy. Vaccines and immunosuppressive therapies to prevent beta-cell destruction are alluring, but have yet to be proven consistently effective.
Targeting a Cure for Type 1 Diabetes by Lisa Rotenstein, Kelly Close, and their colleagues, takes on the demanding task of chronicling the status of the various approaches undertaken to achieve a long-sought-after cure. With a perspective of critical optimism, this book describes the arduous research journey that has been traveled in the pursuit of a cure, as well as the minor successes and failures encountered along the way. This book also reminds us that small steps in understanding immunology, beta-cell biology, glucose sensing, and insulin delivery are what provide people like Jim and Sara the means of improving their diabetes self-management today while they await the grail of a cure. People with diabetes and their caregivers (including physicians and researchers) should read this book to become informed consumers and providers and to generate the next great and innovative approach to turning that vital corner to cure type 1 diabetes.
Robert E. Ratner, MD
Chief Scientific and Medical Officer, American Diabetes Association
Preface
This book has its start in 2006, when Erin Kane, Dan Belkin, and I founded diaTribe. We wanted the publication to be the premier source on therapies, technologies, and research for people living with diabetes—a way for us to share what we learned working at Close Concerns, the healthcare information firm that I founded in 2002. I am proud of how diaTribe (www.diaTribe.org) has grown and feel privileged to have worked alongside so many talented writers, to have interviewed so many brilliant doctors, educators, and scientists, and to have met so many passionate patients, families, and friends. However, diaTribe’s focus has always been on what is immediate or near—novel drugs, cutting-edge technology, late-stage clinical trials—rarely the more ambitious, longer-term work to cure diabetes.
This changed thanks to Lisa Rotenstein, who had just finished her sophomore year of college at Harvard when she spent the summer of 2009 at diaTribe and Close Concerns. Lisa believed strongly that we should write about efforts to target a cure, and she worked long, thoughtful hours that summer (and nearly four years since then) to turn that belief into a reality. Benjamin Kozak, who became Managing Editor of diaTribe in 2010, believed in this book as well, and his vision, insights, and incredibly hard work has guided months of revisions, expansions, and collaborations. Adam Brown put together a comprehensive section on the artificial pancreas, and Dr. Michael Dougan infused the book with his expertise in endocrinology and immunology. Hannah Deming used her passion for basic science to update and expand the book’s immune therapies and transplantation sections and to offer thoughtful commentary throughout. Dr. Aaron Kowalski, fellow type 1 patient and a top scientist at JDRF, wrote a very personal and extremely thoughtful introduction to our book.
What follows is an update on progress toward curing diabetes. We hope that you will enjoy and learn from it whether you are a person living with diabetes, a parent or caregiver, a researcher or clinician, or anyone else looking for clear, definitive information on an issue that inspires strong opinions and stirring hopes. We assume that you are reading this book because you are interested in the details. However, we don’t assume deep experience in science or medicine, and we try to provide the background necessary to understand complex ideas. Though no one knows exactly when a cure will come or what it will look like, we hope that this book will give you a better idea of the possibilities and the promise of that search. Fortunately, the search for a cure is moving faster than a book’s publication cycle, so we will write and post updates on the progress made to finding a cure—and one day on the discovery itself—at the website www.diatribe.org/latebreakers.
We are not blind optimists. We know the hurdles are high. But we know that the gains made have been substantial, we think that today’s research is heading in the right direction, and we believe that a cure will arrive in our children’s lifetimes. For most patients and their loved ones, the development of new drugs and devices moves slowly, whether because of the nature of technology development or abounding regulatory pressure. But as part of our work we see tremendous activity under the surface: immense creativity, good funding (though it could be much better), many laboratories, many companies, many trials.
Of course, the advances discussed in this book have materialized because of the hard work and perseverance of doctors, scientists, and researchers who have worked to cure diabetes throughout the past century, and it is because of these individuals that our hopes for a cure remain high today. We dedicate this book to all of these doctors and researchers, and we wish them every success along the way. We would like to give special recognition to the late Dr. George Eisenbarth who gave so much brainpower to the search for a cure and who was so generous with his thought and time, particularly with us patients.
And lastly, we also dedicate this book to the ten million people around the world who live with type 1 diabetes every day.
Gratefully,
Kelly Close
February 2013
How To Use This Book
This book covers a lot of ground, which befits the scope of research to cure type 1 diabetes. We realize that you may have time to only read parts of the report, so we’ve added several features to make it as accessible as possible.
Icons: At the top of nearly every section (except in Chapter 1), you will find a set of icons. They provide key information about particular therapies and are also meant to guide you to sections that may be most relevant to you. The icons provide the following information:
Who: 
These icons specify who would benefit from the cure-targeted therapy, if proven effective.
When: 
These intervals estimate the earliest time in which the cure-targeted therapy may become available. They apply to the first product of a particular type of therapy, not later-generation products that would likely have greater efficacy.
Bottom Line: 
These icons denote our assessment of how likely it is that a particular cure-targeted therapy will work and will eventually become available. Note that we don’t like to over-promise: these are our best estimates, and our estimates are relatively conservative.
“Kelly’s Take” Sections: Throughout the book, Kelly draws conclusions and provides her own opinions on the promise of each cure-targeted therapy. These sections are written in particularly patient-friendly language and, used alongside the summary boxes, encourage readers to quickly learn about particular topics. Look for italics and the icon of a contemplative Kelly!
Summary Boxes: Colored boxes with bulleted summaries of the above text are located at the bottom of each section. You can use them as concise tools to learn about a given topic.
Targeting a Cure: The Takeaways: If you are looking for a short, yet comprehensive summary of the entire book, look no further. Located near the back, this section features a bulleted chapter-by-chapter compilation of all the key takeaway messages. Following Dan Pink’s approach in one of Kelly’s favorite books, Drive, we also offer a Cocktail Party summary of “Targeting the Cure” as well as a Tweet explaining our take on this book.
The Glossary: We have put in boldface a number of terms that are used frequently in the book and often represent key ideas. Our glossary defines these terms.
Introduction
Dear Reader,
How soon will there be a cure for type 1 diabetes? My family asked this very question when my brother Steve was diagnosed with the disease at age three in 1977, and again when I was diagnosed at 13 in 1984. More than 30 years later, important advances in research have been made. We have a better understanding of what causes type 1 diabetes and have improved treatments that enable individuals to live healthier and longer lives. In fact, I have grown to rely so much on my continuous glucose monitor that I can’t imagine not having instant feedback on my blood glucose levels at all times. But scientific progress is incremental, so while this progress has led us to refine and broaden our vision of a cure, we are not there yet.
The past few decades have led us to identify new avenues of research that may lead to a cure, such as vaccines that have the potential to shut down the parts of the immune system that attack beta cells, and we are advancing in the development of novel ways to regenerate or replace beta cells that do not involve invasive transplantation surgeries. As a scientist and as the current leader of our treatment research team at JDRF, I have more hope than ever before that we will indeed cure diabetes.
In the interim, I see promise for several new treatments on the horizon that will change how individuals manage living with this disease. We’re seeing momentum toward the development of closed-loop systems that will monitor and dispense appropriate amounts of insulin as needed. We also see progress toward new treatments for serious diabetic complications. In fact, European regulatory authorities just approved the first system that senses blood glucose levels and turns off insulin delivery from the pump automatically if a person is hypoglycemic, and they approved the first drug to treat diabetic macular edema. These advances will significantly ease the burden of living with diabetes, reduce the risk of complications, and keep people healthy while we drive toward a cure.
In the past 30 years, tremendous progress has been made in our understanding of diabetes, but answers often lead to more questions. Diabetes is an incredibly complex disease. Today, many experts believe that devising a cure will be an evolutionary process. Initially, we may start with mechanical approaches which improve glucose control and ease the burden of diabetes management through automation of insulin delivery. We may then see bio-mechanical approaches; that is, combinations of artificial pancreas technologies and drugs that begin to stimulate insulin production in people with diabetes. Eventually, we will see cures that free individuals entirely from the burden of the disease. Each of these steps will be clinically meaningful, improving the quality of life and health of those directly affected. Beyond this evolution, I want to emphasize that a cure can take many different forms and what exactly a cure is may vary among individuals at various stages of the disease. A cure for someone at risk for the disease is likely to look very different than a cure for someone who has been living with diabetes for years and has very little, if any, beta cell function. Being able to describe the stages of diabetes more accurately is a priority at JDRF. By describing each of these stages clearly, we can focus on developing meaningful interventions that will have the most impact for all people at all stages of diabetes.
This book includes an overview of ongoing research efforts for type 1 diabetes as diligently compiled by Kelly Close and her team. Below I will take the opportunity to give you a little of JDRF’s perspective on some of the exciting research developments underway.
Cures
For those people who have lived with type 1 for a long time, research on beta cell regeneration is extremely promising. We’ve learned that even people who have been living with type 1 diabetes for 50 years sometimes have a small but detectable number of beta cells in their pancreas and are capable of producing small amounts of insulin! We also know that beta cells can be re-grown from within individuals with type 1 diabetes; in fact, pregnant women with type 1 diabetes experience beta cell mass growth during pregnancy in response to the increased insulin demands on the body. This gives us hope that we may be able to develop a drug that can replicate this process and re-grow beta cells safely. Once the autoimmune attack that causes type 1 diabetes takes place, your immune system remembers beta cells for life. This speaks to the need for better immune modulating treatments that can help fend off a recurring immune attack on the regenerated beta cells.
Islet cell transplantation from human cadaver organs is another way to replace and restore beta cell function. Unfortunately, the demand for human cadaver organs far exceeds the supply. Stem cell research provides a promising approach to address the limited cell source for transplantation. As we know, islets are generated from stem cells in early development. Although nature knows how to coax stem cells into islets, coaxing stem cells outside of the body to become stable, glucose-responsive, insulin-secreting cells is tremendously difficult. Stem cells hold the potential to become any type of cell in the body, and we need to be sure they become beta cells—and only beta cells. The regulatory and commercial aspects of such a therapy are daunting, because we need to develop it in a way that is reproducible and safe.
Once beta cells are replaced, they must be kept alive long-term with the help of treatments that prevent the immune system from attacking them again. Today’s immune treatments are moderately effective, but they are also toxic and dampen the entire immune system, making individuals vulnerable to infection. The immune system, however, is very specific in its ability to attack the insulin producing beta cells in the pancreas. What this means is that if we could target just the parts of the immune system that trigger the destruction of beta cells, we may be able to preserve and possibly even restore those beta cells (perhaps with some coaxing) in individuals with type 1 diabetes, while eliminating the harsh side effects of system-wide immunosuppressive drugs.
A nearer-term answer may be the use of pig islets, or xenotransplantation. Human clinical trials of encapsulated pig islets are ongoing outside the United States, and their preliminary results to date have been promising: they show that transplanted cells seem to function in recipients, improving their glucose levels and staving off severe hypoglycemia. Additionally encapsulating pig islets or stem cell-derived beta cells creates a physical barrier that prevents the immune system from rejecting and destroying them, thus eliminating the need for chronic immunosuppression. Although the concept of encapsulation has been around for a long time, these recent studies give us hope that this may be a viable treatment in the future. So xenotransplantation and encapsulation, when married, successfully offer another promising avenue of research that JDRF is actively pursuing in our effort to develop a cure.
Treatments
If you or a loved one is living with type 1 diabetes, you can appreciate the incredible challenge of managing the disease. Sometimes getting it all right seems impossible: from eating the right foods to adjusting insulin doses, all the while trying to juggle a crazy schedule. We clearly need better tools that not only help us improve glucose control, but also help us do that in a way that reduces some of the burden of the disease. The goal of treatment therapies is to help us get to a cure healthily and happily, and to minimize the risk of developing the devastating complications of diabetes in a way that is not overly burdensome.
In the near term, the artificial pancreas will be a treatment that may provide a bridge to a cure. For many years, developing an artificial pancreas seemed to be an “easy” near-term solution. In fact, in the 1970s, the FDA approved an artificial pancreas, which is still used in some labs for research purposes. This device is called the BioStator and it works quite well; the problem is, it’s as big as a refrigerator, making it impractical for daily use outside of a hospital. We need systems that can be worn comfortably by people on a day-to-day basis. Today, we are on the cusp of the first artificial pancreas systems. We have small continuous glucose monitors that provide robust data. We have small insulin pumps. In the near term, we can clearly use the data from these continuous glucose monitors to turn down insulin delivery before someone’s glucose level gets low. Although we may not be able to prevent every low, preliminary studies show we can prevent the majority of them. We also may be able to reduce very high glucose levels by using a so-called “treat-to-range” approach. This would not be a fully automated system, but would act as a failsafe, giving insulin only when a person’s glucose was too high, or withholding insulin only when glucose dropped too low. Ultimately, technological advances will be necessary to achieve a fully automated system, but these near-term solutions may provide significant help in lowering A1cs, reducing hypoglycemia, and improving quality of life for people with diabetes and their loved ones.
Other promising treatments that could also have a major impact are under development. These include opportunities to improve hormone regulation, which could decrease the need for insulin and/or help more easily keep blood glucose levels in an optimal range. We also appreciate that even today’s rapid-acting insulin does not work nearly as fast as the insulin made in the pancreas. By identifying treatments that improve the way insulin works and by re-balancing glucagon and other hormones in people with diabetes, we may be able to dramatically improve the health of individuals. More advanced treatments, such as a glucose-responsive insulin, could also be revolutionary. Although not a cure, the once-a-day injection of insulin that comes with minimal risk of high or low blood sugar would be a transformative step in diabetes management.
Treatment therapies have the potential to significantly improve quality of life and to minimize the risk of developing complications. Unfortunately, we have not yet eliminated those complications, and this area of research should not and cannot be ignored. Although not a cure for diabetes, providing better treatments to help those individuals who have eye damage, kidney damage, nerve damage, or any other damage due to diabetes complications, must remain a priority in the research and development community.
Summary
When I think back to 1977, the year my brother was diagnosed, the world was a much different place for people with diabetes. We did not have glucose testing or insulin pumps. Complications were much more prevalent. And the prognosis for a person with type 1 diabetes was frightening. Although we haven’t seen the silver-bullet cure that we have been hoping for, we’ve come a long way. Treatments have advanced significantly, and we are on the cusp of the first artificial pancreas systems. We are also moving very quickly toward biological approaches that will ultimately, one day, allow us to finally walk away from diabetes and prevent our next generation from developing this terrible disease. The future is bright. This book details some of the very promising research that will help us get there. I commend Kelly and the diaTribe team for providing a thorough and understandable view of the state of diabetes research. These are exciting and hopeful times.
Sincerely,
Aaron Kowalski
Juvenile Diabetes Research Foundation

1.1
Where Are We Today?
When insulin was discovered in 1922, it was hailed as the “miracle drug.” The claim seemed to be justified. Until then, no sustained treatment for diabetes existed, and a diagnosis was essentially a death sentence. Insulin suddenly gave patients the opportunity to live lengthy, productive lives. Today, over 90 years later, insulin remains the principal treatment for people with type 1 diabetes, and is also an important component of therapy for many with type 2 diabetes.
But insulin does not remove diabetes; it is no cure. The goal of finding a cure continues to elude researchers, though not for lack of effort. Some of the world’s finest scientists have invested countless hours (and dollars) into finding a cure, and progress has been made. Work in animals has enabled a better understanding of the science behind the condition, and curative therapies have been used successfully in some animal models. Indeed, our understanding of the immune system and the molecular underpinnings of diabetes grows each year. As a result, the scientific assault on diabetes is able to take place on many fronts and in many different forms.
1.2
Glucose and Insulin
Our bodies need nutrients to survive. Nutrients provide fuel for our cells to grow and carry out activities that allow us to function properly. Most of our nutrients come from the food that we eat. After eating, food is broken down into three basic nutrients: carbohydrates, proteins, and fat. While all three nutrients can be used for energy, carbohydrates are the most important energy source in our body. Carbohydrates are found in high quantities in foods such as rice, pasta, fruit, potatoes, and bread, and include both simple sugars and long chains of sugars (referred to as starches). As carbohydrates pass through our stomach and into our intestines, a variety of processes break them down into simpler components, culminating in the conversion of all digestible sugars, however complex, into glucose. Glucose is transported in the bloodstream where it becomes accessible to cells throughout the body, acting as the body’s principal energy source. Although the hormone insulin has several important functions, its most crucial role involves enabling cells to remove glucose from the blood, in effect “unlocking” cells to feed on this energy source.
A hormone is defined as a “long range” chemical messenger. Hormones secreted from one part of the body travel through the bloodstream to deliver messages or signals to other distant cells. Insulin is a hormone produced by specialized cells called beta cells, which are located in the pancreas in structures commonly referred to as the islets of Langerhans (see Figure 1, below). A single islet typically contains around two thousand beta cells, and a healthy adult pancreas usually contains around one million islets (about 1-2% of the total volume of a pancreas)[1].
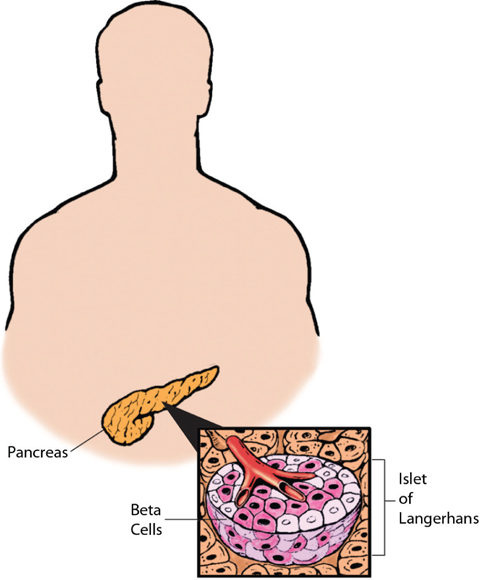
Figure 1. Anatomy of the pancreas, islets of Langerhans, and beta cells.
The pancreas is located in the abdomen. Islets of Langerhans, which contain insulin producing beta cells, are located throughout the pancreas. Islets comprise about 1-2% of the entire mass of the pancreas. In response to rising blood glucose levels, beta cells secrete insulin into the blood. A number of other cells are located in islets including alpha cells, which produce glucagon, another hormone involved in regulating blood glucose levels.
(Image courtesy of Diabetes Research Institute, University of Miami; Artist: Robert Margulies).
Throughout the day, beta cells respond to rising blood glucose levels by secreting just the right amount of insulin to maintain blood glucose levels in the normal range—approximately 70 to 140 mg/dl [2]. Insulin travels through the bloodstream and acts on cells throughout the body (such as liver, muscle, and brain cells), leading to the transport of glucose into these cells. Once the glucose enters, cells can immediately use it to satisfy energy requirements or, as in liver and muscle cells, store the glucose in a long chain of glucose molecules called glycogen. Glycogen can be broken down and released back into the bloodstream later if blood glucose levels drop, such as between meals or during sleep.
Maintaining precise control over glucose in the blood is important to ensure that cells continually receive a proper supply of energy and that the brain (which relies exclusively on glucose for energy) functions properly. If too much insulin is secreted over a period of time, blood glucose levels become too low (hypoglycemia) and cells may have insufficient access to glucose between meals. Hypoglycemia can lead a person to experience lethargy, convulsions, a coma, brain damage, or even death. On the other hand, if insufficient insulin is produced, blood glucose levels will become elevated (hyperglycemia), and not enough glucose will enter cells. Incapable of taking in glucose, muscle and liver cells begin looking elsewhere to satisfy their energy needs and start breaking down muscle and fat to form molecules called keto-acids. Keto-acids are a source of energy that can be used in the absence of insulin, and serve as an energy source of last resort when glucose is unavailable. Although keto-acids temporarily satisfy a cells’ energy needs, as keto-acid levels rise in the blood, a person can develop diabetic keto-acidosis (DKA), a dangerous short-term complication that can lead to dehydration, vomiting, confusion, swelling of the brain (cerebral edema), and coma. Over the course of many years, if uncontrolled, hyperglycemia causes serious damage to blood vessels, nerves, and the kidneys. Overall, maintaining normal blood glucose levels is extremely important for long-term health, and insulin plays a dominant role in blood glucose regulation.
1.3
The Course of Type 1 Diabetes
Diabetes develops when the body is unable to produce sufficient insulin to meet its needs. In type 1 diabetes, this insufficient production results from the destruction of beta cells by the immune system. Normally, our immune system keeps us healthy by fighting off infections, protecting us from cancer, helping our bodies repair wounds, and more. However, in people with type 1 diabetes, the immune system attacks its own beta cells just as it would attack infected or foreign cells. This type of an inappropriate immune attack on a person’s own cells is called “autoimmunity.” No one knows what starts the autoimmune process in type 1 diabetes, though an environmental trigger (possibly an infection or a toxin) has been proposed to play a role in genetically susceptible people [3, 4].
Scientists have found that blood tests can be used to detect early immune assaults on the pancreas, helping to predict who will eventually develop type 1 diabetes. These blood tests detect antibodies, a type of protein produced by the immune system that can identify and mark foreign objects for attack. Specifically, these tests identify antibodies that recognize components of a person’s own beta cells and are termed “autoantibodies.” Normally, antibodies target foreign material such as bacteria and viruses, but in the case of type 1 diabetes, self-reactive autoantibodies are associated with beta cell destruction [3].
Two main autoantibodies are often measured in clinical settings to assist in type 1 diabetes diagnosis; these glutamic acid decarboxylase (GAD) and insulinoma associated-2 (IA-2) autoantibodies. In research settings and on occasion, in clinical settings, an additional two autoantibodies, insulin (IAA) and zinc transporter (ZnT8) autoantibodies, are utilized. These autoantibodies can appear months to well beyond a decade before type 1 diabetes is diagnosed, and the risk for type 1 diabetes has been shown to increase with the number of autoantibodies detected. Yet, the role of autoantibodies in the development and progression of type 1 diabetes remains unclear, because we know that not everyone who expresses one or more autoantibodies will develop type 1 diabetes [3, 5].That said, according to the late Dr. George Eisenbarth (Barbara Davis Center for Childhood Diabetes, Denver, CO), most people who have two or more autoantibodies generally progress to diabetes.
The onset of type 1 diabetes can occur suddenly and is characterized by recurring episodes of high blood glucose (hyperglycemia). The destruction of beta cells begins months to years or even decades before diagnosis. In fact, people have typically lost a significant proportion (~60-90%) of their beta cell mass at the time of diagnosis, and many of the beta cells that remain are not working normally [6–8]. As significant beta cell mass is lost, hyperglycemia develops because little to no insulin is being produced. Hyperglycemia can cause a number of different symptoms including extreme thirst, frequent urination, sudden vision changes, increased appetite, sudden weight loss, drowsiness, stupor, rapid breathing, and diabetic ketoacidosis (see above).
For several months after the onset of type 1 diabetes and starting of insulin therapy (see below), some people experience a period of improved blood glucose control and lower insulin requirements. This period, referred to as the “honeymoon,” occurs when beta cells undergo a temporary recovery, regaining the ability to produce insulin so that the body’s external insulin demands are temporarily reduced [9]. As with most honeymoons, however, the one for type 1 diabetes inevitably ends as beta cell loss (driven by ongoing beta cell autoimmunity) continues, and insulin production resumes its progressive decline. Eventually, nearly every person with type 1 diabetes is left with few, if any, remaining beta cells and certainly not enough to make sufficient insulin. This stage is known as established diabetes.
Because people with type 1 diabetes can no longer produce enough insulin by themselves, administration of insulin becomes necessary. Insulin therapy allows for uptake of glucose into cells and the lowering of blood glucose levels. But as any person with type 1 diabetes can tell you, insulin therapy creates significant lifestyle barriers and does not completely normalize glucose control. Most people on insulin therapy still experience occasional or even frequent blood glucose highs and lows. Because of these limitations, no one would characterize insulin therapy as a “cure.”
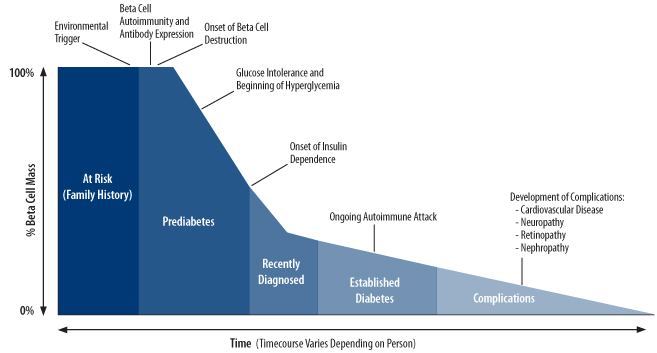
Figure 2. The progression and stages of type 1 diabetes (Adapted from Atkinson and Eisenbarth, Lancet 2001).
In Summary…
• Glucose is an important source of energy for our bodies, and glucose levels in the blood must be regulated properly to ensure correct brain function and to avoid long-term organ damage.
• Insulin is a hormone produced by beta cells in the pancreas that allows cells to use or store glucose, thereby lowering blood glucose levels.
• In type 1 diabetes, the body produces little or no insulin because beta cells are inappropriately destroyed by the body’s immune system.
• To make up for insulin deficiency, insulin administration is required. Insulin therapy, however, is not a “cure.” To date, type 1 diabetes cannot be “cured.”
1.4
Targeting a Cure
Targeting a cure for type 1 diabetes is now the goal of many ongoing research efforts. Broadly, these approaches attempt to either restore or maintain normal glucose control in people with type 1 diabetes or those at risk of developing the disease. With improvements in glucose control, both the day-to-day burden of diabetes management and the risk for developing long-term complications could be reduced. The specific goals of a cure and the strategies used to achieve these goals differ, however, depending on the stage of disease (see Figure 3).
For people at risk for developing type 1 diabetes, such as those with a family history, the ultimate goal is to prevent diabetes from starting in the first place. In some people, this will mean preventing the immune system from attacking beta cells (primary prevention); in others, the immune system may have already begun destroying beta cells (a condition sometimes referred to as prediabetes1), and preventive therapies will try and stop beta cell destruction before insulin therapy becomes necessary (secondary prevention).
For people with recently diagnosed type 1 diabetes, independence from insulin therapy and normal glucose control could be achieved if the immune system’s ability to properly recognize beta cells was restored and a method for regenerating beta cells in the pancreas was developed.
For those with established diabetes, successful cure-targeted strategies must emphasize restoration of beta cell function. Introducing and/or creating brand new beta cells would be a first step towards this, but developing methods to protect and preserve these cells from the autoimmune response will also play a critical role.
Therapies are also being developed to prevent, reverse, or halt the development of diabetes-related complications. These complications include neuropathy (nerve damage), retinopathy (eye disease), nephropathy (kidney disease), and heart disease. Treating complications is an interesting topic, which we hope to cover in a future report.
There are many different approaches that researchers are taking to achieve a cure for type 1 diabetes. Over the last few years, notable progress has been made in both the clinic and lab, and we are excited about the creativity and the strong rate of progress. However, at the time of writing (fall 2012), there is no full cure close to being approved. The types of solutions described above are years and most likely decades away. But researchers are following many exciting and fruitful paths. We will be describing these approaches in the rest of this report to give you a clearer picture of how we are targeting a cure.
In Summary…
• Cure-targeted approaches attempt to restore or maintain normal glucose control in order to ease the burden of daily diabetes management and to reduce the risk for long-term complications.
• There are two main aspects to a cure—(1) stopping the autoimmune response which causes diabetes in the first place, and (2) replacing or renewing beta cells as necessary.
• The strategies used to achieve these objectives differ depending on stage of disease:
» For people at risk for developing type 1 diabetes, the goal is to prevent the immune system from attacking beta cells in the first place; if the immune system has already begun to damage beta cells (prediabetes), the goal is to stop this attack before insulin therapy becomes necessary.
» For people with recently diagnosed type 1 diabetes, the goal is to preserve remaining beta cells by halting ongoing autoimmune attack and by lowering beta cell stress. Therapies that generate new beta cells may also help in removing the need for insulin therapy in this group.
» For people with established type 1 diabetes, the goal is to introduce or create new beta cells while simultaneously protecting these cells from immune destruction.
1.5
Cure-Targeted Therapies
We have divided current efforts to find a cure into four main approaches, which are the focus of the remaining sections of this report (see Figure 3).
Immune Therapeutics
The first group of cure-targeted therapies, immune therapeutics, aims to stop the immune system from destroying beta cells, which could potentially halt disease progression and allow for beta cell recovery. While a number of strategies are being pursued, these therapies generally either try to disrupt the pieces of the immune system involved in beta cell destruction or restore the immune system’s ability to recognize beta cells in a non-harmful manner.
Because these therapies address the underlying cause of type 1 diabetes, they could one day be used to prevent the initiation of beta cell autoimmunity in people at risk for developing type 1 diabetes or halt the progression of beta cell destruction in those with prediabetes even before they require insulin therapy. Immune therapies could also promote beta cell preservation in individuals recently diagnosed with type 1 diabetes and could prevent the destruction of newly generated beta cells in individuals with recently diagnosed or established disease. Used in this way, immune therapies might aid in the restoration of beta cell function and normal glucose control.
Islet and Pancreas Transplantation
Islet and pancreas transplantation, the second group of therapies, attempts to replace lost beta cells directly by transplanting either a whole functioning pancreas or new functioning islets into the body.
Both islet and pancreas transplantation techniques are currently available and in some people with established type 1 diabetes, they have been effective in restoring insulin secretion and insulin independence (at least temporarily) as well as in reducing hypoglycemia. This sounds quite a lot like a cure.
Unfortunately, there are many limitations of current transplant technology. Some of these limitations include a shortage of transplantable human islets and pancreases, surgical risks (for pancreas transplants), the need for strong, often toxic broad immunosuppressive therapies to avoid transplant rejection, and high transplant costs. That’s why transplants are currently restricted in number, and go only to people with extremely uncontrollable hypoglycemia, to those who have great difficulty managing the disease, and to those who already take immunosuppressive drugs (e.g. because they have had a kidney transplant).
Current research efforts are attempting to provide alternative sources of islets for transplantation as well as ways to protect the transplanted islets from rejection without the need for broad immune suppression (which brings many side effects of its own, including the risk of cancer).
Because beta cell replacement is only beneficial once people have lost a significant portion of their natural beta cells, transplantation strategies are not suitable for people at high risk for developing diabetes or with prediabetes, nor are they likely to be the optimal strategy shortly after diagnosis.
Beta Cell Regeneration and Survival
A third therapy group uses beta cell regeneration and survival therapies to encourage the growth of new beta cells or to preserve existing ones.
Using regeneration agents, new beta cells could be grown inside or outside the body, so that ultimately any type 1 patient’s beta cells could be restored, removing the need for insulin therapy. However, to reach a lasting cure, we would need to develop further strategies to protect these new cells from autoimmune destruction.
Beta cell survival agents could one day help delay or prevent the onset of type 1 diabetes in people with prediabetes, slow or stop progression of beta cell death in those with recent onset type 1 diabetes, and promote the survival of beta cells that are transplanted or regenerated.
The Artificial Pancreas
Lastly, the artificial pancreas (AP) replaces the insulin secreting and glucose regulatory functions of a normal pancreas. The key components of the AP are an insulin pump, a continuous glucose monitor, and a control algorithm—all of which are available today. Rapid progress is being made towards creating an AP that provides perfect glucose control, but a fully functioning AP has not yet been achieved. One day, an AP that administers other hormones involved in the regulation of glucose levels in addition to insulin (such as glucagon) may be developed to provide even greater blood glucose control.
While the AP may not target the underlying cause of type 1 diabetes or attempt to replace lost beta cells, some people still consider the AP to be a potential cure because, if optimized, an AP could allow people to normalize their blood glucose. Improvements in glucose control made possible by an AP may promote beta cell survival shortly after diagnosis by lowering beta cell stress and could also be useful immediately after transplantation or regeneration by helping preserve new beta cells until they fully recover or become functional.
If nothing else, the AP will help maintain a good A1c and greatly reduce long-term complications while progress to a more fundamental cure is being made. And on a practical note, an AP could eventually eliminate the majority of patient involvement in glucose control, greatly improving the daily management of diabetes.
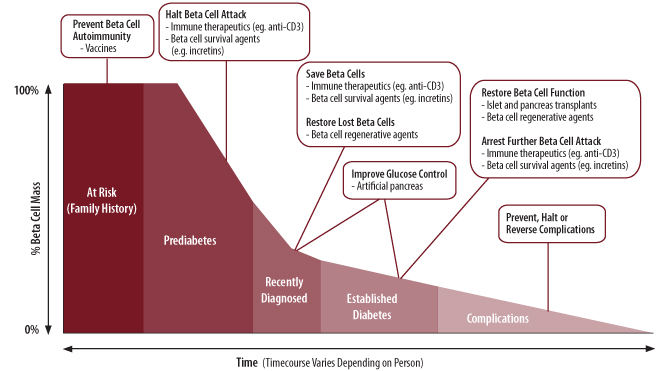
Figure 3. Cure targeted strategies and therapies for type 1 diabetes broken down by stage of disease (Adapted from image provided courtesy of JDRF.)
In Summary…
• Immune therapeutics aim to stop the immune system from attacking beta cells; these therapies may be useful at all stages of disease, including in the prevention of type 1 diabetes.
• Islet and pancreas transplantations replace lost beta cells with beta cells from an outside source. Although a few advances may eventually improve upon the limitations of these therapies, they cannot prevent the development of type 1 diabetes, nor will they likely be the optimal therapy for people who have been recently diagnosed. Their use may become more widespread when new sources of beta cells and/or improved ways to protect transplanted beta cells are established.
• Beta cell regeneration therapies promote the growth of new beta cells, while survival therapies maintain beta cell function. Regeneration therapies may one day allow any person with insufficient beta cells to control blood glucose. Survival therapies have the potential to benefit people at all stages of type 1 diabetes, including people with prediabetes, by preserving the function of both natural and transplanted beta cells.
• The artificial pancreas (AP) uses an insulin pump, continuous glucose monitor, and a control algorithm to mimic and replace the glucose sensing and insulin secreting functions of the pancreas; once optimized, the AP could potentially help anyone with type 1 diabetes achieve glucose targets.
1.6
The Drug Development Process
To grasp the efforts underway to develop a cure, it is helpful to have an understanding of the steps involved in creating and bringing a new drug to market. The drug development process is a long and expensive one, normally taking over a decade and requiring close to a billion dollars. The process is also full of risk and uncertainty: only 5 in every 5,000 drugs that begin preclinical testing (studies in test tubes and animals) will ever make it to human trials, and out of these five, only one is likely to ever be approved for human use.
Preclinical Studies
Drug development usually begins in the lab where researchers try to identify a “target”– something in the body that if acted on will have a positive effect on a particular disease. Next, the pharmaceutical industry works to create a drug (usually a compound that is made by chemists) that will act on this target. Before this new drug can be evaluated in people, it must first be tested for safety and effectiveness in test tubes and in animals (such as mice, primates, and dogs). These non-human studies are termed preclinical studies.
The primary goal of preclinical studies is to establish that the drug is both effective enough to warrant human study and safe enough so that human volunteers won’t be putting themselves in serious danger by using the drug.
Human Studies
In the US, human clinical trials for a particular drug are initiated in volunteers after all lab and animal work has been completed and data from this work has been presented to the US Food and Drug Administration (FDA) for assessment. Clinical trials are designed to assess the safety, efficacy, and action of a drug in human subjects. There are three phases of human clinical trials (phase 1, phase 2, and phase 3), and each phase asks a different question about the drug.
Most clinical trials are randomized, which means participants are randomly assigned to receive the test therapy, a comparator therapy, or a placebo, which is an inactive pill, injection, or infusion. Additionally, trials may also be blinded. In blinded trials, participants are not made aware of which therapy they are receiving. Randomization and blinding allows researchers to eliminate any bias from the trial as as well to make the groups as similar as possible other than the treatment they are receiving. With this kind of design, the efficacy and safety of a test therapy can be most accurately assessed. When a trial’s results are reported, a therapy’s efficacy is often compared to that of a placebo.
A phase 1 trial is the first stage of testing of a drug in people. There are usually two of these trials and they generally involve small groups of healthy people without the disease (usually 20-80 individuals). They explore what dose of a drug should be given by looking at the safety of different doses. Phase 2 trials typically have larger groups of participants (100-300 individuals), and the people in these trials usually have the disease the drug is intended to treat. They look to see how well the drug works for its specified purpose, and during this phase, data are still collected about the drug’s safety. This is the first time the company sees whether the drug works as planned, but also the first time when a drug can fail based on a lack of effectiveness. This type of trial examines several potential doses of the drug within the range identified in phase 1 to determine at which dose levels the drug is efficacious. Phase 3 trials are usually much larger than phase 1 and phase 2 trials–typically, several thousand patients will be enrolled. Phase 3 trials help to provide large-scale efficacy and side effect data so that researchers can understand how best to use the drug. They help to make sure that the drug works in a range of different patients, and that it doesn’t cause rare side effects that might have been missed in smaller trials. These trials are large, expensive, and are the last hurdle before sending the drug’s information to the FDA for approval.
Submission and Approval
Upon completion of phase 3 trials, the company must submit an application for approval to the FDA. The application typically contains information from the entire development program of the compound (preclinical and clinical), and also includes information on plans for manufacturing and marketing the drug to consumers. The company then waits for the FDA to reach a decision on whether the drug is marketable. The review process may take a year or longer, and additional studies are sometimes required to further convince the FDA of the safety or efficacy of a candidate drug. If a drug is approved, the company may begin to sell the drug and advertise to consumers and health care professionals in the US. Notably, regulatory processes must take place in every single country or region in which a company wants to sell a drug.
In Summary…
• The drug development process is long and expensive, normally taking over a decade and requiring close to a billion dollars. Only 1 in every 5,000 drugs that begins preclinical testing (studies in test tubes and animals) will likely be approved for human use.
• Studies in animals and test tubes (preclinical studies) determine whether the drug is safe and effective enough to begin human trials.
• There are three phases of human studies:
» Phase 1 trials represent the first stage of testing of a drug in people. They explore what dose of a drug should be given by looking at the safety of different doses in healthy people without the disease the drug treats.
» Phase 2 trials are typically larger than phase 1 trials and explore how well the drug works in people with the disease it treats; data are still collected about the drug’s safety during this phase.
» Phase 3 trials are the last stage of human studies; these trials help provide efficacy and side effect data on a larger population and over a longer period of time so that researchers can understand how best to use the drug.
• In the US, after completing phase 3 studies, the company developing a drug must submit an application to the FDA for drug approval. The agency usually takes at least a year to decide on whether to approve the drug. If approval is granted, the company may begin to sell the drug and advertise to consumers and health care professionals.
1.7
Where To Learn More
About Type 1 Diabetes
Type 1—The American Diabetes Association
Information for Study Participants—Type 1 Diabetes TrialNet
About Drug Development
Overview of Clinical Trials—CenterWatch
Clinical Trials Registry—ClinicalTrials.gov
JDRF Type 1 Clinical Trials Connection

2.1
Background
Immune therapeutics are drugs for type 1 diabetes that attempt to halt the progression of the disease by modifying or blocking destructive immune responses against beta cells. If administered early enough in the course of the disease, such as during prediabetes when the immune response against the pancreas is just beginning, or even in people at high risk for developing type 1 diabetes, immune therapies could theoretically prevent the onset of disease altogether. Immune therapies will likely have the greatest effect (providing maximal beta cell preservation) for people already diagnosed with type 1 diabetes if given shortly after diagnosis. If used later on, when more beta cells have been lost, immune therapies may still be beneficial, provided that enough functional beta cells remain. Lastly, in people with recently diagnosed or established type 1 diabetes, by preventing further beta cell destruction by the immune system, immune therapies could have an important role in maintaining the survival of newly regenerated (see Chapter 4) or transplanted beta cells (see Chapter 3).
A number of strategies are being explored to modify the inappropriate immune attack on beta cells that underlies all stages of type 1 diabetes. Several companies and organizations are even investigating immune therapeutics in human trials. While some of these treatments have shown indications of beta cell preserving effects, no drug has yet been demonstrated to prevent or permanently halt type 1 diabetes progression in humans. One possible explanation for this relatively slow progress is that scientists still lack a clear understanding of the incredibly complex immune processes involved in type 1 diabetes, making it difficult to identify and target the disease’s most important immune system components. Additionally, because each of the different immune therapeutics under investigation targets different aspects of the immune system, a combination of these therapies may turn out to have greater efficacy than any one therapy alone. To date, very few trials have specifically addressed the potential value of combination therapy, but this approach has shown promise in animals [10] and many experts believe this is the future of immune therapies.
Nonetheless, progress with immune therapeutics has been made, and furthermore, our understanding of the immune system continues to grow. Because they address the underlying cause of type 1 diabetes, immune therapeutics remain attractive and will likely be an important component of future cure-targeted approaches.
In Summary…
• Immune therapeutics aim to address the underlying cause of type 1 diabetes by altering the immune pathways responsible for beta cell destruction.
• While a number of immune therapies are being investigated in human studies and several have shown initial promise, no candidate has demonstrated a conclusive ability to prevent the initiation of diabetes or durably alter ongoing destruction of beta cells.
2.2
The Immune System and Type 1 Diabetes
Every day, our bodies are exposed to foreign material (such as bacteria and viruses) that can make us ill. Our immune system protects our bodies from these potential threats through the elimination of damaged, infected, or malfunctioning cells. The immune system can be divided into two intertwined arms: the innate immune system and the adaptive immune system. The innate immune system is our first line of defense against foreign invaders. Although specifics about this system will not be discussed here, in general terms, through its ability to recognize a variety of molecules typically located on the surfaces of infectious organisms, the innate immune system is able to mount an immediate attack on foreign material. The adaptive immune system, on the other hand, plays a large role in the elimination of invading organisms that have evaded destruction by the innate immune system and is the major target of immune therapeutics for type 1 diabetes. The adaptive immune system is highly specific: cells that make up this system recognize and remember particular foreign agents. This ability allows the adaptive immune system to mount a powerful attack against certain viruses or bacteria, while ignoring others. It also gives the adaptive immune system a form of memory, allowing the body to quickly eliminate an invading organism if it is encountered again. This memory is responsible for lifelong immunity to certain infections after an initial illness, such as chicken pox as well as all childhood vaccines.
B cells and T cells are the cells of the adaptive immune system that have the ability to target and remember specific foreign material. They are thought to play a central role in the development of type 1 diabetes. B cells and T cells contain molecules on their surface that recognize small parts of a specific foreign agent; the specific foreign agent targeted by T cells or B cells is referred to as an “antigen.” There are very many variants of B cells and T cells, and most individual B cells and T cells produced by the body recognize a different antigen from other such cells. In other words, there are B and T cells specialized just for recognizing a particular antigen.
When the appropriate B cells and T cells come into contact with their specific antigen, they become activated and begin performing a variety of functions that set off further immune responses (see Figure 4). T cells function both as coordinators of immune responses and as potent inducers of cell death. Most of the immune coordinating function of T cells is carried out by a subset of T cells referred to as “helper” T cells or “CD4” T cells. CD4 T cells modify the function of many other immune cells including B cells and other T cells. Much of this function is carried out through the secretion of chemical messengers called cytokines, which can directly influence the activity of many other cells. A specific type of CD4 T cell, called a “regulatory” T cell, works to suppress the activation of the immune system. Regulatory T cells play a critical role in helping the immune system distinguish between a body’s healthy cells and foreign material, protecting normal cells from inappropriate destruction. Because type 1 diabetes is caused by the inappropriate destruction of normal cells (the beta cells), some scientists believe that defects in regulatory T cells may be important in initiating type 1 diabetes [11]. In addition to coordinating immunity, some T cells have the ability to kill infected or damaged cells; these T cells, referred to as “CD8” or killer T cells, are extremely important in immunity to viruses. Because type 1 diabetes ultimately involves the death of beta cells, CD8 T cells are also hypothesized to play a central role in type 1 diabetes [12, 13].
B cells function primarily as a source of antibody production. Antibodies are secreted proteins that B cells produce after encountering foreign material; antibodies circulate throughout the body and bind to foreign material, serving as “flags” to direct further immune attack. B cells can also produce some cytokines and can have a role in activating some T cells [14]. Although B cells produce the autoantibodies associated with the development of type 1 diabetes (see Section 1.3), these autoantibodies are not thought to play a direct role in the autoimmune attack against beta cells [15–17]. Rather, in type 1 diabetes, B cells likely function as facilitators of T cell activation, and through cytokine production, influence ongoing responses against beta cells [14].
Most immune responses begin when phagocytes, cells specialized for eating small particles, or B cells swallow foreign material, digest it, and then present antigens from this material to T cells (see Figure 4, below). This antigen presenting step is critical for proper immune responses to viruses and bacteria, and in type 1 diabetes, it may play an important role in the initiation of beta cell destruction. In humans, as part of normal cell death and turnover, beta cell particles are regularly digested by phagocytes and B cells and presented to T cells. Usually, in reaction to beta cell antigens, T cells activate immune regulatory processes that suppress immune attack against beta cells. In type 1 diabetes, however, for reasons that are not well understood, these same regulatory responses are not triggered (or triggered less strongly), and destructive immune responses are activated instead.
Given the alterations of normal immune function that occur in type 1 diabetes, the major goals of immune therapeutics are to disrupt the cells that directly kill beta cells, such as killer T cells, and to restore the proper regulation of immune responses to beta cells so that they are again recognized as part of the body (self) rather than as potential foreign invaders.
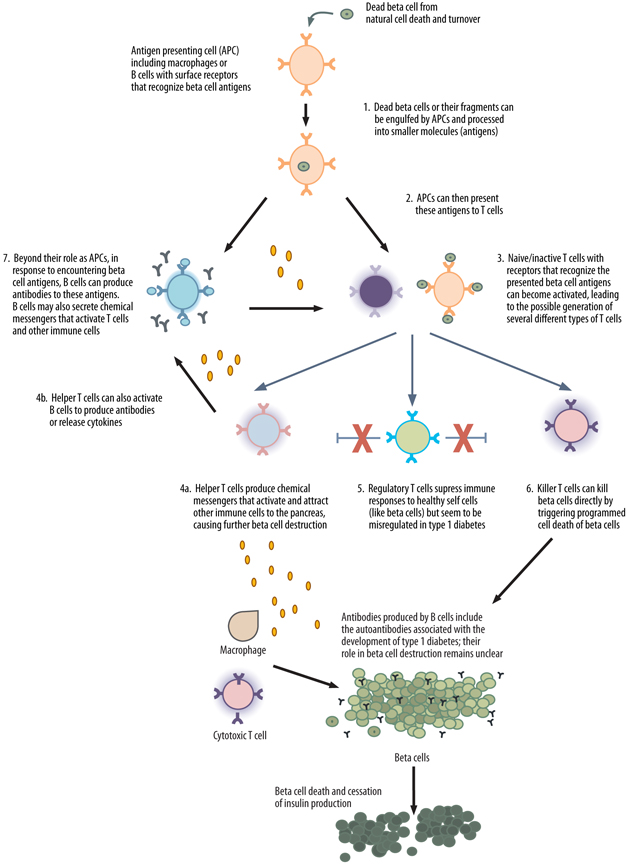
Figure 4. A proposed model of the autoimmune attack on beta cells and the development of type 1 diabetes. 1) Fragments of beta cells that result from natural cell death may be engulfed by a number of different cell types called antigen presenting cells (APCs). B cells are one type of APC. Only APCs with receptors that can recognize small beta cell particles (called antigens) can engulf beta cells. 2) Once inside the APC, the fragment is broken down further into smaller pieces (antigens) that are then displayed on the APC’s surface. 3) As these APCs circulate in the body, they may encounter T cells. If the receptor on the T cell recognizes the antigen presented by the APC, the T cell can become activated, potentially resulting in the production of helper T cells, killer T cells, or regulatory T cells. 4) Helper T cells produce chemical messengers that activate and attract other immune cells (such as killer T cells, macrophages, and B cells) to the pancreas, coordinating further beta cell destruction. 5) Regulatory T cells also produce chemical messengers, but these messengers suppress immune responses (such as by helper T cells and killer T cells) to healthy self cells (like beta cells); these T cells may be dysregulated in people who develop type 1 diabetes. 6) Killer T cells can induce the direct death of beta cells in type 1 diabetes. 7) In addition to their role as APCs, B cells can become activated in response to beta cell antigens and produce antibodies and chemical messengers called cytokines. Although B cells produce the autoantibodies associated with the development of type 1 diabetes, these autoantibodies are generally not thought to play a direct role in the autoimmune attack against beta cells. Additionally, cytokines released by B cells can cause the activation of other immune cells, such as T cells.
In Summary…
• The immune system is largely responsible for fighting off foreign invaders, such as bacteria and viruses. It’s really complex.
• B cells and T cells are important cells of the immune system and are thought to be involved in the development of type 1 diabetes.
• The major goals of immune therapies for type 1 diabetes are to disrupt the immune cells that directly kill beta cells and to restore the ability of immune cells to recognize beta cells as normal components of the body.
2.3
Diabetes Vaccines
Who: 
When: 
Bottom Line: 
Over the past century, vaccines have been instrumental in preventing and nearly eradicating a number of diseases, including diphtheria, measles, mumps, smallpox, and polio. The goal of vaccination is to alter immune responses against specific proteins, carbohydrates, or even whole microorganisms such as bacteria and viruses. In their traditional form, vaccines are designed to build the immune system’s familiarity with potentially dangerous infectious material prior to actual infection. This familiarity will lead to quick eradication of the infectious material if a person is exposed to it again. This is often done by exposing the immune system to a fragment or a less potent or dead version of the dangerous infectious agent. In the case of a virus or bacteria, once a person’s immune system has been exposed the first time, the next time it sees that organism, it usually destroys it quickly enough to prevent disease. This is the “protection” that vaccines provide.
The idea behind type 1 diabetes vaccination is actually the inverse of traditional vaccination (see Figure 5, below). The idea is to create tolerance, much like with allergy shots. In diabetes vaccination, certain beta cell and islet proteins targeted by the immune system in type 1 diabetes are administered to the body to prevent harmful immune responses against those proteins and, therefore beta cells, in the future. To inhibit these harmful immune responses, type 1 diabetes vaccines aim to both inactivate the T cells responsible for beta cell attack and to restore the ability of the immune system to properly recognize and respond to beta cells as normal self cells. At least in theory, the restoration of proper immune regulation will stop ongoing beta cell destruction or even prevent this destruction from occurring in the first place. So a working vaccine would be a cure, even if people might need to take booster doses over time.
In contrast to other immune therapies for type 1 diabetes, a unique advantage of vaccines is that they attempt to modify or inactivate just those immune cells and processes involved in beta cell attack, reducing the possibility of side effects caused by the immune system being altered in a more general way.

Figure 5. Comparison of traditional vaccination with vaccination for type 1 diabetes.
A vaccine aimed at prevention will likely be most effective if given early in the course of type 1 diabetes development or even before a person develops antibodies (for family members at risk, for example) [18]. However, because screening for these antibodies today is typically reserved for first or second-degree relatives of people with diabetes, few at-risk people are currently identified at an early enough stage for preventive measures to be useful. According to Dr. Richard Insel, MD (Chief Scientific Officer, JDRF, New York, NY), universal childhood screening for the presence of autoantibodies would be one way around this limitation. However, because current antibody assays are relatively expensive and don’t predict all cases of diabetes, such population-wide screening is not cost-effective. Dr. Insel alternatively suggests that if diabetes vaccines were demonstrated to be safe, the use of immunization for universal childhood primary prevention of type 1 diabetes (before the onset of beta cell autoimmunity) may be increasingly justified given the rising incidence of childhood type 1 diabetes worldwide. Aside from prevention, vaccines may also be used to reset the immune system and stop autoimmune attack at type 1 diabetes onset, as is being explored in the current vaccine clinical trials, or even later in the course of the disease.
In animals, several vaccine strategies have been effective at preventing future immune responses against beta cells. So far, no such vaccines have been proven to work in humans with type 1 diabetes. Two of the more common proteins known to be targeted by the immune system in type 1 diabetes are the insulin and proinsulin proteins and GAD65; vaccines under development target these proteins (discussed below). Because our experience with type 1 diabetes vaccines is limited, we still lack a clear understanding of their mechanisms of action and side effects. Theoretically, type 1 diabetes vaccines should be safe in part because they attempt to modify just those aspects of the immune system involved in beta cell attack, and so far, in clinical trials, they have been well tolerated. Rare, mild side effects have been seen in early trials (headache, upper respiratory infection), but whether or not these were actually linked to vaccination is unclear.
In Summary…
• Diabetes vaccines attempt to modify or inactivate the immune system’s response to proteins in the pancreas that are thought to trigger the autoimmune attack against beta cells.
• By restoring the ability of the immune system to properly recognize these beta cell proteins, vaccines may prevent or stop beta cell destruction.
• Vaccines may one day be used as both preventative therapies for people at risk for type 1 diabetes and as therapies for people who are insulin independent, but show evidence of ongoing immune responses against beta cells (prediabetes). In conjunction with beta cell regeneration therapies in people with recent onset or established disease, vaccines may restore beta cell function and remove the need for insulin therapy.
Glutamic Acid Decarboxylase (GAD65) Immunization
Glutamic acid decarboxylase (GAD65) is a protein found in pancreatic islet cells [19, 20]. People with type 1 diabetes typically have antibodies to GAD65, and the measurement of these antibodies is often used in diagnosis. Additionally, identification of GAD65 antibodies in family members of people with type 1 diabetes indicates an increased risk for developing type 1 diabetes in the future [21].
GAD65 vaccination is under development as a method for potentially halting immune attack against pancreatic beta cells [22]. The GAD65 vaccine, Diamyd, is composed of the GAD65 protein and alum, a substance that helps deliver the protein to the immune system without triggering a destructive immune response. It is made by Diamyd Medical. Two phase 2 trials and one phase 3 trial for the compound have been completed in people with recently diagnosed type 1 diabetes.
In one phase 2 trial that included 70 children and adolescents diagnosed with type 1 diabetes in the past 18 months, Diamyd was shown to help preserve some natural beta cell insulin secretion over a 30-month period. The preservation of insulin secretion was determined by the measurement of higher levels of stimulated C-peptide (a fragment of natural insulin, which can be used as a measure of insulin secreted by the pancreas) in people who received the vaccine. This study also showed that the effectiveness of the vaccine was decreased in people with a longer duration of type 1 diabetes [23]. Unfortunately, the vaccine was not shown to decrease insulin therapy requirements or A1c, effects that are expected if beta cells are preserved and their function is meaningfully improved. Mild adverse events (side effects) were reported in the trial, but only two cases of hypoglycemia were deemed as possibly related to the treatment.
Despite these positive phase 2 findings, Diamyd announced in May 2011 that a phase 3 trial conducted in Europe failed to show indications of beta cell preservation at 15 months as measured by changes in C-peptide levels. The study had enrolled approximately 334 people ages 10-20 that had been diagnosed with type 1 diabetes within three months of entering the study. Although a small effect on C-peptide levels was observed, this improvement was not statistically significant. C-peptide levels were preserved in certain groups of people, but further studies in these groups are needed before any conclusions can be drawn. Diamyd, however, did appear to be well tolerated as a similar number of adverse events were observed in both the vaccine treated and control groups [24].
In June 2011, it was reported that a phase 2 GAD65 vaccine trial sponsored by TrialNet and conducted in the US and Canada had also failed to cause improvements in C-peptide levels at one year as compared to placebo. The trial enrolled 145 people ages 3-45 who were either given three separate injections of vaccine, two injections of vaccine and one of placebo, or three injections of placebo. At one year, no differences in any study measurements of beta cell preservation were observed between the three groups and participants in all groups lost C-peptide at a similar rate over the course of the year [25].
In the wake of the results from the European phase 3 and TrialNet phase 2 trial, Diamyd Medical announced in June 2011 that it had suspended dosing in its phase 3 US trial, DiaPrevent, and in the fall of 2012 it announced that it was limiting spending on the vaccine for the next three years (due to Diamyd Medical’s challenges with its program and funding, we doubt it will successfully move forward with Diamyd). Though the drug had a good safety profile, the company felt it was unlikely to be effective with the particular dose and dosing schedule being tested. However, a researcher-initiated pilot prevention study with Diamyd is still underway. This study enrolled 50 children as young as age 4 who are at risk for developing type 1 diabetes (as determined by their expression of GAD65 antibodies and at least one additional type 1 diabetes associated antibody). The estimated study completion date is January 2017.
“Kelly’s Take”

Of all the immune therapeutics, vaccines appear to hold the most promise for the prevention and treatment of type 1 diabetes. As someone with type 1 diabetes, I know that the risk my children have also developing it is higher than average (slightly!), and the prospect of providing them with a preventive vaccine, like that for chicken pox or measles, is truly exciting.
Like others, we had placed great hopes on the Diamyd vaccine based on the outcomes of initial trials. The recently reported phase 2 and 3 results for the vaccine were a significant disappointment; however we hope these trials can provide some hint as to how to make the GAD65 vaccination more effective.
Insulin Immunization
The insulin hormone (itself a protein) is found in high concentrations within islets. Evidence suggests that insulin may play an important role in beta cell destruction by the immune system. Insulin vaccination is conceptually analogous to vaccination with GAD65. The insulin vaccines currently under investigation include insulin, but do not include alum (a component of the GAD65 vaccine previously discussed). Although successful preclinical work with insulin vaccination has been accomplished in mice, the relevance of these experiments to people with diabetes remains uncertain, and the results of early clinical trials will be true gauges of these vaccines’ potential.
A major area of study in insulin vaccination has been determining the optimal method for insulin delivery. It’s unclear whether vaccine administration through the nose (intranasal insulin) or mouth (oral insulin) is more effective. So far, oral insulin has not been clearly demonstrated to prevent type 1 diabetes. In the Diabetes Prevention Trial-1 (DPT-1), neither oral nor systemic insulin was shown to delay the onset of type 1 diabetes better than placebo among people who had first- or second-degree relatives already diagnosed with type 1 diabetes. However, in this trial, oral insulin was associated with a four-year delay in disease onset in people who had very high levels of insulin autoantibodies (IAA) at the start of treatment [26, 27]. A benefit of treatment was still seen in this group after a median 9.1 year follow-up. While unfortunately, after therapy was stopped, rates of diabetes development in this group were the same whether participants had previously received oral insulin or placebo [28], the delay was nonetheless still welcome and may bode well for future prevention efforts. The TrialNet Oral Insulin Prevention Trial, which focuses on subjects similar to those in the DPT-1 trial, was designed to confirm these preliminary results; this trial is currently recruiting at centers around the US, and is expected to finish in 2014. JDRF is also recruiting for its own small oral insulin study (called the Pre-Point Study) which will examine whether dose influences the effectiveness with which oral insulin prevents type 1 diabetes in children 18 months to 7 years of age with at least two first-degree relatives or an identical twin with type 1 diabetes.
Similarly, studies conducted thus far have not shown the ability of oral insulin administration to preserve remaining beta cell function in people newly diagnosed with type 1 diabetes [26, 27].
Administration of insulin through the nose (intranasal) is being investigated by Dr. Leonard Harrison MD, DSc (Walther and Eliza Hall Institute of Medical Research, Victoria, Australia). Dr. Harrison and his colleagues studied the efficacy of intranasal insulin in a three-year trial of 52 adults with recent-onset type 1 diabetes who did not require insulin injections at time of enrollment. Unfortunately, the study found no significant difference in progression to insulin treatment between the intranasal arm and placebo. However, the insulin antibody response to insulin injections was significantly reduced in those who had received nasal insulin. Thus, nasal insulin may be inducing immune tolerance to insulin and it could be a method for preventing type 1 diabetes in at-risk people [25]. Intranasal insulin has also been studied through the Intranasal Insulin Trial I (INIT I) trial. INIT I found that intranasal immunization was safe and associated with a modest decrease in immune responses to insulin, suggesting that intranasal insulin can modify destructive immune responses to insulin. A study conducted in 52 adults with recently diagnosed diabetes provided further evidence for the vaccine’s immune modulating effects when it showed that nasal insulin vaccination significantly lowered levels of insulin autoantibodies in those who had been treated [25]. The treatment didn’t, however, slow loss of beta cell function. Now, the Intranasal Insulin Trial II (INIT II) is investigating whether nasal delivery of insulin can actually delay or prevent type 1 diabetes. The trial, being supported by the Diabetes Vaccine Development Centre (DVDC) in Australia, is enrolling healthy at-risk relatives of people with type 1 diabetes ages 4 to 30 who have detectable levels of two or more diabetes-associated antibodies [18]. Conducted in Australia and New Zealand, the primary outcome of the study is whether or not diabetes has been diagnosed after five years, and the trial is expected to finish in late 2016. The ability of intranasal insulin administration to prevent type 1 diabetes was also recently examined in a Finnish study enrolling children at risk of developing the disease. Unlike INIT I, however, this study found that intranasal insulin had no effect on diabetes prevention and may have even accelerated the development of type 1 diabetes in children expressing three or four diabetes associated antibodies. It should be noted though, that a lower dose of insulin than that used in INIT II was given in this trial and a substantial portion of the children in the trial may already have had impaired beta cell function.
In terms of what is being explored commercially, Bayhill Therapeutics’ insulin vaccine, named BHT-3021, is an injectable vaccine that delivers a ring of DNA that ‘encodes’ proinsulin, a building block of insulin. When this DNA enters cells, proinsulin is created [29]. Results from an early human trial with the vaccine were promising. In the phase 1/2 trial, people who had been diagnosed with diabetes within the last five years were given either BHT-3021 or placebo once a week for 12 weeks. Notably, those who received a 1 mg dose of BHT-3021 had significantly improved C-peptide levels at 15 weeks compared to those who received placebo (indicating preserved beta cell function) [30]. However, C-peptide levels declined after this time point, suggesting that the vaccine may need to be readministered over time in order to be effective [30, 31]. BHT-3021 was reported to be safe and well tolerated in the trial. Bayhill Therapeutics is currently planning a phase 2 trial that will examine the efficacy of BHT-3021 in pediatric or adolescent subjects with recent-onset type 1 diabetes [30].
Early stage work is also being done with the delivery of proinsulin peptide (rather than the DNA that can make it) through repeated skin injections; this method is being explored by Dr. Mark Peakman, PhD (King’s College London, London, UK) [32]. Trials testing this method’s ability to preserve beta cell function in people with recent onset type 1 diabetes are currently recruiting in the United Kingdom.
“Kelly’s Take”

We clearly haven’t yet cracked the code with oral and intranasal insulin. Only certain people appear to respond to these therapies, and we don’t really know the best dosing strategy for these vaccines. Though the work that Bayhill has done is compelling and its vaccine seems safe, in the near-term we don’t think that we’ll see a complete reversal of diabetes with this therapy. Still, there might be a slowdown in the rate of progression of early diabetes. Though the vaccine might not be a cure, and a cocktail of vaccines rather than one vaccine may ultimately be necessary to halt diabetes in its tracks, we know that many parents would welcome a safe treatment that could delay the onset of diabetes by several years. We’re looking forward to seeing more results from insulin vaccination trials.
In Summary…
• Insulin is another protein found in islets that is thought to play an important role in the immune response leading to beta cell destruction.
• Insulin immunization has been explored in several trials as both a preventive therapy for type 1 diabetes and a therapy that could halt the progression of beta cell destruction in people with established disease.
• Researchers are still trying to determine whether administration of an insulin vaccine through the nose, mouth, or by injection will be the most effective.
• In a recently completed phase 1/2 trial, Bayhill’s injectable insulin vaccine BHT-3021 was shown to significantly improve C-peptide levels compared to placebo, and to be safe and tolerable.
• Insulin immunization still has much to demonstrate in terms of efficacy and safety before its use in the clinic is approved.
2.4
Other ‘Immune Modulating’ Strategies
Because diabetes vaccines are designed to restore the immune system’s ability to tolerate specific beta cell proteins, and therefore to prevent ongoing or future destructive responses to these proteins and beta cells, these vaccines are thought to have a minimal effect on broader immune function. This means that a person’s protective responses to bacteria or viruses should theoretically not be altered after the administration of diabetes vaccines.
Other approaches to type 1 diabetes immune therapy typically have broader effects than vaccines do. These ‘immune modulating’ therapies modify groups of cells, specific immune regulatory molecules, or pathways of the immune system that are thought to contribute to beta cell destruction; however, each of these targeted approaches will likely influence other immune functions as well. Because of these immune modulating therapies’ broader effects on the immune system, a greater number of side effects, such as increased risks for infections or the reactivation of particular viruses, are possible. Ideally, these immune modulating therapies will also have the ability to reinitiate the normal regulatory processes of the immune system that prevent the development of type 1 diabetes in most people.
It should be noted, however, that because many components go into every immune response, interrupting any one pathway may be insufficient to block or slow the progression of diabetes, and individual therapies may have to be used in combination. Similar to diabetes vaccines, these approaches, whether used alone or in combination, will likely be most useful as a preventive therapy or as a treatment to preserve remaining beta cells in people with recent onset disease. In the future, immune therapies may also be used in combination with beta cell regeneration therapies to restore lost beta cells.
Anti-CD3 Therapies
Who: 
When: 
Bottom Line: 
As described in Section 2.2, T cells likely play an important role in the development of type 1 diabetes. Anti-CD3 therapy targets T cells with an injectable synthetic antibody against the T cell surface protein CD3. CD3 plays a critical role in T cell activation and function, and binding to it with an antibody (which is what anti-CD3 therapy does) induces several effects on T cells, although variability in these effects is large and governed in part by the particulars of the antibody as well as the dose used [33]. As with vaccines, the goals of anti-CD3 therapy are to interrupt the T cells that directly attack beta cells and to initiate natural regulatory responses that restore the ability of the immune system to properly recognize beta cells as self-cells, thereby further inhibiting T cell attack on the pancreas.
Two companies are developing anti-CD3 compounds. MacroGenics is developing a compound called teplizumab. The Protégé phase 3 trial for teplizumab enrolled people recently diagnosed with type 1 diabetes with the intention of slowing the progression of disease. However, on October 21, 2010, MacroGenics announced that the trial had failed to achieve both its primary target outcome (a combination of insulin usage and A1c levels) and secondary outcomes (change from baseline in A1c, insulin dose requirements, and C-peptide levels) after one year [34]. However, more recent analysis suggested that the strongest dose may have been effective in preserving C-peptide or reducing insulin doses as compared to placebo in particular groups of people (specifically children 8-11 years old or people from the US) or when given earlier in the course of disease. Shorter courses of treatment or lower doses did not seem to be effective compared to placebo in any group. However, these findings need to be verified in futher, larger studies [35]. After announcing that the trial had failed to meet its primary target, the company suspended enrollment and dosing in the phase 3 Protégé ENCORE trial (which was similar in design to Protégé) and the phase 1b SUBCUE Trial (which was looking at the safety and efficacy of subcutaneous teplizumab administration, rather than IV infusion). No unanticipated safety issues were reported, though rashes and reductions in lymphocyte levels were observed. What these results mean for teplizumab’s future is currently unclear. While they’re clearly a setback, they may help the company better hone what teplizumab doses to use and who the drug is useful for.
Different dosing regimens for teplizumab have been considered. In the phase 2 trial that preceded Protégé, C-peptide levels improved and A1c and insulin requirements were reduced when a slightly higher dose of teplizumab than was used in phase 3 was given within six weeks of diagnosis. With this higher dose, however, people experienced a number of side effects including fevers, anemia, and rashes [36, 37]. A second phase 2 trial found that administering a second course of teplizumab a year after the first does not increase the drug’s beta cell preserving effects [38].
While Macrogenics has not yet revealed its plans for further development of teplizumab, investigation of the compound has continued. A recent phase 2 trial not affiliated with Macrogenics found that teplizumab is effective even outside the immediate new-onset period. Immune therapy trials have typically only enrolled people who have had diabetes for less than three months because it was thought that interventions are not as efficacious once significant beta cell mass has been lost. However, this study found that teplizumab has a significant effect on C-peptide secretion even in subjects diagnosed within the last four to twelve months [39].
An ongoing TrialNet study is now investigating the ability of teplizumab to prevent or delay the onset of type 1 diabetes in people who are at high risk of developing the disease. The trial is recruiting people who are either eight to 45 years old and have a first degree relative with type 1 diabetes, or are eight to 20 years old and have a second degree relative with type 1 diabetes. All participants must have detectable levels of at least two autoantibodies associated with type 1 diabetes.
Tolerx, in partnership with GlaxoSmithKline, was previously developing the anti-CD3 therapy otelixizumab. However, much like with teplizumab, Tolerx and GlaxoSmithKline announced in March 2011 that the phase 3 trial testing otelixizumab in people recently diagnosed with type 1 diabetes, DEFEND-1, had failed to achieve its primary target outcome (change in C-peptide levels) at one year [40]. There was also no difference in insulin usage at twelve months between the otelixizumab and placebo treated groups. Generally, side effects were significantly reduced at the dose used in this study. However it is possible that this improved side effect profile may have come at the expense of therapeutic efficacy [41]. Although no major safety concerns were noted, Tolerx suspended recruitment and dosing in the follow up phase 3 study DEFEND-2 after DEFEND-1 results were released. Subsequently, in late 2011, GlaxoSmithKline discontinued development of otelixizumab for type 1 diabetes and Tolerx shut down its operations. This was a surprising and disappointing turn.
DEFEND-1’s results were surprising because the phase 2 trial TTEDD had had positive results with the same dosing regimen (3.1 mg administered over eight days). TTEDD showed some preservation of beta cell function after 12 months of treatment as determined by C-peptide levels in people with recently diagnosed type 1 diabetes, while significantly reducing the risk for adverse events compared to earlier dosing schemes. Before TTEDD, another phase 2 study had demonstrated that a much higher dose of otelixizumab (48- 64 mg administered over six days; about 15 times more than the dose used in DEFEND-1 and TTEDD) preserved beta cell function and insulin production at 18 months and 36 months post-treatment, and it maintained lower insulin use in the otelixizumab group compared to the placebo group at 48 months post-treatment. However, those taking otelixizumab experienced side effects such as headaches, nausea, body aches, other flu-like symptoms, and infection with Epstein-Barr virus (mononucleosis) or transient Epstein-Barr virus reactivation in those previously infected [42, 43].
Because the CD3 protein is found on many T cells, anti-CD3 therapy likely also exerts effects on other T cells beyond just those involved in beta cell destruction, raising the risk for unwanted side effects. In early human trials that examined the use of older versions of anti-CD3 therapies (not otelixizumab or teplizumab) to prevent the rejection of various transplants, significant adverse effects were observed [44]. These adverse effects decreased following the development of techniques that allowed for precise engineering of CD3 antibodies, but as detailed above, moderate side effects have still been experienced with teplizumab and/or otelixizumab [42, 43, 45]. While the companies developing these therapies have used several strategies (including the use of lower doses) to minimize the side effects, it may be that these efforts are coming at the expense of how well the treatment works, potentially explaining the results observed in the Protégé and DEFEND-1 trials.
“Kelly’s Take”

Even just two years ago, excitement surrounded anti-CD3 therapy. Given the sobering results from phase 3 trials with Tolerx’s otelixizumab and Macrogenics’ teplizumab, the future of anti-CD3 therapies remains uncertain. Part of the issue is that our understanding of anti-CD3 therapies remains incomplete. No one is clear on how permanent the effects of the therapy are and whether additional dosing is necessary. While some side effects have been reported in trials, we don’t yet know how severe they might be in the short, medium, and long term. Gaining a better understanding of these side effects will be particularly important as researchers, people with type 1 diabetes, and health care providers try to decide if the side effects associated with higher doses are worth the additional efficacy they appear to have. This said, we are hopeful that anti-CD3 therapies will eventually be useful in preventing or stopping diabetes from progressing. We especially look forward to hearing about the results from the ongoing prevention study for teplizumab.
In Summary…
• Anti-CD3 therapy modulates the function of T cells with the goal of reactivating immune regulatory responses, restoring the immune system’s ability to tolerate beta cells, and preventing further beta cell destruction. But it may have broader effects on the immune system, leading to side effects.
• Teplizumab and otelixizumab are two anti-CD3 therapies that have been explored for commercialization.
• Macrogenics halted teplizumab phase 3 trials, which had enrolled people with recently diagnosed type 1 diabetes, due to lack of efficacy; however, a prevention trial with teplizumab has been launched by TrialNet.
• Tolerx’s otelixizumab also failed to demonstrate efficacy in a phase 3 trial enrolling people recently diagnosed with type 1 diabetes. Unfortunately, otelixizumab is no longer being developed for type 1 diabetes.
Anti-CD20 Therapies
Who: 
When:  2
2
Bottom Line: 
Several studies using animals that have type 1 diabetes have implicated B cells in the mechanisms leading to beta cell destruction [15, 46]. As we discussed above, B cells may help trigger beta cell destruction by aiding in the activation of T cells [15, 17]. B cells may also release chemical messages called cytokines that can promote beta cell damage by T cells and other immune cells [16, 46]. Anti-CD20 antibody therapy targets CD20, a protein found on the surface of most B cells. When anti-CD20 antibodies stick to the outside of the B cells, they lead to B cell depletion. In phase 2 trials, the anti-CD20 antibody rituximab (which has been used to treat a wide range of other diseases, such as leukemias, lymphomas, and rheumatoid arthritis) has been shown to increase C-peptide levels, lower A1c, and lower insulin requirements in patients with newly diagnosed type 1 diabetes for approximately one year after four doses of the drug were administered 7 days apart. However, after the first three months, similar declines in beta cell function were observed between those taking rituximab and placebo, suggesting that that the therapeutic effects of anti-CD20 may be short-lived [16]. Although more frequent dosing of anti-CD20 may avoid this problem, high dose rituximab can lead to significant immune suppression, increasing infection risk. To confirm the findings of this phase 2 trial, TrialNet is currently conducting a similar study in people 8 to 45 years of age who had been diagnosed with type 1 diabetes within 3 months of the trial’s start. Plans for further investigation of rituximab in type 1 diabetes have not yet been released.
Even if the phase 2 results are eventually confirmed, precisely how rituximab might be contributing to improved beta cell responses is not clear, leaving open concerns about balancing therapeutic benefit with long-term toxicity (such as decreased immune function, and increased risk of infection). While rituximab has been found to increase susceptibility to rare infections, recent studies in patients with diabetes have predominantly shown reactions such as fever, cough, and rash after infusion [47]. Additionally, previous trials using rituximab to treat other diseases saw concerning side effects such as low white blood cell counts, though whether these effects will occur at the doses currently under investigation for diabetes treatment is not known [48].
“Kelly’s Take”

Although rituximab has shown initial promise as a treatment for type 1 diabetes, we think that concerns over balancing the therapy’s potential side effects with its moderate effectiveness have dampened interest in developing rituximab much further. We are still very eager to hear about what could happen when different doses of the drug are administered or if it is used as a preventive therapy for type 1 diabetes.
In Summary…
• Studies in animals have suggested that B cells may play a role in the development of type 1 diabetes.
• Anti-CD20 therapies attempt to prevent or halt the autoimmune destruction of beta cells by depleting B cells from the blood.
• Rituximab has been shown to help preserve beta cell function, decrease A1c, and lower insulin requirements one year after treatment; however, these therapeutic effects do not appear to be durable and repeat dosing may be required.
• Rituximab has been associated with a variety of side effects, including susceptibility to rare infections, fever, cough, rashes, and decreased white blood cell counts.
Abatacept
Who: 
When: 
Bottom Line: 
As described in Figure 4, inactive or naïve T cells need to be activated before they can become killer T-cells, which are responsible for beta cell attack, or helper T cells, which coordinate beta cell destruction. In general, naïve T cell activation requires two signals. Abatacept (also known as CTLA4-Ig) is a drug that blocks one of these signals, thus inhibiting T cell activation [49]
Abatacept can blunt autoimmune diseases in both animals and humans. In clinical studies, it has been shown to have positive effects in psoriasis, psoriatic arthritis, rheumatoid arthritis, and lupus [49–51]. Abatacept has been approved in the United States for treatment of adult rheumatoid arthritis and juvenile idiopathic arthritis, and its safety profile in these conditions is generally tolerable [52, 53]. Abatacept is sold by Bristol-Myers Squibb under the name Orencia for the treatment of rheumatoid arthritis.
More recently, researchers have become interested in using abatacept as a treatment for type 1 diabetes. A phase 2 trial in people ages six to 45 diagnosed with diabetes within three months of the onset provided evidence that abatacept slow beta cell functional decline. In the study, participants received monthly injections of abatacept or placebo for two years. After two years, the abatacept group had a significantly lower A1c than the placebo group. Additionally, the abatacept group had a significantly higher level of C-peptide secretion. The positive effects were still present a year after the treatments had been stopped, and abatacept was calculated to have delayed the decline in beta cell function by an average of 9.6 months. However, similar to what was observed with anti-CD3 and anti-CD20 therapies in clinical trials, the drug only appeared to slow loss of beta function at the beginning of treatment. While beta cell function declined less with abatacept treatment during the first six months of the study, it declined similarly in both the abatacept and placebo groups for the rest of the study [49, 54]. These results suggest that abatacept is only useful during the earliest stages of diabetes, when the body is still producing new beta cell reactive T cells [55]. The study also suggests that shorter durations of treatment with abatacept may have similar efficacy to long-term treatment while minimizing adverse effects [54]. Abatacept was well tolerated during the study, and showed no association with increased risk of infection or low blood cell counts. However, in rheumatoid arthritis, abatacept has been associated with side effects such as headache, upper respiratory tract infection, sore throat, and nausea [56].
While these results are promising, additional, larger trials will now have to confirm abatacept’s effectiveness, safety, and optimal dosing. Whether abatacept’s short-lived effects on C-peptide have enough clinical significance (in terms of hypoglycemia rates, insulin requirements, or A1c changes, for example) to make treatment worthwhile remains to be seen.
Because abatacept acts on naive T cells and appears to have the greatest effect early after treatment initiation, this drug may be particularly useful in people at high risk of developing type 1 diabetes) [49, 57]. In mice, abatacept has been shown to prevent type 1 diabetes when administered before immune attack on beta cells begins [49]. Researchers are now considering testing abatacept’s ability to prevent type 1 diabetes in people who are at high risk for the disease [58].
Abatacept may also be beneficial when used alongside other immune therapies in order to target multiple pathways involved in type 1 diabetes development. Drugs included in combination therapies typically have already been shown to be safe and effective. Since abatacept has been approved in adult and juvenile arthritis and has an established safety profile in these indications, abatacept is an intriguing candidate for combination testing [49, 59]. Combining abatacept with other immune therapies has thus far had mixed results in animal models. When administered with thymoglobulin (a drug that eliminates T cells circulating throughout the body), abatacept restored normal glucose control in mice with diabetes [60] (we note that the practicality of this approach in humans is limited by the significant toxicity of thymoglobulin). In contrast, combining abatacept with an insulin vaccine did not enhance efficacy [61]. Further studies will have to identify which immune therapies are most complementary to abatacept in the context of type 1 diabetes. Safety of various combinations containing abatacept will also be a key issue. In general, modulating two parts of the immune system has the potential to cause deleterious side effects not seen when only one component is altered [59]. In rheumatoid arthritis, use of abatacept plus an immune therapy is associated with a greater risk of adverse events, and is not recommended [62]. The relative risks of using abatacept in conjunction with other immune therapies will thus have to be carefully investigated prior to any large scale clinical use.
“Kelly’s Take”

Early testing has shown that abatacept has a number of positive effects in type 1 diabetes. Unfortunately, it seems to only have an impact at the beginning of treatment, presumably when significant T cell activation is still occurring. For this reason, we think it’s unlikely that abatacept will be used alone for treatment of recent onset type 1 diabetes. However, we are interested to see if its effects will be longer lasting if it is given earlier in the course of disease, with other immune therapies, or possibly for prevention.
In Summary...
• Abatacept prevents the full activation of T cells by blocking one of the signals immature T cells need to become helper or killer T cells.
• In a phase 2 trial, two years of abatacept treatment significantly preserved C-peptide secretion relative to a placebo; however, the drug’s effect was short-lived.
• Additional trials will now have to confirm abatacept’s safety, efficacy, and optimal dosing, as well as the clinical significance of its C-peptide effects.
• Abatacept has characteristics that make it appealing for testing in a prevention trial or in combination with other immune therapies.
IL-1 Therapies
Who: 
When: 
Bottom Line: 
IL-1 is a protein that is linked with inflammation, and has a central role in producing fevers. Because inflammation is believed to contribute to beta cell failure in type 1 diabetes, therapies that block IL-1 are under investigation as possible beta cell preserving treatments. Inflammation is a natural response to infections, damaged tissue and cells, or irritants. Through acute inflammation, the body attempts to remove the harmful stimuli and start the healing process. But inflammation that continues for a long period of time (chronic inflammation) can sometimes have harmful effects. Chronic inflammation associated with IL-1 is believed to contribute to beta cell destruction in type 1 diabetes: as beta cells are destroyed by the immune system and insulin production becomes insufficient to control blood glucose levels, beta cells are thought to become stressed due to hyperglycemia. In response, the beta cell produces IL-1, leading to the development of inflammation around islets that causes further beta cell failure and death [63].
Current methods to target IL-1 include an injectable version of a natural IL-1 inhibitor (IL-1Ra, Amgen’s anakinra), as well as antibodies directed against IL-1. In a recently completed phase 2 trial testing anakinra in adults with type 1 diabetes, the compound did not improve beta cell function (as measured by C-peptide secretion), A1c, or insulin requirements compared to placebo [64].
Two anti-IL-1 antibodies are under development, one produced by XOMA Pharmaceuticals (called gevokizumab [previously XOMA 052]), and another one by Novartis (called canakinumab). TrialNet recently completed a phase 2 trial testing the use of canakinumab in people with recently diagnosed type 1 diabetes. Unfortunately, in this trial, canakinumab was not found to preserve C-peptide secretion or improve A1c compared to placebo in subjects ages six to 45 [65]. Gevokizumab is in a phase 2 trial (supported by JDRF) in people with established type 1 diabetes. Like the canakinumab trial, this trial is examining whether the antibody can prevent further beta cell destruction. In a recent trial in people with type 2 diabetes, however, gevokizumab failed to significantly reduce A1c over control treatment at 6 months. IL-1 is thought to play a similar role in the progression of type 1 and type 2 diabetes, and a positive effect on A1c would be expected if the therapy had reduced beta cell dysfunction and destruction. Whether these same results will also be observed in the type 1 diabetes trial is still unclear. On a more positive note, no safety issues were identified in the trial, and the therapy reduced inflammation, improved cholesterol, and improved markers of heart disease risk. IL-1 may also contribute to beta cell death during prediabetes. However, the use of IL-1 therapy in the period after the onset of autoimmunity has not yet been explored in human trials.
“Kelly’s Take”

IL-1 therapies appear to have benefits that extend well beyond diabetes; however, we are still in the early days of studying IL-1 therapies in diabetes. It’s important to remember that IL-1 is just a small component of the immune response against beta cells in type 1 diabetes, making IL-1 therapy, even if successful, more likely a treatment than a cure.
In Summary…
• Although inflammation is a natural response that helps remove harmful stimuli and initiates the healing process, chronic inflammation can be harmful.
• In type 1 diabetes, hyperglycemia due to beta cell loss triggers the production of a molecule called IL-1, which initiates inflammation around remaining beta cells. This inflammation appears to cause further beta cell dysfunction and death, so blocking IL-1 might have a therapeutic benefit.
• However, in recent phase 2 trials, both the IL-1 inhibitor anakinra and the anti-IL-1 antibody canakinumab failed to significantly improve beta cell function in people with recently diagnosed type 1 diabetes.
• Because many components go into every immune response, interrupting any one pathway may be insufficient to prevent or stop the progression of diabetes, making the use of a combination of immune therapeutics potentially valuable.
2.5
The Interface of Vaccines and Immune Modulation
Two therapies under investigation attempt to combine facets of both type 1 diabetes vaccination and the other immune modulatory therapies discussed above.
DiaPep277
Who: 
When: 
Bottom Line: 
DiaPep277 is a fragment derived from the human Heat Shock Protein-60 (Hsp60), which is currently in phase 3 testing for its ability to prevent or slow the progression of type 1 diabetes. It is being developed by Andromeda Biotech. In contrast to other immunization methods, where the actual function of the protein in the vaccine is not important for the vaccine’s therapeutic effect, Hsp60 itself is thought to have a direct immune modulating function [66, 67].
In a phase 2 trial of DiaPep277 in 35 adults recently diagnosed with type 1 diabetes, treatment with DiaPep277 significantly improved C-peptide levels as compared to placebo at 10 months; however, A1c levels were not different between the two groups. In addition, following DiaPep277 administration, T cell responses to Hsp60 were altered in a way that suggested cells expressing Hsp60 (like beta cells) would likely be more protected from immune destruction than before [66]. A year-long extension study later showed that the benefit of the drug declined over time [68]. Two other phase 2 trials in recently diagnosed adults demonstrated slight, though not statistically significant, delays in C-peptide decline after DiaPep277 treatment, but showed no effect of DiaPep277 administration on A1c or insulin requirements [69, 70].
Notably, in late 2011, Andromeda announced that a phase 3 trial for DiaPep277 named DIA-AID1 had met both its primary and secondary endpoints. In the trial, which enrolled people recently diagnosed with diabetes ages 16 to 45, subjects were given nine doses of either DiaPep277 or placebo over the course of 21 months. At the end of two years, treatment with DiaPep277 was associated with higher levels of C-peptide secretion than treatment with placebo. Additionally, a higher proportion of individuals treated with the drug had an A1c of < 7.0% by the end of the trial. DiaPep277-treated patients also had lower rates of hypoglycemia than placebo-treated patients. Overall, DiaPep277 was well tolerated during the study [71]. These early results are encouraging, and we look forward to seeing the final results for a fuller understanding of DiaPep277’s potential. DiaPep277 is now being further explored in a confirmatory phase 3 trial, DIA-AID 2, which began enrolling patients at 100 medical centers in the US, Europe, and Israel in May 2010. The two-year study will ultimately include 450 people with recently diagnosed type 1 diabetes ranging in age from 20 to 45 years old.
Since two trials of DiaPep277 in recently diagnosed children showed no effect of treatment on C-peptide decline [70, 72], the current target population for DiaPep277 is newly diagnosed adult patients. Further studies will need to establish whether this drug has any potential children and in people with established diabetes. At this time, whether its immune modulatory effects could play a role in preventing the disease altogether remains unclear.
“Kelly’s Take”

Recent phase 3 results certainly look promising, and give us hope that DiaPep277 could have an impact in type 1 diabetes, although unfortunately, only in adults. Further studies will now have to establish what DiaPep277’s long- term effects are, as well as whether it can significantly affect the course of type 1 diabetes. If the compound cannot ultimately change the course of type 1 diabetes or improve glucose control, we believe its chances of becoming approved for clinical use are minimal.
In Summary…
• DiaPep277 is being developed by Andromeda Biotech as a therapy to prevent or slow the progression of type 1 diabetes.
• In a recent phase 3 study, DiaPep277 treatment was associated with significantly higher levels of C-peptide secretion than placebo. A confirmatory phase 3 study is underway.
• The therapy’s effectiveness appears best in recently diagnosed adults. It seems to have limited (if any) efficacy in children.
BCG Administration
Who: 
When: 
Bottom Line: 
Bacillus Calmette-Guerin (BCG) vaccination uses a killed bacterium (similar to the one that causes tuberculosis) to induce low-level inflammation across the body [73]. This is an alternative strategy to modify the immune system and is designed to interfere with immune responses against self-cells.
A phase 1 trial with six participants with type 1 diabetes and six without was completed in June 2010. Three in each group were treated with the vaccine, while three were treated with placebo. For comparison purposes, the study also enrolled people with and without diabetes who were not treated. The primary target outcomes of the study were self-reactive T cell levels, inflammatory molecule levels, and C-peptide levels; these endpoints were measured eight weeks after the first doses of the BCG vaccine were given. Preliminary results from the phase 1 trial were announced in June 2011 and full results from the study were published in August 2012. These results showed the vaccine to be safe. Additionally, vaccination seemed to favorably alter both regulatory and killer T cell profiles, and in two of the three treated subjects, it transiently increased C-peptide levels (compared to either subjects’ own baselines or levels seen in untreated subjects with diabetes) [74]. However, significant changes in the course of disease as a result of this treatment, such as improved glucose control or lowered insulin requirements, have yet to be demonstrated. While these results are intriguing, they will have to be confirmed in the larger and longer phase 2 trial currently being planned.
The bulk of this work, both clinical and basic science, is being done in the laboratory of Dr. Denise Faustman, MD, PhD (Massachusetts General Hospital, Boston, MA) with the sponsorship of the Iacocca Foundation. Although the results of this phase 1 trial are promising, prior studies of BCG for both treatment of recent onset type 1 diabetes and for prevention of type 1 have failed to consistently show therapeutic benefit [11, 75, 76], and in one study, BCG vaccination even appeared to increase the risk of developing type 1 diabetes in at-risk children expressing autoantibodies [75]. It is possible that these trials may have failed due to a less than ideal dosing scheme. Since then, insight has been gained into the vaccine’s effects on the immune system, which may help better inform future dosing.
The BCG vaccine is approved in the United States for the treatment of superficial bladder cancer [77]. It is also used around the world as tuberculosis vaccine that is safe, but whose efficacy is highly variable [78].
“Kelly’s Take”

Dr. Faustman indicated in an interview with diaTribe that the key to making this treatment useful for large numbers of patients will be finding the right dose and dosing schedule, which is expected to take a significant amount of time. We are awaiting further results with interest; like with other early-stage work, her likelihood of success is impossible to predict. The fact that her results haven’t yet been widely reproduced does give one pause; however, her strong support from the highly-respected Dr. David Nathan (Massachusetts General Hospital, Boston, MA) is notable.
In Summary…
• By inducing low-level general inflammation, the BCG vaccine is thought to deplete T cells involved in the destruction of self-cells such as beta cells, and may help stop the progression of beta cell death in type 1 diabetes.
• Trials are still in very early stages. A recently completed phase 1 trial showed the vaccine to be safe and suggested it might be capable of altering C-peptide levels and T cells in a favorable way. This compound’s ability to change the course of type 1 has yet to be demonstrated, however. Planning for phase 2 testing is underway.
• Previous human trials have failed to show therapeutic benefit of BCG vaccination.
2.6
Mesenchymal Stem Cells
Who: 
When: 
Bottom Line: 
Mesenchymal stem cells (MSCs) are cells found in the bone marrow that have the ability to turn into bone and fat, as well as other cell types. These cells appear to be able to travel to sites of inflammation and may be able to reduce or alter the inflammation associated with type 1 diabetes, ultimately reducing beta cell destruction [79, 80]. What makes MSCs particularly attractive is that they tend not to provoke immune rejection when transplanted from one person to another. Usually, without immune protein matching and the use of immune suppressive drugs, rejection of transplanted cells occurs rapidly after transplantation because of immune-system triggering mis-matches between the proteins found on donor cells and those on the recipient’s own cells (see Section 3.2) [81]. MSCs, however, appear to avoid immune rejection when transplanted, eliminating the need for broad immune suppression and donor ‘matching.’ Thus far, the use of MSCs for immune modulation has been shown to be safe in clinical trials for a variety of diseases [81–83]. Yet researchers are still not clear on what happens to the cells after transplantation, leaving open the possibility for yet unknown adverse effects [83].
The major diabetes trial with MSCs, conducted by Osiris Therapeutics, is sponsored by JDRF. This phase 2 trial evaluates the effects of treatment with Prochymal, a cocktail of adult bone marrow-derived MSCs, in patients with new onset of disease. The company posits that the mixture will modulate immune responses in areas of inflammation (like the pancreas in type 1 diabetes) and reduce autoimmune beta cell destruction.
Interim results from this phase 2 trial showed that Prochymal was safe and well tolerated after one year. Importantly, Prochymal was not rejected by recipients’ immune systems despite no immunosuppression agents being used, and the MSCs not coming from or being matched to participants. Unfortunately, there were no significant differences in stimulated C-peptide levels between Prochymal and placebo treated patients after one year. There was also no significant difference in hypoglycemia rates between the groups. There was, however, a trend towards less hypoglycemic events in those receiving Prochymal. Full results from phase 2 will be released after participants have been followed for a total of two years [84].
Although Prochymal’s utility in diabetes has yet to be proven, the drug has shown some promise in other diseases. While outcomes from an initial phase 3 Prochymal trial for graft-versus-host disease (GvHD; an immune diseases associated with bone marrow transplantation) did not meet its main target [85], a later phase 3 study in more severe pediatric cases did meet its targets [86], and in the summer of 2012 Canada and New Zealand’s medical regulatory agencies approved the treatment for GvHD in children [87, 88]. These approvals made Prochymal the word’s first approved stem cell therapy. Additionally, interim results from a phase 3 trial exploring Prochymal in Crohn’s disease appeared positive [89]. The ability of MSCs to slow or halt the progression of beta cell destruction in people with recently diagnosed type 1 diabetes is also being examined in clinical trials taking place in China, Sweden, and Brazil.
“Kelly’s Take”

Although this therapy has generated excitement, we remain cautious at this point. Early trial results have not shown a benefit of the drug and much is still unknown regarding the specific effects of MSCs on the immune system and on beta cells. The possibility for side effects is another major concern.
In Summary…
• Mesenchymal stem cells (MSCs) are thought to alter immune responses in areas of inflammation (such as the pancreas in type 1 diabetes).
• Osiris Therapeutics is conducting a phase 2 trial for type 1 diabetes with its MSC cocktail, Prochymal.
• One-year results from this study suggest that Prochymal is safe, but does not slow disease progression in people recently diagnosed with type 1 diabetes.
• We see this approach as potentially limited by a lack of specificity, and whether the possible risks of therapy will outweigh its benefits is currently not clear.
2.7
Overall Potential
Immune therapies are attractive because of their potential to modify and alter the immune processes underlying beta cell destruction and the development of type 1 diabetes. Again, most of these therapies hold the promise of preventing or halting the progression of type 1 diabetes, but not necessarily of restoring beta cell function (which is some people’s definition of a cure). Immune therapies may also prove beneficial in other aspects of diabetes care, including the protection of beta cells newly transplanted or produced by regeneration therapies. There does seem to be a growing consensus that at any of these stages, several therapies may need to be used together for this approach to be effective.
Overall, progress in this area has been steady, but not overwhelming, largely due to a still hazy understanding of the exact immune processes that contribute to type 1 diabetes. Our lack of reliable measures (termed biomarkers) to fully and accurately assess the effects of these therapies on beta cells and the rest of the human body has also limited progress. Progress has not stopped, however. Many notable researchers continue to push forward in this area, including the renowned Dr. Matthias Von Herrath, Professor at La Jolla Institute for Allergy & Immunology and Director of the Novo Nordisk Type 1 Diabetes R&D Center. Officially launched in June 2012, this Seattle-based center is focused on developing immunological treatments for type 1 diabetes and hopes to begin clinical trials in the next five years.
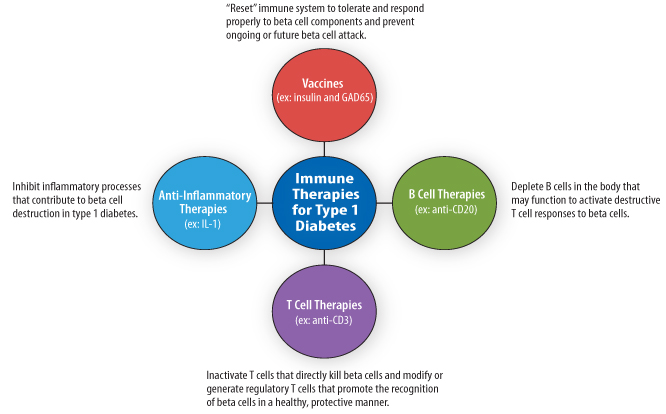
Figure 6. The four main categories of immune therapies for type 1 diabetes and their intended effects.
2.8
Where to Learn More
Immune Therapeutics
The Immune Tolerance Network Clinical Trials
The Immune System and Type 1 Diabetes
The Immune System—The University of California at San Francisco
Diabetes Vaccines
How Vaccines Work—The National Network for Immunization Information
BHT-3021 Program Overview—Bayhill Therapeutics
Intranasal Insulin Trial II—Diabetes Research Centre
Anti-CD3 Therapies
Tolerx’s Otelixizumab Fails in Phase III Trial—Current Research into a Cure for Type 1 Diabetes
Pivotal Clinical Trial of Teplizumab Did Not Meet Primary Efficacy Endpoint—Macrogenics
Anti-CD20 Therapies
Approved Drug May Slow Type 1 Diabetes—The Diabetes Channel
IL-1 Therapies
XOMA-052 Phase 2 Trial Initiated in Diabetes Patients—XOMA
DiaPep 277
BCG Vaccination
Shot May Reverse Type 1 Diabetes—The Boston Channel
Mesenchymal Stem Cells
Prochymal for Type 1 Diabetes—Osiris Therapeutics

3.1
Current Techniques
Who: 
When: 
Bottom Line:  3
3
Islet and pancreas transplantation treats type 1 diabetes by adding new, functioning islets to the body. Every year in the US, roughly 1,500 pancreas transplants are performed at approximately 100 centers; the procedure involves adding a second pancreas without removal of the first. With pancreas transplants, approximately 80% of patients achieve independence from external insulin one year after the procedure [90]; however, whether pancreas transplants improve long-term patient survival as compared to insulin therapy is currently unclear, and some information suggests that, in fact, survival may be worse in people who received transplants [91]. Because transplantation supplies new beta cells, theoretically it can be used to cure any person with existing diabetes. Currently, the procedure is generally recommended for only those whose diabetes is very difficult to control with insulin therapy or those with severe hypoglycemic unawareness.
Pancreas transplantation is a technically demanding major surgery. The number of organs available for transplant falls significantly short of demand. The procedure is expensive (in the vicinity of $150,000) and entails high long-term follow-up costs [92]. Despite the high cost, an advantage of pancreas transplants is that pancreases can last a lifetime without rejection, provided that appropriate immune suppressive drugs are used to block the immune response against the transplanted organ. Many people who received transplants in the 1980s still have fully functioning organs. Most pancreas transplant failures occur from toxicity related to immune suppressive drugs rather than failure of the transplanted pancreas itself.
Because of their less invasive nature, in the future, islet cell transplants will likely be preferred over whole-organ transplants, presuming that new sources of islets can be found (see Section 3.4 below). The islet transplantation procedure is a simple infusion of islets through the major vein feeding the liver, called the portal vein. To perform an islet cell transplant, cadaver pancreases are removed (current approaches usually require at least two, although recent research, such as that by Dr. Bernhard Hering, MD (University of Minnesota, Minneapolis, MN), suggests the use of one pancreas may be possible [93]), the islet cells are isolated and purified, and then infused into the recipient. Instead of settling in the pancreas, the infused islets typically settle in the liver, though research is underway in both animals and humans to identify possible new locations for islet delivery. Once settled in the liver, islets can begin producing insulin in response to rising blood glucose. There’s no surgery involved, and people can generally leave the hospital a few days after the procedure. Often challenging immune suppressive therapy is also needed for islet transplantation.
Unfortunately, islet cell infusion has not been effective in inducing insulin independence over the long term. In 2000, the islet transplant team at the University of Alberta in Edmonton made headlines using a new protocol that allowed all patients to become insulin independent and enabled transplanted islets to survive the associated immune suppression therapy (see Section 3.2) for 12 months. The Edmonton Protocol, as it became known, involved standardization of the transplantation procedure and elimination of steroid-based immune suppression (which had major side effects, including being toxic to beta cells). But, only 10% of people who received islet transplants through the original Edmonton Protocol study were insulin-independent at five years [94, 95]. Despite this drawback, the procedure has been found to markedly reduce rates of hypoglycemia, reverse life-threatening hypoglycemic unawareness, improve blood glucose variability, and lower insulin dose requirements [95, 96]. Evidence has also emerged that people who receive transplants progress more slowly to certain complications [97].
Some changes to the protocol have been made since it was originally published. For example, the use of new immunosuppressants (such as tacrolimus and mycophenolate) has significantly reduced side effects and gynecological problems associated with previously used therapies. New methods to alleviate some of the complications that accompany transplantation have also been introduced [97]. Additionally, several smaller studies have suggested that the use of newer transplantation protocols (which incorporate alternative immune suppressive drugs and agents that could potentially help beta cell survival) may improve long-term insulin independence following islet transplantation [98–101]. Although promising, these results are still preliminary and will need to be confirmed in much larger and longer trials. Nevertheless, as transplantation methods have evolved since publication of the Edmonton Protocol, rates of insulin independence following islet transplantation have significantly improved [102].
Dr. Peter Senior, MD, PhD (University of Alberta, Edmonton, Alberta), Medical Director of the Clinical Islet Transplant Program in Edmonton, has noted that overall, there is now a better understanding of the transplantation procedure’s risks, who the procedure is beneficial for, and when patients should or should not receive another transplant. More emphasis is being placed on avoiding hypoglycemia and achieving stable blood glucose and good glycemic control through the Protocol, while less emphasis is being placed on achieving insulin independence with it [97].
In contrast to pancreas transplantation, another limitation of islet transplantation is that it may not restore a person’s ability to release pancreatic hormones other than insulin (such as glucagon, amylin, and somatostatin) that are also involved in blood glucose control; the most important of these hormones is glucagon, which helps promote the synthesis of new glucose molecules and the release of stored glucose into the blood when blood glucose levels become too low [103–105]. Islet transplantation costs roughly $150,000 (about as much as a whole-organ pancreas transplant), although costs are higher if more than one infusion is needed. As with whole-pancreas transplantation, the biggest limiting factor for islet transplantation is the availability of islets. Only about 6,000 organs from deceased donors are available for islet transplantation each year (an additional 2,000 are used for whole-organ transplants), limiting how many procedures can be performed; consequently, establishing a new source of islets could dramatically increase the availability of this treatment [91, 106].
In Summary…
• Because of easier delivery, islet transplantation will likely be preferred to pancreas transplantation in the future.
• Difficult immune suppressing treatments and a shortage of islets available for transplantation are major limitations of current islet transplantation techniques.
• While most people eventually return to requiring insulin therapy at some point post-procedure, islet transplantation can be very effective at reducing insulin dose requirements, rates of hypoglycemia, hypoglycemic unawareness, and glucose variability.
3.2
Immune Suppression in Transplantation
The biggest risk of islet cell and pancreas transplantation is the accompanying immune suppressive therapy necessary for both procedures. People take immune suppressing drugs both before and after transplantation to prevent rejection of the foreign cells (caused by the recipient’s body reacting to genetic differences between donor and recipient cells, and essentially attacking what they see as ‘foreign’) and to prevent recurrence of ‘autoimmune’ destruction of the transplanted beta cells [107]. In comparison to the immune therapeutics under investigation to prevent or halt type 1 diabetes progression that are discussed in Chapter 2, these immune suppressive therapies have a much broader effect on the immune system. Compliance with treatment is critical, because if these drugs are stopped, attack against the transplanted organs or cells by the immune system will begin, leading to transplant rejection. Stopping this attack once it has begun is quite difficult. Despite the fact that these drugs are necessary to preserve transplants, these drugs come with a corresponding increased risk for infections [91, 108, 109] and certain types of cancer [106].
The new drugs that the Edmonton Protocol introduced into islet transplantation were derived from other organ transplantation protocols, and include daclizumab (Zenapax), sirolimus (Rapamune), and tacrolimus (Prograf). These drugs have side effects that many people find challenging, including mouth sores, increased infections caused by decreased immune cell numbers, and gastrointestinal problems such as diarrhea. Immune suppressive drugs may also worsen islet function, possibly negating some of the benefits of transplantation [110]. According to Dr. David Sutherland, MD a pioneer in pancreas and islet transplantation, the trade-offs for transplantation are tricky. He emphasized to us that islet transplantation can only become widespread once we can bypass the very strong immunosuppressive therapies currently needed to preserve transplanted islets. The immune therapeutics under development for type 1 diabetes (see Chapter 2) may one day help prevent the autoimmune processes that underlies diabetes from destroying transplanted islets [111]; however, their use alone is unlikely to completely block islet transplant rejection since rejection involves different parts of the immune system. Consequently, scientists have focused on two alternative strategies to avoid the concurrent use of immune suppression in islet transplantation: 1) the encapsulation of transplanted islets behind a barrier that lets glucose and insulin through but shuts out the immune system, and 2) the transplantation of beta cells derived from other cells in the transplant recipient’s body, leading to perfectly genetically matched donor cells. Both strategies are discussed in greater detail below.
In Summary…
• The need for immune suppression to ward off transplant rejection is a major obstacle to islet transplantation.
• Current immune suppression regimens have many challenging side effects and carry noteworthy risks.
• Researchers are focused on encapsulating islets to protect them from the immune system, or creating islets from other cells in the patient’s body so they are ‘matched.’
3.3
The Intended Population for Islet Transplantation
As the field currently stands, islet transplantation is really intended for a small audience of people with type 1 diabetes. The limited supply of islets means that transplantation criteria, while varying from center to center, are generally very restrictive. For example, Dr. Sutherland performs pancreas or islet transplants only for the following reasons: 1) if a person has chronic pancreatitis; 2) a person has chronic, brittle type 1 or 2 diabetes that is poorly controlled with insulin; 3) a person has a high incidences of hypoglycemia or severe hypoglycemia unawareness; 4) when diabetes treatment severely impairs a person’s lifestyle.
How the procedure will be used if and when islets are ever abundant is not yet clear, and will likely depend on the relative risks and benefits of the new technology. At the moment, for transplantation to be worthwhile, the inconvenience and risks of a person’s diabetes must be greater than the inconveniences and risks that would accompany immune suppressive therapy. Because many people control diabetes well with insulin therapy, this only happens rarely. The procedure’s monetary cost will also likely be an issue when weighing relative benefits. For the general diabetes population, Dr. Sutherland sees islet transplantation as a secondary solution: the first line of treatment will be strategies that prevent or reverse diabetes (see Chapter 2) or regenerate beta cells (see Chapter 4).
In Summary…
• Due to costs, a shortage of islets, and the risks associated with current immune suppressive therapies, islet transplantation is currently recommended only for people whose diabetes that is very difficult to control with insulin therapy or for those who have severe hypoglycemic unawareness.
• The target population for islet transplantation may expand if islet availability is no longer problematic and safer strategies to protect transplanted islets become available.
3.4
New Sources of Islets
We describe below several ideas for alternate sources of islets. All of these ideas are in an early experimental stage, but are important to understand because of the scarcity of currently available islets. If we could grow new islet cells in the lab using cells from the person who will be receiving the islet transplant, rejection would cease to be a general concern, since rejection occurs when the recipient’s immune system recognizes that the donated islets are genetically different than cells in the recipient’s body. Some degree of immune therapy will still likely remain necessary for a complete cure (rather than repeated transplants) since type 1 diabetes is caused by immune attack on “self” islets as discussed above. Despite their early stage, these ideas fuel optimism for the potential of islet transplantation.
Creating Beta Cells from Stem Cells
Who: 
When: 
Bottom Line: 
Stem cells are cells that are uniquely capable of two special things—replicating indefinitely without changing cell type (i.e. stem cells can continue to divide to produce a continuous supply of identical stem cells) and differentiating into or becoming “daughter cells” of other cell types. Such a daughter cell could be destined to become part of the pancreas, the heart, the brain, or a number of other tissues. A basic diagram of this process is shown in Figure 7.
A few days after conception, a fertilized egg divides into a mass of stem cells. When this mass contains 256 cells or less, each cell is classified as an embryonic stem cell (ESC). These ESCs continue to divide and change into other cell types along paths prompted by the expression of certain genes. A single ESC has the potential to differentiate into all cell types of the body (referred to as a pluripotent stem cell); other types of stem cells can have more limited potential; for example, stem cells in the skin can contribute to most types of skin cells (referred to as a multipotent stem cell), but cannot turn into blood or bone.
The basic approach to making beta cells from stem cells involves delivering particular molecular messages to the ESCs that encourage the development of certain daughter cells. Ideally, if we could discover the right messages, we would be able to drive ESCs toward the development of beta cells. (Note that particularly in the US, the use of ESCs in this way has often been controversial). The proof of principle experiment in this field came in 2001 when Dr. Suheir Assady, MD (Rambam Medical Center, Haifa, Israel) successfully created a small number of cells that secreted insulin from a starting population of ESCs [112]. Since then, work has been done by academic groups around the world, as well as several companies (including ViaCyte, Geron, and BetaLogics) to use ESCs to create a variety of islet cells such as beta cell precursors—cells that can mature into glucose responsive and insulin producing beta cells once inside the body. Ultimately, these companies hope to create a product consisting of encapsulated beta cell precursors which can be delivered into the human body. This approach has proven successful in some animal studies. When such cells have been introduced into mice with diabetes, blood glucose control has improved and diabetes has even been reversed, but some animals also developed non-cancerous stem cell tumors called teratomas [113]. These teratomas were thought to originate from contaminating ESCs that had not yet differentiated into beta cell precursor cells. This highlights the need for adequate purification methods to ensure the safety of these treatments (an important regulatory concern for future stem cell-based therapies). Unfortunately, as research currently stands, the beta cells that have been made from human ESCs are not nearly ready for clinical usage. Additionally, once true beta cells are created, scientists will have to think of ways to grow enough of these cells to effectively treat diabetes. A better understanding of the interaction between beta cells and their neighboring cells is also needed before therapies can be optimized. New research is showing that the co-transplantation of pancreatic cells other than beta cells along with islets can improve islet survival. However, how best to exploit this knowledge is not yet clear.
As discussed above, we do not currently have methods for matching donor beta cells, whether those currently obtained from cadavers or those eventually created from ESCs, to a transplant recipient, making the use of broad immune suppressive therapies necessary to avoid rejection. A series of recent breakthroughs, however, has resulted in a method that may eventually allow scientists to generate beta cells from stem cells that would be perfectly matched for each person. Additionally, this approach would not require the use of ESCs, allowing the controversies associated with ESCs to be avoided. The method involves taking a person’s adult cells, such as skin cells, and transforming them into stem cells (called induced pluripotent stem cells or iPS cells). In principle, these iPS cells could then be coaxed into becoming beta cells. If successful, the creation of beta cells from iPS cells would be a major advancement for islet transplantation therapy. However, as mentioned above, the use of iPS-derived beta cells in transplantation procedures will not avoid the autoimmune destruction of “self” beta cells characteristic of type 1 diabetes. Consequently, immune modulating therapies (such as those discussed above) or encapsulation (see Encapsulation in Section 3.5 below) in combination with iPS beta cell transplantations will likely remain necessary to promote the long-term survival of the transplanted cells.
Finally, iPS-derived beta cells will likely face significant safety hurdles if and when they are successfully developed. Because iPS beta cells are derived from each person’s own adult cells, effective purification and testing procedures will be needed to ensure that each iPS-derived cell is in fact a healthy, genetically normal beta cell. The use of a genetically abnormal cell could be a potential trigger for cancer development.
“Kelly’s Take”

Creating brand new beta cells from stem cells could be a godsend for people with diabetes. Although the approach won’t treat the underlying autoimmune problem, the transplantation of these beta cells could help significantly improve glucose control (at least for a period of time) and hypoglycemic unawareness. Still, the science is at an extremely early stage—no one has produced a true beta cell yet, and even the best protocols for producing insulin-secreting cells are far from efficient enough to be effective in humans. Even if creation of a beta cell from a stem cell could be acheived, multiple other hurdles would remain, including lengthy testing to ensure their safety when used in transplantation.
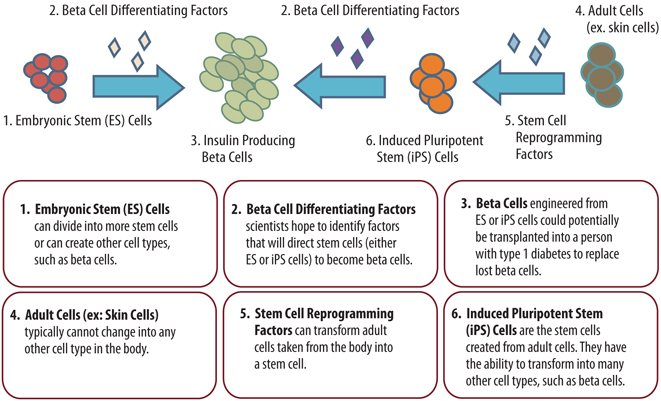
Figure 7. Creation of new beta cells from embryonic stem cells (left) or adult cells (right).
In Summary…
• Creation of beta cells from stem cells, particularly from those that are self-derived, would be a major leap forward for islet availability and transplantation.
• This technology is being worked out in labs and has a long way to go before reaching the clinic.
Pig Islet Transplants
Who: 
When: 
Bottom Line: 
Because the supply of human islets is limited, the use of animal islets (especially from pigs) for transplantation has generated significant interest. Animal transplants are known as xenotransplants. While primate islets (e.g. chimpanzees) would seem appealing for use in xenotransplantation at first glance, the use of primate organs has many disadvantages. Primate organs are very expensive because no truly domesticated species of primates is available. Primates also harbor a large number of viruses that can infect humans; the fear is that these viruses could be transferred upon organ transplant. Also, harvesting organs from primates poses ethical concerns because of primates’ close relation to humans. Alternatively, the potential use of pig islets has generated significant interest. Pigs are easy to breed (generation time of about a year) and produce large litters, allowing for large numbers of islets to be harvested. Pig islets also have the same physiological response to glucose as human islets, and the insulin they produce is very similar to human insulin [114]. As with primates, however, pigs carry many viruses that are potentially harmful for humans (especially those with immune systems weakened by the use of immune suppressive therapies). To make use of pig islets feasible, pathogen—free animals must be identified, carefully farmed, and used. Such ‘medical-grade’ species have been created in the US by the Spring Point Project in Minnesota and in New Zealand by Living Cell Technologies (LCT) [115]. As of now, it seems that until islets can be grown in the lab, transplantation of animal tissue, and pig tissue in particular, will be the most viable option for obtaining large quantities of islets [116].
Two main constraints limit the use of pig islets. Firstly, they are both chronically and rapidly rejected (the latter is called hyperacute rejection) if strong immune suppressive therapies are not used. Secondly, their transplantation is associated with significant side effects [114, 117]. Hyperacute rejection is analogous to rejection of blood when blood-types are mismatched in a transfusion and is a barrier to transplantation of organs from nearly any animal. Hyperacute rejection of pig islets in humans is thought to occur, at least in part, because we lack an enzyme (called alpha 1,3 galactosyltransferase) found in pigs that attaches particular sugars on the surfaces of cells. When our bodies detect these sugars on transplanted pig cells, the immune system is triggered to attack the transplanted cells, leading to their death. Researchers are currently pursuing three ideas to eliminate the risk of hyperacute rejection and the need for strong immune suppression after pig islet transplantation: 1) the use of encapsulation; 2) the use of pigs that are genetically engineered so that their islets are as compatible for transplantation as human islets; and 3) the use of pancreatic tissue from pig embryos.
Encapsulation (see Encapsulation in Section 3.5 for more details) entails the placement of transplanted islets within a membrane that allows nutrients such as glucose, oxygen, and insulin to move in and out, but which blocks out cells of the immune system, preventing destructive immune components from reaching the transplanted cells. Thus far, New Zealand-based Living Cell Technologies (LCT) has progressed the farthest with developing encapsulated pig islet therapy for the treatment of diabetes. The company has already received approval to provide its encapsulated pig islet transplantation therapy DIABECELL in Russia, but LCT is unlikely to receive approval in many other countries, including the US, until longer studies are conducted. A recently completed phase 2 trial conducted in New Zealand showed that though use of DIABECELL in people with type 1 diabetes did not significantly lower insulin doses, it did reduce the incidence of severe, unaware hypoglycemia. LCT recently received permission from the New Zealand Minister of Health to expand this trial to two further patients who will receive the maximal dose of DIABECELL [118]. A similar phase 2 trial is ongoing in Argentina. LCT plans to begin phase 3 testing of DIABECELL in 2013 and to complete clinical trials by 2015 [119].
Progress towards making pig islet transplantation more feasible has also been made with the development of islets from genetically modified pigs. Most notably, the company Revivicor has created pigs that lack alpha 1,3 galactosyltransferase and that produce human CD46, a protein that helps protect cells against attack by the immune system [120]. Although islets obtained from pigs containing both genetic modifications have not yet been tested in primates, hearts transplanted from these pigs into primates survived and remained functional for over eight months. Encouragingly, it was reported in 2009 that diabetic primates transplanted with islets obtained from Revivicor’s genetically modified CD46 pigs achieved normal glucose control and insulin therapy independence for 3 to 12 months [121]. Although immune suppressive therapy was still necessary to promote the survival of these islets, the primates remained generally healthy and side effects were mild with no indications of reactivated viruses or blood clot formation (a serious side effect observed in previous studies of pig islet transplantations in primates). In the future, Revivicor plans to add additional genetic modification in its pigs to further improve the survival of their islets when used in transplantation. These modifications include genes that encode proteins which may decrease the diabetes T cell response against islets [120]. While immune suppression therapy will likely still be needed to preserve these cells over a long period of time, these modifications (along with the elimination of alpha 1,3 galactosyltransferase and addition of CD46) aim to make pig islets nearly as compatible as human islets so that similar immune suppression protocols can be used with pig islet transplantations as with human islet transplantations. No indications have been given as to when Revivicor will begin clinical trials with its genetically modified pig islets.
Finally, researchers are also investigating a novel approach that may minimize or eliminate the need for immune suppression therapy with pig islet transplantation entirely. In early studies, researchers found that the transplantation of pancreatic cells obtained from pig embryos into diabetic rats and primates resulted in long-term improvements in blood glucose control without the need for immune suppression [122]. Surprisingly, when these researchers transplanted rats with adult pig islets several weeks after the initial transplantation of embryonic pig pancreatic cells, they found significant improvements in the survival of the adult islets. This improvement was achieved without use of immune suppressive therapies [123]. These results suggested that embryonic pig pancreatic cells might improve the ability of the transplant recipient’s immune system to tolerate adult pig islets. In fact, while the rats that received both transplantations achieved normal glucose control, the rats that only received the transplantation of adult pig islets retained similar glucose control as diabetic rats that had received no transplantation.
Researchers have since been able to replicate this transplantation procedure in non-human primates (specifically rhesus macaques). The procedure did allow islets to properly establish themselves within primates’ bodies and secrete insulin. However, unlike in rats, it did not normalize responses to glucose. This is likely because larger quantities of islets are needed to correct glucose intolerance in non-human primates than in rats, and not enough islets were used. Before moving this procedure into human studies, researchers will have to refine it and conclusively show it is effective in non-human primates [124].
“Kelly’s Take”

Pig islets could be a near-term solution for the shortage of islets available for use in islet transplantation. When used in combination with encapsulation, the transplantation of pig islets could eliminate the need for toxic immune suppressing drugs. For that reason, many notable researchers and doctors are quite enthusiastic about pig islet transplantation. There’s quite a bit of activity in this area. In the US and New Zealand, efforts are already underway to obtain a safe supply of medical grade animals. Living Cell Technologies has been flying under the radar by doing its initial human clinical trial work in less heavily regulated places such as Russia, but it has received promising grants from the New Zealand government and JDRF. Phase 2 trials are taking place in New Zealand and Argentina. At this stage, it is hard to know how long the transplants from pigs will last, but advances in encapsulation technology and genetic engineering may make pigs the preferable source of islets in the near future.
In Summary…
• Pig islets are the most viable animal islet source. Currently, their use is not widespread because of rapid rejection, the need for harsh immune suppressive regimens, and possible inter-species virus transmission.
• Living Cell Technologies has identified a pathogen-free animal and is thus the first major player in this field. They are making progress with their encapsulated pig islet therapy DIABECELL, and we await Phase 2b data to see how well it works in humans.
• Revivicor is attempting to develop pigs whose islets are genetically engineered to be as compatible as human islets when used in transplantation. Initial results in animals have been reasonably promising.
3.5
The Bioartificial Pancreas
Who: 
When: 
Bottom Line: 
The need for broad immune suppression is a major limitation of islet transplantation procedures, regardless of whether the islets are obtained from human cadavers, animals, or will eventually be obtained from ESCs in the future. Over the last several decades, researchers have attempted to develop a number of devices that could eliminate the need for immune suppression therapies and improve the viability of transplanted islets in other ways. Generally, these devices use biomaterials (which are natural or synthetic materials suitable for use in the human body) to create a protective or supportive unit around the transplanted islets. These units promote islets’ survival while still allowing islets to continually monitor blood glucose levels and secrete proper amounts of insulin (and possibly other pancreatic hormones) so as to restore and maintain normal glucose control. Ideally, when perfected, these devices will mimic the glucose regulatory functions of a healthy pancreas, and therefore could eventually serve as “bioartificial pancreases.” Currently, a number of different variations of the bioartificial pancreas concept are being explored, several of which are discussed below.
Most of the potential components of a bioartificial pancreas remain in laboratory or animal studies, and many of these are likely a decade or more away from possible human use. Nevertheless, the prospect that a bioartificial pancreas that can significantly improve upon the safety and effectiveness of current transplantation techniques (i.e., by eliminating the need for broad immune suppression, enhancing the survival of transplanted islets, and allowing for easy implantation and replacement of transplanted islets) is certainly exciting. Along with the development of alternative sources of islets (see Section 3.4), the creation of a bioartificial pancreas may one day significantly expand the use of islet transplantation among people with type 1 diabetes. Even if the islets inside these devices require replacement as often as every two years, we believe this technology has significant potential to be considered a “cure” for type 1 diabetes.
We note that the bioartificial pancreas is a separate concept from the artificial pancreas (AP), which exclusively uses mechanical devices to mimic the glucose regulatory functions of the pancreas and is discussed in Chapter 4.
Encapsulation
Who: 
When: 
Bottom Line: 
As discussed in Section 3.4, the need for broad immune suppression to ward off the immune attack on transplanted islets is a major limitation of current islet transplantation techniques. Thus, technologies that protect islets from cells and components of the immune system will likely form an important part of any bioartifical pancreas. One such technology is islet encapsulation. Islet encapsulation aims to “wall off” transplanted islets from immune cells while still allowing beta cells to secrete insulin in response to glucose.When islets are encapsulated, they are wrapped in a selectively permeable barrier, which allows blood, oxygen, glucose, and insulin to move in and out, but which also blocks the infiltration of cells or large molecules from the immune system [125].
Two basic methods of encapsulation are under development: microencapsulation and macroencapsulation, both of which are illustrated in Figure 8. Macroencapsulation packages a large cluster of cells into a single device. A downside to macroencapsulation is that the free flow of nutrients into cells and insulin out of cells can be more challenging, which potentially results in increased beta cell starvation and death and less effective glucose control. This limitation is caused partially by the large size of the capsules themselves and partially by the tendency of islet cells to clump together. However, macrocapsules do remain relatively stationary after transplantation, facilitating later access if the device needs to be removed or replaced. Microencapsulation coats single islets or small groups of islets to obtain a smaller capsule. With this strategy, blood, oxygen, and glucose can more easily move into the islet cells, and insulin can readily move out. One drawback is that microcapsules are often hard to extract because they rarely remain stationary after implantation [125]. To make microcapsule removal easier, current research is also working on placing microencapsulated beta cells into devices that would confine the microcapsules to one area but which wouldn’t hinder nutrients from getting to the beta cells. Finally, the biocompatibility (not invoking an immune or inflammatory response to the capsule material itself) of the materials used to construct the capsules is another limiting factor facing both micro- and macroencapsulation. In the past, most macrocapsules and many microcapsules were made of poly-L-lysine or PLL, a material which is not very biocompatible and which, for this reason, often triggers unwanted immune responses that wall off capsules with natural scar material. This process can lead to cell starvation, oxygen deprivation, and cell death [126]. However, the use of ultra-pure alginate, a substance commonly derived from seaweed, along with other polymers, such as PLO, has appeared to minimize these unwanted side effects [127].
While most encapsulation research is being conducted in academic laboratories, Living Cell Technologies’ (see Pig Islet Transplants in Section 3.4) microencapsulation method IMMUPEL (which uses ultra-pure alginate) is currently being evaluated in humans in New Zealand and is supposedly both more stable and more selective than other available encapsulation materials. Initial results have suggested that IMMUPEL can eliminate the need for immune suppression after transplantation [128]. It remains unclear whether this technology will truly allow for the long-term survival of transplanted pig islets in humans.
In a slightly different variation of encapsulation, Cerco Medical is developing a macroencapsulation device called the Islet Sheet. Reportedly the size of a business card, the Islet Sheet consists of a layer of human islets surrounded by mesh, which, in turn, is bound to an ultra-pure alginate encapsulating membrane. The added mesh layer aims to provide the device with extra strength and durability to protect islets from physical damage. This product is still being evaluated in animal studies (studies with dogs started in early 2012) and the first human studies are expected in 2013 [129, 130].
In Summary…
• Islet encapsulation may prevent immune attack on transplanted islets without the use of immune suppressive therapies.
• Macroencapsulation packages large clusters of islets together, while microencapsulation packages them singly or in much smaller clusters.
• Macro and microencapsulation approaches may eventually be combined by placing microencapsulated beta cells into macrocapsules.
• Advances in this field have been slow, though LCT’s IMMUPEL microencapsulation technology and Cerco Medical’s Islet Sheet may hold promise.
Scaffolding
One limiting factor for macroencapsulation, as noted above, is the tendency for islets to clump together, limiting the flow of nutrients to and insulin away from the islets located near the center of the device. Additionally, in the pancreas, the organization of islets and the contacts they make with other pancreatic cells play important roles in maintaining beta cell health and function. When islets are transplanted, this organization and these contacts are lost, a result that appears to contribute to poor islet function after transplantation. To address both of these limitations, researchers are investigating the use of structures called scaffolds that could be combined with microcapsules or even used independently from any encapsulation technique. Scaffolds can be compared to the frame of a house before it is built, where wood beams provide the framework for the three dimensional structure (see Figure 8). Instead of wood, scaffolds are made of biomaterials that form compartments for transplanted islets to reside within. This compartmentalized structure helps provide support for the transplanted islets, protecting them against damaging forces produced by body movements, and ensuring proper spacing between each islet. Proper spacing ensures that each islet receives an adequate supply of nutrients, and provides surfaces to place other pancreatic components or cells to help recreate a pancreas-like environment [20, 131]. Research into optimal scaffold design is ongoing at the Diabetes Research Institute (DRI) as well as academic labs worldwide. To date, several studies conducted in animals or in cells cultured outside the body have shown promise for the use of scaffolds in a bioartificial pancreas [221]. Testing of scaffolds in humans, however, has only just begun. Currently, Dr. Denis Dufrane, MD, PhD (Université Catholique de Louvain, Brussels, Belgium) and Dr. Pierre Gianello, MD, PhD (Université Catholique de Louvain, Brussels, Belgium) are conducting a phase 1 study in Belgium that is enrolling people with established type 1 diabetes; the study will examine the safety and effectiveness of a macroencapsulation device that has a scaffold-like design in the interior. The trial was initiated in 2008 and plans to finish in 2013.
In Summary…
• Scaffolds are compartmentalized devices that aim to improve survival of transplanted islets by providing physical support for the islets, as well as proper spacing between islets. Scaffolds ensure that each islet receives an adequate supply of nutrients and also provide space to place other pancreatic components or cells that help recreate a pancreas-like environment.
• Scaffolds can be used with microcapsules or independently from any encapsulation strategy.
• A phase 1 trial with human islets placed into a scaffold-like device is currently taking place in Belgium in people with type 1 diabetes.
Improving Blood Supply
Another important requirement for islet survival is close proximity to blood vessels, which deliver oxygen and nutrients to the islets and carry secreted insulin to the rest of the body. Several strategies that could eventually be incorporated into a bioartificial pancreas are being explored to improve the blood supply to transplanted islets. In general, these strategies implant islets into devices made of materials that contain pores, which promote the infiltration of blood vessels near the islets (see Figure 8). A porous metal cage implanted under the skin is an example of this type of device; in this case, the pores are larger than the pores in micro—and macrocapsules. Over a period of days or weeks, these cages are colonized by blood vessels and can be subsequently loaded with islets. In a study with diabetic rats, the transplantation of islets housed in this type of cage was shown to reverse diabetes. Although not protected from the immune system in any way, the transplanted islets were able to secrete insulin, become surrounded by blood vessels, and remain functioning and healthy for more than 160 days [126, 132]. A variation of this strategy is used in Viacyte’s encapsulation device Encaptra. With Encaptra, macroencapsulated islets are surrounded by a layer of mesh that serves to facilitate the growth of islet progenitors [133]. Using a slightly different approach, Sernova is developing a porous scaffold device called the Cell Pouch. Although no encapsulation technique is used, the scaffold has been shown to improve the survival of transplanted islets in animals by quickly promoting the growth of blood vessels into the device. Whether immune suppression will still be necessary to promote the long-term survival of the transplanted islets, however, remains unclear [134]. While Encaptra is still in preclinical testing, the first human implant of the Cell Pouch occurred in August 2012 as part of a phase 1/2 trial; results from this trial are expected in 2015 [135].
Alternatively, blood supply to transplanted islets may be enhanced through the use of certain substances that promote the growth of new blood vessels. These substances can be incorporated into the material of the scaffolds or micro- and macrocapsules themselves, or can be released locally from depots attached to these structures. In several early animal studies, the addition or delivery of these substances (such as Vascular Endothelial Growth Factor or VEGF) from several types of devices was shown to improve blood vessel density around encapsulated islets [136–138], and even increase encapsulated islet survival [125, 131].
In Summary…
• Because blood delivers essential nutrients to islets and carries insulin produced by islets to other cells of the body, a close proximity to blood vessels is an important factor for transplanted islet survival.
• To improve blood supply to transplanted islets, researchers are developing a number of devices that contain pores or materials which allow or promote the infiltration of blood vessels near islets.
• Several devices, including Viacyte’s Encaptra and Sernova’s Cell Pouch, have shown promise in animals. Phase 1/2 studies of Cell Pouch began in June 2012, with the first human implantation occurring in August.
Additional Strategies
Finally, significant work is underway by a number of academic groups and institutions, including the DRI, to improve the survival of transplanted islets through the attachment of a number of different chemicals or slow releasing depots of chemicals to the surfaces of micro- or macrocapsules and scaffolds. These chemicals can include anti-inflammatory or immune suppressive drugs that could help further limit harmful responses by the immune system against the transplanted islet cells or the synthetic material of the device itself (see Figure 8) [139, 140]. These chemicals may also include compounds (called PEG or polyethylene glycol chains) that are commonly used to mask proteins and materials from the immune system [141, 142]. Unlike in current islet and pancreas transplantation procedures, all of these therapies will only be delivered locally to the transplanted islets, allowing for significantly smaller overall doses to be used and for exposure to these drugs throughout the rest of the body to be very limited, lowering the risk for serious side effects. Beyond anti-inflammatory, immune suppressive, and immune masking therapies, the ability of a variety of other chemicals to promote islet function and survival is also being explored in animal studies. For example, the company Beta-O2 is developing a macroencapsulation device called ßO2 that is able to produce and supply oxygen to the encapsulated islets itself. In diabetic rats, the device was shown to return glucose levels to normal levels after subcutaneous implantation, though it’s not clear whether the capsule’s oxygen supply was responsible for improving islet survival [143].
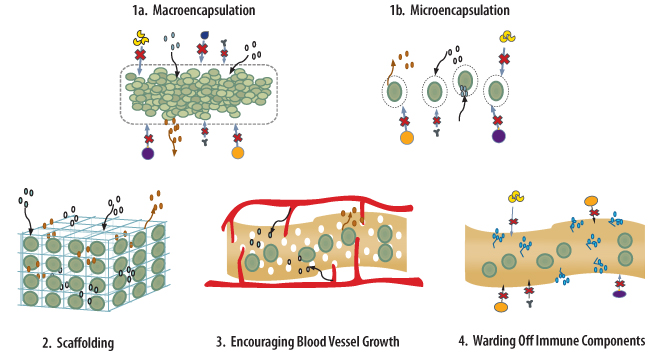
Figure 8. Illustration of beta cell protection and survival enhancement strategies. 1) Transplanted beta cells or islets may be protected from immune attack by placing them inside porous capsules. The holes in these devices are too small to let immune cells (such as T cells (orange), B cells (purple), and macrophages (blue)) or antibodies (black, Y-shaped) pass through, but nutrients such as glucose and oxygen (blue and white) can move into the capsule and insulin (red) produced inside the capsule can pass out into the blood. 1a) In macroencapsulation, many beta cells are placed into one large capsule. 1b) In microencapsulation, just one beta cell or a few beta cells are placed into much smaller capsules and many capsules are placed in the body. 2) Beta cells can be placed inside scaffolds, which can be compared to the frame of a house but are made of natural or synthetic materials that can be placed inside the body. In these scaffolds, beta cells are properly spaced and receive proper nutrients and oxygen. It might also be possible to place other pancreatic cells and components in these scaffolds to create a pancreas-like environment. 3) Close proximity to blood vessels is important for ensuring that transplanted islets receive proper supplies of oxygen and nutrients and insulin produced by the islets becomes distributed to the rest of the body. This can be accomplished by placing beta cells on materials whose pores allow for infiltration of small blood vessels and/or present factors that encourage blood vessel growth. 4) By presenting anti-inflammatory or immune suppression chemicals (blue) on the natural or synthetic materials that beta cells are placed on, harmful immune responses against beta cells could be avoided. These chemicals could act over a short range, possibly lowering the risk of serious side effects associated with other immune suppression therapies.
“Kelly’s Take”

An implantable device that promotes the survival of transplanted islets is sometimes termed a “bioartificial pancreas,” and it’s an exciting idea. At this point, we have many unanswered questions about the quality and durability of the different approaches being taken to create a bioartificial pancreas, such as micro- and macroencapsulation. We don’t know how long islets will keep working in these pancreas-like devices. But even if the device needed changing every few years, it would no doubt be welcomed by many patients, especially if it eliminated the use of harsh immune suppressing agents that are needed for the survival of transplants currently. Work on the “bioartificial pancreas” is being done mostly in the research labs, with the exception of a few pioneering companies.
In Summary…
• Researchers are also working to improve the survival of transplanted islets by attaching a number of different chemicals or slow releasing depots of chemicals to micro—or macrocapsules and scaffolds.
• These chemicals could include anti-infammatory or immune suppressive drugs that may help further suppress harmful responses by the immune system against the transplanted islet cells or the synthetic material of the device itself. These chemicals could also encourage beta cell growth and survival.
• Beta-O2 is developing a macroencapsulation device called βO2 that is able to produce and supply oxygen to the encapsulated islets itself. In diabetic rats, the device was shown to return glucose levels to normal levels after subcutaneous implantation.
3.6
Overall Potential
At the moment, the use of transplantation therapy for the treatment of type 1 diabetes is limited by shortages of islets and pancreases, high costs, and risks associated with current immune suppressive therapies. It is unlikely that the availability and use of transplantation therapy will increase unless new sources of islets are identified and new, safe, and minimally invasive strategies to protect transplanted islets from rejection are developed. Despite repeated alterations of protocols, most people that undergo these procedures today are unable to eliminate the need for insulin therapy over the long term. At least in the near future, we see the modification of immune suppression protocols for islet and pancreas transplantation as an area of declining focus while research on alternative beta cell sources and the use of devices to improve islet survival continues to expand and progress. Still, for some people whose diabetes is very difficult to control with insulin therapy or those who have severe hypoglycemic unawareness, transplantation remains an attractive option because of its effectiveness in reversing hypoglycemic unawareness, improving blood glucose variability, and lowering insulin dose requirements.
3.7
Where To Learn More
Pancreas and Islet Transplantation Procedures
Pancreatic Islet Transplantation—The National Institutes of Health
Pancreas Transplantation—MedScape
Benefits and Risks of Pancreatic Islet Transplantation—The National Institutes of Health
Creating Beta Cell From Stem Cells
Stem Cell Basics—The National Institutes of Health
Induced Pluripotent Stem Cells—The National Institutes of Health
Stem Cells and Diabetes—The National Institutes of Health
Pig Islet Transplantation
Xenotransplantation Overview—MedScape
DIABECELL—Living Cell Technologies
Bioartificial Pancreas
Biohybrid Devices—The Diabetes Research Institute
IMMUPEL—Living Cell Technologies
Tissue Scaffolds—The Diabetes Research Institute
Reducing Inflammation—The Diabetes Research Institute

4.1
Background
Who: 
When: 
Bottom Line: 
Especially in light of the challenges of islet transplantation, the discovery of drugs that prompt the creation of new beta cells and protect their health and survival would represent a major leap forward for cure-targeted therapies. If successfully developed, beta cell regeneration agents could be used by any person with type 1 diabetes to restore lost beta cells and to remove the need for insulin therapy. In theory, these treatments may even be useful in delaying the onset of diabetes in people with prediabetes.
Functional beta cell mass (beta cell number, size, and function) has been shown to increase in response to a range of physiological conditions including pregnancy, childhood growth, and obesity. In mice, several different mechanisms have been proposed for generating new beta cells; these include the generation of completely new beta cells (neogenesis), the division of mature beta cells to create additional cells (replication), and the transformation of another adult cell type into a beta cell (transdifferentiation). The type of mechanisms that play a role in beta cell expansion in normal people is still an area of active investigation; replication of existing beta cells is thought to be the method most commonly used in adult animals, but neogenesis hasn’t been ruled out [144]. At this point, most of the research on beta cell regeneration has been conducted in diabetic mice. Humans’ ability to generate new beta cells appears to wane after adolescence, and whether humans possess the same spontaneous beta cell regeneration capabilities as mice, particularly in response to injury or with aging, is not yet understood [145].
Much research interest also focuses on discovering drugs that could block beta cell loss or death. Beta cell survival therapies could be used to preserve beta cells remaining in the pancreas, slowing or halting the development or progression of type 1 diabetes in people with prediabetes or newly diagnosed disease. Additionally, survival therapies could be used to protect newly regenerated or transplanted beta cells and islets from autoimmune destruction, which could be beneficial for people with established diabetes. In contrast to immune therapeutics, which try to preserve and protect beta cells by modifying the immune system, survival therapeutics generally have little impact on the immune system. While several cellular components thought to be involved in beta cell survival have been identified from studies in animals and cultured human beta cells (beta cells grown outside the body), the development of survival therapies is still in early stages and to date only a few therapies have been explored in human trials.
In Summary…
• Beta cell regeneration therapies would stimulate the generation of new beta cells; when coupled with therapies that halt the immune response against beta cells, regeneration therapies could help a person with type 1 diabetes restore lost beta cells and regain independence from insulin therapy.
• Beta cell survival therapies would preserve and protect existing beta cells.These drugs could promote the survival of remaining beta cells in people with prediabetes or recent onset disease. Survival therapies may also be used to preserve beta cells or islets that have been newly regenerated or transplanted.
4.2
Beta Cell Regeneration and Survival Factors
Scientists have figured out many of the signals regulating islet replication and neogenesis through studies in animals, providing us with potential targets for beta cell regeneration drugs in humans. A variety of molecules (including a number of growth factors and hormones) have been able to induce neogenesis, beta cell proliferation, or insulin production in animals [136, 144]. A particularly interesting example of such a factor is the Human proIslet Peptide (HIP), which is being developed by a company called Cure DM. In mice with type 1 diabetes, HIP was able to improve glucose control, reduce insulin requirements, and induce new islet formation [137]. Given that there is not yet convincing evidence of the regenerative capacity of human beta cells [146], results with HIP and all of the other factors under investigation, though exciting, must be verified in human studies.
Of all the factors explored so far, the most clinical focus has centered on incretins and incretin-related compounds as both potential regeneration and survival agents. Incretins, including GLP-1 (Glucagon Like Peptide-1), are hormones that increase insulin secretion only when glucose levels in the blood are increased. GLP-1 and its analogs (analogs have a similar function as the original molecule but are chemically different) have been shown to increase islet neogenesis, beta cell proliferation, and beta cell survival in animal models [138, 147]. Three GLP-1 analogs, Byetta (exenatide) and Bydureon (exenatide ER), made by Amylin, Bristol-Myers Squibb, and AstraZeneca, and Victoza (liraglutiude), made by Novo Nordisk are currently approved as treatments for type 2 diabetes in the United States. Additionally, DPP-4 inhibitors act to block the break-down of GLP-1 in the body and, thus, could also have positive effects on beta cell mass, proliferation, and survival as has been shown in early studies [148]. Januvia (sitagliptin), made by Merck & Co., Nesina (alogliptin), made by Takeda, Onglyza (saxagliptin), sold by Bristol-Myers Squibb and AstraZeneca, and Tradjenta (linagliptin), produced by Eli Lilly and Boehringer Ingelheim, are four DPP-4 inhibitors currently approved for sale in the United States; these drugs are used as treatments for type 2 diabetes.
Experiments in cells cultured in the lab and diabetic animals have demonstrated the potential of GLP-1s and DPP-4 inhibitors to promote beta cell regeneration and survival [146, 149, 150]. However, human clinical results have not been definitive. Several trials have suggested that GLP-1 analogs are capable of improving glucose control4 in people with established type 1 diabetes. In some of these trials, the drugs also reduced insulin requirements and suppressed glucagon secretion. GLP-1 analogs have not, however, been shown to increase C-peptide levels (which would indicate beta cell regeneration or positive changes in beta cell function) [151–156]. Meanwhile, the DPP-4 inhibitor Januvia has been found to have positive effects on glucose control and insulin requirements in adults with type 1 diabetes. The effects of GLP-1 agonists on the survival and function of transplanted islets have also been examined in several small trials. Two of these studies investigated the long-term use of Byetta following a supplemental islet transplantation procedure (a second infusion of islets after failure of the initial transplant) and found stable improvements in C-peptide levels, insulin independence, and blood glucose control while the drug was being used, suggesting positive effects of Byetta on beta cell function and survival [98, 157]. Another suggested the ability of Byetta to stimulate and preserve insulin secretion after islet transplantation [158]. These results are promising, but because of the small size of the study populations in these trials, they must be confirmed in larger and longer studies. Nevertheless, if positive study results do continue, the fact that these compounds are known to be safe and are already approved for use in type 2 diabetes may hasten their approval process for use in type 1.
Human clinical studies with factors other than incretins are also at an early stage. For example, in initial trials in both type 1 and 2 diabetes, Islet NeoGenesis Associated Protein (INGAP), a factor thought to stimulate islet neogenesis) did cause moderate improvements in C-peptide levels [159], but it did not lead to reductions in insulin doses. In studies with analogs of gastrin, a digestive hormone, in combination with other factors, an increase in functional beta cell mass was observed in animals, but the same effect has not yet been supported by human clinical trials [160, 161].
Since inappropriate cell growth and tumor formation could result if regeneration or survival therapies accidentally promote the generation of other types of cells in the body, it will be important to assure that these factors limit their effects to beta cells [161].
Beta cells have been found to replicate specifically when demands on insulin production are increased (such as during pregnancy, growth, or obesity), so it’s highly possible that we can find the specific molecules and mechanisms by which to very selectively promote beta cell growth without affecting the growth of anything else. HIP and INGAP are examples of compounds that might be able to promote specific beta-cell specific regeneration [144, 162].
It’s worth noting that in future studies, beta cell mass and function will need to be better measured in order to assess the regeneration and protective potential of specific therapies. But it’s currently hard to measure beta cell mass in living people, because of the lack of accurate and reliable tools such as imaging techniques. This remains a notable limitation of current beta cell regeneration and survival research.
In summary, while several compounds have been shown to regenerate or preserve islets and improve glucose control in animals, results from clinical trials have not yet repeated these positive findings. Although important biological differences between the animal models and humans also exist, discrepancies between human and animal trials could also potentially be due to the way these trials have been designed.
In Summary…
• Research efforts to develop both regeneration and survival therapies for beta cells largely remain in early stages.
• Studies in animals and with human beta cells cultured outside the body have expanded our understanding of the processes regulating beta cell regeneration and survival.
• A number of compounds, including GLP-1 agonists, DPP-4 inhibitors, gastrin analogs, CureDM’s Human proIslet Peptide (HIP), and INGAP, have been shown to promote beta cell regeneration and/or preservation in animals with diabetes.
4.3
Current Trials
Many trials are continuing to explore the use of incretins and other factors as beta cell regeneration and/or survival-promoting agents. REPAIR T1D, a phase 2 trial supported by JDRF, is examining the effect of sitagliptin plus lansoprazole (which increases gastrin levels) on beta cell survival and regeneration in people who have been diagnosed with type 1 diabetes within the last six months. Additionally, a Swedish study will soon begin looking at the effects of liraglutide on A1c levels in adults with poorly controlled diabetes, and a similar trial is being conducted in Israel. There also are two trials recruiting at the Albert Einstein College of Medicine that are examining the use of exenatide in type 1 diabetes. The first is evaluating exenatide’s effect on post-meal hyperglycemia, post-meal glucagon, and gastric emptying in people who have recently been diagnosed with type 1 diabetes. The second is studying the glycemic effects of adding either exenatide or pramlintide (which suppresses glucagon secretion) to insulin therapy in people who have had diabetes longer than a year.
Incretins are also being actively studied on the transplantation front. A trial at the University of Miami is evaluating whether exenatide can improve survival and function of transplanted islets. Additionally, a currently-recruiting trial sponsored by Novo Nordisk is looking at whether liraglutide treatment can increase the proportion of subjects who do not require insulin one year after receiving a transplant.
Finally, a phase 2 trial is examining the safety and tolerability of INGAP injections, as well as the therapy’s effects on C-peptide, glucose, glucagon, and insulin in people with established type 1 diabetes.
“Kelly’s Take”

Restoring or protecting beta cells in a way that is non-invasive and does not require the strong immune suppressing therapies used in transplantation has real potential to revolutionize the treatment of type 1 diabetes. We are encouraged that multiple companies have ongoing programs focused on developing compounds that achieve just these goals. Still, research on beta cell regeneration and survival agents remains at an early stage, and we still have much to learn about how beta cells are normally generated, protected, or regenerated in the body. Since these therapies could have an impact on many groups of people with type 1, we hope that the positive results found in animals so far are also observed in ongoing and future human studies.
4.4
Where To Learn More
Beta Cell Regeneration
An Overview of Beta Cell Regeneration—Ann Bartlett
Beta Cell Regeneration and Survival Agents
Human Islet Propeptide—Cure DM
Januvia Lowers Blood Sugar in People with Type 1 Diabetes

5.1
Background
Who: 
When:  5
5
Bottom Line: 
After half a century of research into the artificial pancreas (AP), the majority of patients with type 1 diabetes are still taking injections, counting carbohydrates, and spending a significant portion of the day in states of hypo—and hyperglycemia. For example, in an oft-cited 2005 study by Dr. Bruce Bode MD, FACE (Emory University School of Medicine, Atlanta, GA) type 1 patients were found to be “in the optimal ADA glycemic zone” only 28% of the time, with the average patient spending over two hours per day in hypoglycemia [163]. However, many experts believe that an early form of the AP—an automated device that can sense glucose and secrete an appropriate quantity of insulin in response—is coming in the next five years, although regulatory hurdles have already slowed its clinical deployment, and we are concerned about the impact of the FDA’s caution on this timeline.
Although some people don’t consider the AP or any mechanical intervention a cure, we believe that it has the potential to significantly change the diabetes landscape, since its effects on everyday life could be so significant. We should note that our vision of the AP differs substantially from the current diabetes technology some people wear routinely—we are not just thinking of the AP as a slightly more advanced version of an integrated pump/CGM combination. As currently envisioned, with the “real” AP, there would be minimal thinking or user input—or ideally none. A “hybrid” AP would fall somewhere between today’s systems that rely solely on user input, and a fully automated system requiring no user input at all.
At its heart, the AP will involve an insulin pump, a continuous glucose monitor (CGM), and a control algorithm. The hope is that this “closed-loop system” will act as a “cure” in that it will mimic the functions of a normal pancreas; it will detect changes in blood glucose (via CGM) and regulate insulin delivery (via a control algorithm and pump) in order to maintain blood glucose within a normal range. By controlling blood glucose, the AP will help reduce a person’s chance of developing long-term complications, and by eliminating some or all patient involvement, it will ease the daily management of diabetes. Additionally, if adopted soon after diagnosis or immediately post-transplantation, the AP may even help preserve beta cells because lowering blood glucose levels helps lower beta cell stress. Since the ideal AP would effectively replace pancreatic function, this device could be used at any stage of diabetes after diagnosis, but would not slow the progression of prediabetes, or prevent the onset of beta cell destruction in people at high risk for type 1.
We believe that a combination subcutaneous pump-subcutaneous CGM sensor system [164] has the greatest potential for approval and widespread application. We approach the following section with this focus in mind. However, we note that research to develop AP-associated technologies that are further away from clinical use, such as intravenous glucose sampling and next-generation implantable insulin pumps, is also ongoing.
In addition to its commitment to curing type 1 diabetes, JDRF has emerged as a key leader in the development of the AP, which will help individuals with type 1 diabetes live healthier lives today. The JDRF Artificial Pancreas Consortium aims to get closed-loop products in the hands of people with diabetes as quickly as possible, by getting components from multiple companies approved and reimbursed, while encouraging investment in the next generation of technologies. The Consortium consists of leading AP researchers at 13 sites, eight of which are in the US. Beyond being able to attract a wide range of scientists, companies, and countries to its cause, its unique role as a patient advocacy organization has positioned JDRF well to advance the AP cause with both the government and industry.
The Leona M. and Harry B. Helmsley Charitable Trust has also made substantial commitments to funding artificial pancreas research and development. The Trust has a $10 million Emerging Technologies Initiative (ETI) focused on accelerating the development of novel technologies to improve the management of type 1 diabetes. Additionally, on June 22, 2011, JDRF and the Helmsley Charitable Trust announced the launch of a major funding initiative (up to $20 million per project) aimed at accelerating the development and delivery of advanced continuous glucose monitors. The goal of the initiative will be to develop sensors that are capable of driving closed-loop systems to a glycemic range seen in those without diabetes.
In Summary…
• The artificial pancreas (AP) will consist of an insulin pump, a continuous glucose monitor (CGM), and a control algorithm, which will act together to regulate glucose like a “normal pancreas.” The ideal AP would bypass all user involvement.
• This device could be used at any stage of diabetes after diagnosis.
• JDRF and the Helmsley Charitable Trust, in particular, are key drivers toward the goal of developing an artificial pancreas.
5.2
Current State of Closed Loop Components
Insulin Pumps
Insulin pumps have been around for decades and are generally considered safe and effective for the management of insulin dependent diabetes. Studies have shown that the use of pumps can drop A1c (~ 0.21%—0.76%) and reduce the risk for hypoglycemia as compared to standard multiple daily injection (MDI) therapy [165]. Two types of pumps are currently available: standard durable pumps (e.g., Medtronic’s Paradigm Revel) and disposable patch pumps (e.g., Insulet OmniPod). Standard durable pump systems consist of the pump and insulin reservoir attached via tubing to the infusion site. Patch pumps are tubeless, and consist of a remote wireless controller and a wearable unit filled with insulin.
Timing of insulin action is an issue when using insulin pumps. With currently available pumps, insulin is dosed subcutaneously (under the skin). This creates delays in the timing of insulin action as compared to normal insulin production, where insulin goes directly from beta cells into the blood. Despite this issue, most experts would argue that current insulin pump technology is sufficient for incorporation into hybrid versions of the artificial pancreas (i.e., still requiring patient input). More fully automated versions of the artificial pancreas would require advancements in insulin delivery (see Section 5.3).
In Summary…
• Insulin pumps deliver designated doses of insulin and can reduce A1c as well as hypoglycemia.
• Overall, these devices are considered safe and effective for use in diabetes management.
Continuous Glucose Monitors
Continuous glucose monitoring (CGM) technology provides real-time, continuous glucose readings to the person wearing the device. Real-time CGM was approved by the FDA in 2005 and is comprised of a sensor that is placed just under the skin (where it detects glucose from the interstitial fluid that fills the spaces between blood vessels (capillaries) and cells, rather than directly from the blood), a transmitter that wirelessly sends the values, and a receiver that displays real-time glucose values, trends, and graphs. Changes in blood glucose levels take time to show up in the interstitial fluid, so a 5-20 minute delay exists between readings from this fluid and current blood glucose values.
In the US, there are three currently marketed CGM systems that exist as stand-alone products: Dexcom’s G4 Platinum (approved by FDA on October 5, 2012), Dexcom’s Seven Plus, and Medtronic’s Guardian Real-Time. Additionally, Medtronic has an integrated insulin pump-CGM system in the US: the Paradigm Real-Time Revel. An integrated system uses the insulin pump as the CGM receiver, requiring only one device to be carried at a time. However, separate sites on the body are still required for the pump infusion set and CGM.
Standalone CGM sensors currently available outside the US include Abbott’s FreeStyle Navigator (discontinued in the US in August 2011) and FreeStyle Navigator II, Dexcom’s G4 Platinum, and Medtronic’s Enlite. US FDA approval of the newer and more accurate Medtronic Enlite sensor is expected in the first half of 2013. No FDA approval timeline has been announced for Abbott’s FreeStyle Navigator II. CGM-integrated insulin pump systems currently available outside the US include Medtronic’s Veo low glucose suspend insulin pump/Enlite sensor and the Animas Vibe insulin pump/Dexcom G4 sensor. FDA approval of the Medtronic system is expected in the first half of 2013, while approval of the Animas system could come sometime in the second half of 2013. See Section 5.3 for further discussion of these and other new technologies.
All current CGMs require daily fingerstick blood glucose calibrations. Therefore, they do not eliminate traditional blood glucose monitoring, but instead provide additional information to the user in the form of real-time trends, more comprehensive analysis of historic blood glucose levels, hypoglycemia alerts, etc. In terms of days of wear, CGMs are FDA approved for use between three and seven days (depending on the product), though according to our data, most CGM users wear the sensors for longer periods of time. Additionally, as CGM sensor durability and reliability improves with newer generations, users will likely be able to wear the sensors for longer periods of time. We anticipate that some companies will ultimately show success in trials that support a longer wear indication.
CGM allows for intensified glycemic control without an accompanying increased risk for hypoglycemia. The JDRF-CGM Trial showed that CGM was able to improve A1c by 0.5% in regular users, while the STAR-3 study, which compared sensor-augmented pump therapy (use of both CGM and an insulin pump) to traditional blood glucose monitoring and MDI, showed A1c improvements of 1.2% in adults who used the CGM sensor and pump more than 80% of the time [166, 167]. In both studies, exposure to hypoglycemia in those wearing the CGM was equivalent to or lower than in those who didn’t use the CGM.
For a number of different reasons relating to reimbursement as well as hassle, initial patient uptake of CGM devices was relatively low. However, acceptance of the technology has recently accelerated, and we believe that eventually most insulin users will monitor their glucose levels with CGM. While many early adopters of CGM fell into the 25-30% of people with type 1 diabetes who used pumps, individuals on multiple daily injections are increasingly using a CGM. Despite the fact that, in a recent study, over 70% of all CGM users believed that CGM helped them better manage their diabetes, people sometimes discontinue CGM because of equipment problems, insurance coverage, and perception of device inaccuracy [168] (however, as we understand it, the percentage of users who discontinue for these reasons is becoming smaller and smaller as perception of value increases with every generation of the product). In an analysis of individuals from the JDRF-CGM trial, study participants who had the best glycemic outcomes while wearing the CGM were those who coped well with frustrations, used historic data analysis, and involved their spouse or significant other in CGM use, suggesting that adaptation is a big determinant of success [169]. Technical and financial issues are also important in their own right. Ease of use, accuracy, and reimbursement have improved dramatically since CGM was first marketed, but they could still improve further.
Accuracy of CGM devices is an especially critical issue in closed-loop development, and is considered by many to be one of the major technological stumbling blocks to creating an ideal system. Several factors affect how well CGM readings match up with true blood glucose levels. One is how accurately CGM sensors can measure the glucose levels in the interstitial fluid, and another is the inherent time lag between glucose levels in this fluid and the blood. Mismatches between true versus CGM reported levels may also occur because CGMs are calibrated based on traditional blood glucose meters, which themselves don’t represent true blood glucose levels 100% of the time. Finally, CGM accuracy can also vary based on the specific user, glycemic state (e.g., hypoglycemic, hyperglycemic, rapidly changing), the manufacturer of the device, and the length of time a person has been wearing the sensor (i.e., sensors tend to get more accurate the longer they are worn, until at a certain point, they cease to function due to biological interference or sensor breakdown).
The three CGMs currently available in the US have an average error of around 13-20% across the range of 40-400 mg/dl, with the greatest percentage inaccuracy during hypoglycemia. Average errors for each system can be obtained from the respective user guides, which are available online. These numbers are based on the clinical trials used by the three companies to obtain regulatory approval for each of the devices.6 Data from the pivotal studies for each of the four devices found average errors of 13.2% for Dexcom’s G4 Platinum [170], 12.8% for Abbott’s FreeStyle Navigator [171], 16% for Dexcom’s Seven Plus [172], and 19.7% (in adults) and 19.0% (in children ages 7-17) for Medtronic’s Guardian Real-Time [173]. As of August 2011, the FreeStyle Navigator has been discontinued in the US, though it is still available internationally. Newer CGM systems are currently available internationally, but are not yet approved by the FDA (see Section 5.3 for a discussion of these systems).
Opinions are divided over whether CGM technology is already accurate enough to run an AP. Dr. Kowalski believes that available CGMs are accurate enough to drive insulin pumps, provided proper boundaries are set (i.e., a more-conservative “control-to-range approach” that would steer a person’s glucose to a range of acceptable glucose values (80-150 mg/dl) instead of targeting a specified level like 100 mg/dl [174, 175]; for more on control-to-range research, see Section 5.3 below). Others in the field question this approach, noting that there is a potential danger of over—or under-infusing insulin based on CGM values that may not reflect true blood glucose.
Another issue for CGM is reliability. We recall that blood glucose meters often stopped working or gave error messages in the early 1980s, whereas today they rarely give error messages. Although CGM reliability has improved since CGMs came on the market, some researchers have suggested that a fully closed-loop system will require two or more separate CGM sensors. In one version of the system, one sensor would serve as the primary control sensor for the closed loop and the other would serve as a redundant sensor for safety. In such a system, the second sensor would provide a separate, independent monitor of a patient’s glucose level. If the glucose values reported by the primary and secondary sensors are not in agreement, automated delivery of insulin would cease and the insulin pump would revert to a preprogrammed basal rate of insulin delivery like that used in current open-loop insulin pump systems. Multiple sensors could also be averaged or otherwise combined to serve as inputs in more complicated AP algorithms to further boost the accuracy and reliability of CGMs. For instance, studies have shown that a single Dexcom Seven Plus sensor chosen at random has on average 19 hours per month of large errors (greater than 50%). Using the input from two sensors can reduce this to seven hours per month [176]. Of course, many believe that single-sensor reliability and accuracy can be improved such that one sensor could be sufficient to drive a closed loop.
In Summary…
• Continuous glucose monitors (CGM) provide real-time, continuous glucose readings to the person wearing the device. If worn six or more days per week, they can improve A1c by 0.5% or more.
• Due to cost, accuracy, and greater demands placed on people wearing the devices, uptake of CGM has been slow, but will increase.
• As technology and reimbursement improves in coming years, we see patient acceptance of CGM rising substantially.
Control Algorithms
The third major component of the AP is a control algorithm, which takes readings from a CGM, interprets them, and instructs insulin pump dosing accordingly. The development of these algorithms is challenging. Delays are inherent in any algorithm-driven system, as it takes time for that system to realize a glucose change has happened, time for the insulin to work, and then more time to see if the effect was what was expected. Additionally, in such a system, if too much insulin is given, nothing can currently be done about it except to warn the patient (future systems may include glucagon or remote monitoring; see Section 5.3 for a discussion of these topics).
There are three major types of algorithms currently being studied: Model Predictive Control (MPC), Proportional, Integrative, Derivative Control (PID), and MD-Logic Control [177–179]. These were previously tested on animals (such as diabetic pigs or dogs). Then, in 2008, a landmark FDA approval allowed the testing of control algorithms with computer simulations, making it far easier and faster to initially test their safety and effectiveness [180].
In this report, we won’t get into the mathematical pros and cons of the different types of algorithm, except to say that over the last three years we’ve made remarkable progress in developing algorithms that can control blood glucose well and can adapt to variations from patient to patient and from day to day or week to week.
The simplest artificial pancreas algorithm approach is low glucose suspend (LGS): when the CGM hits a set threshold for hypoglycemia (e.g., 70 mg/dl), the pump suspends insulin delivery and alarms the user. When the alarm is not responded to – typically while the patient is sleeping – insulin delivery will remain suspended for two hours (or until the alarm is cleared). This algorithm is currently part of the Medtronic Veo insulin pump, and in an eight-week home study in 24 patients with type 1 diabetes, it reduced time spent in hypoglycemia by 42% without increasing overall mean blood glucose [181]. Predictive LGS systems are also in development, which would suspend insulin delivery when a low blood sugar is predicted. Using a Medtronic insulin pump, CGM sensor, and a bedside laptop with predictive LGS, more than 20 patients have used the system at home for over 250 nights. Thus far, the predictive algorithm has halved the number of nights during which patients go below 60 mg/dl [182].
Control-to-range algorithms are another major area of academic and company research. These systems seek to maintain glucose in a zone such as 70-180 mg/dl – in other words, the algorithm will increase insulin infusion if glucose is trending too high and will decrease insulin infusion if glucose is trending too low. Such algorithms would still require bolusing for meals, though they would be able to help compensate for over- or under-dosing insulin. In a series of studies at the Universities of Virginia, Padova, and Montpellier, 11 adolescents and 27 adults participated in 22-hour sessions comparing use of the artificial pancreas with a control-to-range algorithm to standard therapy. Studies included meals, overnight rest, and 30 minutes of exercise. Participants wearing the artificial pancreas spent 74% of the time between 70 and 180 mg/dl, compared to 61% during standard pump therapy. Encouragingly, the closed-loop algorithm reduced hypoglycemia nearly three-fold [183].
Medtronic and Animas have also presented similarly encouraging data on their control-to-range systems in development. Medtronic’s Portable Glucose Control System, which includes two Enlite CGM sensors, a Paradigm Veo insulin pump, and a BlackBerry cell phone, kept eight patients’ glucose in the range of 70-144 mg/dl for 78% of the time overnight, translating to a mean blood sugar of 115 mg/dl [184]. And in a recent feasibility study of Animas’ Hypoglycemia-Hyperglycemia Minimizer System, consisting of a Dexcom Seven Plus CGM, a OneTouch Ping insulin pump, and a controller algorithm running on a laptop computer, patients spent 70% of a 20-hour period in the range of 70-180 mg/dl, with only 0.2% of the time spent in hypoglycemia. This was despite two high-carb meals and deliberate under- and over-bolusing of insulin (in some cases up to 50%) [185].
Because ‘real world’ events such as illness, stress, large meals, exercise, and malfunction of a CGM or pump can cause unexpected fluctuations in blood glucose levels, they may pose challenges to current algorithms. Fortunately, there are a number of approaches to dealing with these obstacles. For instance, certain algorithms can detect pump or CGM malfunctions if blood sugars begin rising abnormally quickly. Other algorithms are able to learn over time and constantly adapt and improve based on their past performance. It is hoped that eventually, algorithms will be able to quickly identify and address any factors that change blood glucose, provided the rest of the system can respond quickly and accurately enough. For now, exercise and meals remain some of the toughest challenges for algorithms to overcome [186].
Control algorithms generally perform well during overnight studies, when few external factors affect blood sugar. However, Dr. Roman Hovorka, PhD (University of Cambridge, Cambridge, UK) and his colleagues found that closed-loop control could also deal with ‘a late night out.’ In their experiment, 12 adults with type 1 diabetes were studied on two different nights: one night, they used their standard insulin pump settings and another night, they used a closed-loop system (Abbott’s FreeStyle Navigator CGM, a Smiths’ Deltec Cozmo pump and a control algorithm). On both nights, subjects slept at a hospital under supervision (as is standard in overnight studies of the closed loop). However, unlike many previous studies, subjects ate a large, carbohydrate-heavy dinner (100 g carbohydrates) along with several glasses of wine (7.2 ml/kg [0.1 oz/lb]), which translates to roughly three glasses of wine for a 150-pound person). Despite these challenging conditions, the closed-loop outperformed standard control, with subjects spending 22% more time in their glycemic target range [187]. In another study by Dr. Stuart Weinzimer (Yale University School of Medicine, New Haven, CT) and his colleagues, a closed-loop system kept twelve subjects’ blood sugar in target (80-140 mg/dl) 91% of the time overnight on sedentary days and 79% of the time overnight on exercise days (compared to just 60% in both open-loop conditions) [188]. These results suggest that algorithms’ good nighttime performance can continue even in more challenging conditions.
In Summary…
• Control Algorithms are the middleman of the artificial pancreas, using CGM readings to calculate appropriate insulin pump doses.
• Algorithms can acheive impressive glucose control, keeping patients in the range of 70-180 mg/dl over 70% of the time with very little hypoglycemia.
• While algorithms may be stressed by ‘real world’ fluctuations in blood glucose caused by meals, exercise, or stress, this is being improved and algorithms generally do a good job of controlling glucose overnight.
5.3
The Path Ahead
Based on our conversations with Dr. Aaron Kowalski, PhD, Research Director of the Artificial Pancreas Project at JDRF, and JDRF CEO Jeffrey Brewer, we believe the path to a closed-loop system will occur in small steps, with technological advances at each stage [189, 190]. The first step would be the development of a device that suspends insulin delivery whenever a user does not respond to a CGM low-glucose alarm. Such technology is part of the Medtronic Paradigm Veo Insulin Pump, which was first approved in the EU in 2009. After significant regulatory delays, the device – renamed the MiniMed 530G in the United States—was recently submitted for FDA approval (expected sometime in the first half of 2013). This submission was made under special arrangement with the FDA and was earlier than expected. The in-home pivotal trial for the MiniMed 530G is being conducted during the FDA review process.
More advanced systems would use predictive CGM alarms to increase or decrease insulin infusion when hyperglycemia or hypoglycemia are expected, allowing more time in the ideal glycemic zone. Animas, Medtronic, and many academic institutions are currently developing these systems. Most experts believe that such hypoglycemia-hyperglycemia minimizers are possible with today’s pumps, sensors, algorithms, and insulins. A semi closed-loop system would likely come next and would feature a fully automated basal system with user-assisted mealtime bolusing. The culmination of closed-loop research, a fully automated, ideal AP, would come sometime after. It would likely include faster acting insulin and might even include glucagon or other hormones in addition to insulin, further improving glycemic control. We include in Figure 9 a diagram based on the steps in Dr. Kowalski’s “Roadmap to an Artificial Pancreas” that outlines the path ahead for the development of the AP. Several aspects of this roadmap are discussed in the sections below.
Some researchers believe that future versions of the AP will not only close the loop, but will also “expand the loop” by incorporating additional sensors and information technology. Dr. Eyal Dassau, PhD (University of California, Santa Barbara, Santa Barbara, CA) and his colleagues at the Sansum Diabetes Center have proposed a system that could use telemedicine to alert loved ones and healthcare providers in the event of severe hypoglycemia [191]. Other experts believe that the AP will one day incorporate sensors for heart rate, motion, and other factors that can help an AP’s control algorithm predict changes in blood glucose. Research on telemedicine has accelerated greatly over the past year, while work on additional sensors is just beginning.
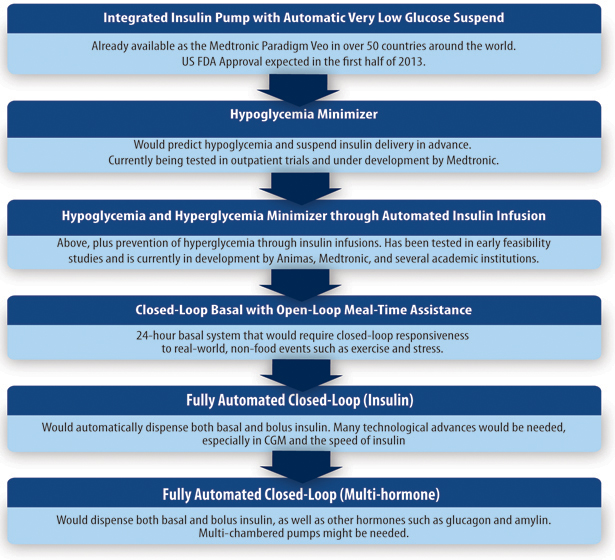
Figure 9. The Pathway to the Closed Loop.
In Summary…
• The approval of a fully automated AP will occur in steps, with increasing innovation, improved glycemic control, and less required user input appearing over time.
• The first step toward the closed loop is an integrated pump/CGM system with a “low glucose suspend” feature that shuts off insulin delivery during hypoglycemia. The Medtronic Paradigm Veo, the only current system with low glucose suspend, is under FDA review (renamed the MiniMed 530G) and is expected to be approved in the US in the first half of 2013. This device is already available in 50 countries outside the United States.
Better and More Accurate CGM Technology
In general, the future of CGM looks bright because the current rate of technological progress and research for AP components has been relatively rapid and reimbursement for AP components has dramatically improved in the last one to two years. Smaller and more accurate CGMs have very recently become available (Dexcom’s G4 Platinum) in the US or will likely receive approval in the next year (Medtronic’s Enlite). Bayer and Becton Dickinson (BD) are studying CGM systems in early clinical trials, and several smaller companies are developing novel technologies for continuous monitoring.
Medtronic’s Enlite is not an entirely new device, but rather a sensor that is compatible with the company’s current CGM systems. However, the Enlite, which launched in 35 countries outside the US in April 2011, features several improvements over Medtronic’s previous Sof-Sensor. When used along with the Veo insulin pump, the Enlite has an average error of 14.2% as compared to 19% for the Sof-Sensor, with especially large improvements in the hypoglycemic range [192]. The implanted volume of the sensor is roughly 70% lower, with decreases in both needle length (from 17.5 mm to 10.5 mm) and width (from 22 gauge to 27 gauge at their thinnest points) as compared to the Sof-Sensor. The Enlite was submitted to FDA along with the MiniMed 530G insulin pump. Approval is expected in the first half of 2013. Another recent advance for Medtronic’s CGM has been CareLink Pro 3.0, a software program for healthcare professionals that treat patients using sensor-augmented pumps (i.e., Paradigm Revel or Veo). The software uses pattern recognition and analytics to propose therapy considerations, such as adjusting pump settings or changing infusion sites, depending on when users experience low and high blood glucose.
Meanwhile, Dexcom has developed a fourth-generation (G4 Platinum) CGM system that has several notable improvements over the third-generation Dexcom Seven Plus. Most importantly, the G4 Platinum system is more accurate, posting a 13% overall average error compared to 16% for the Seven Plus [193]. Notably, this includes a 30% improvement in accuracy in the hypoglycemia range. The Gen 4 transmitter will also have a much longer communication range with the receiver – up to 30 feet normally and up to 50 feet if it’s in line of sight, compared to a range of five feet cited in the label for the Seven Plus. Finally, the new sensor is 50% smaller by volume and has the smallest introducer needle as compared to the Abbott Freestyle Navigator and Medtronic Sof-Sensor. The G4 Platinum CGM received FDA approval on October 5, 2012, and launched in the US three weeks later. The G4 Platinum sensor is also available in Europe as a standalone system and as part of the integrated Animas Vibe insulin pump, which displays CGM readings directly on the pump’s screen. US launch of the Animas Vibe is expected in the second half of 2013. Additionally, Dexcom and Tandem are working to integrate the G4 Platinum sensor into Tandem’s t:slim; US launch is expected in 2014.
Dexcom is also designing a more accurate fifth-generation sensor that will put the CGM’s processing power in the transmitter, not the receiver. This means that the transmitter will be able to send CGM data for display on a variety of devices, such as pagers, hospital monitors, or potentially even cell phones or cell-phone attachments. On April 24, 2012, JDRF and Dexcom announced a one-year research collaboration to develop a “smart” CGM transmitter for use in artificial pancreas studies. The smart transmitter would permit a Dexcom CGM sensor to communicate directly with a variety of AP controller devices (e.g., cell phones running artificial pancreas algorithms that would communicate with an insulin pump). These innovations are a critical part of making artificial pancreas systems more portable, less intrusive, and more practical in the real world.
In early 2011, Bayer announced that it was working on a new subcutaneous CGM designed for seven-day use that could significantly improve upon current CGMs. It will have a needle-free insertion mechanism (the thin sensor wire itself will act like the insertion needle), a one-hour start-up time, and a receiver with a built-in blood glucose meter and USB technology for data downloading. Bayer recently presented results from a weeklong home-use study that showed an average error of around 16% [194]. The new device will also feature improvements in adhesive strength and signal transmission. As of this writing, the company is continuing to develop its technology and has not announced a timeline on when it might come to market.
Attempting a different method to improve CGM accuracy, BD is developing a new optical-based CGM system that involves protein binding rather than the electrochemical reactions used in currently available CGM (which are based on the enzyme glucose oxidase). BD’s sensor uses a fluorescently labeled protein called glucose/galactose binding protein (GBP). When GBP binds glucose, its fluorescence pattern changes; thus, optical sensors allow the CGM to measure the concentration of interstitial fluid glucose based on the GBP fluorescence pattern. The sensor is currently being studied in a subcutaneous version as well as an intradermal version that is placed less than 1 millimeter under the skin. Both prototypes show promise: small width (31-gauge cannula), short warm-up time (accurate within roughly 30 minutes of insertion), low potential for chemical interference, and few required calibrations (possibly only one) compared to current CGM. A recently published 12-hour feasibility study showed that both the subcutaneous and intradermal versions have very good accuracy, especially in the hypoglycemic range [195]. BD is still studying the technology in early-stage trials and has not indicated when it might come to market.
Despite these upcoming sensors’ greater accuracy, multiple redundant sensors may still be required to ensure the accuracy and safety of fully closed-loop technology. As mentioned in Section 5.2, reliability of CGMs is also a major concern, and so CGM companies have also been placing significant emphasis on the reliability of their new systems. It still remains unclear whether concerns about sensor failure and inaccuracy will be addressed by better sensors, backup sensors, or some combination.
Finally, new, possibly more accurate forms of CGM based on fluorescent, implantable, or micro-dialysis sensing are also in the works, but these technologies appear to have a long way to go before they can be used outside of a research setting. Companies developing early-stage CGMs include ArKal (worn like a patch on the skin), GlySens (implantable), Senseonics (implantable), and a number of others. Several CGM companies (including Dexcom, Medtronic, Edwards, Echo Therapeutics, and GluMetrics) are developing products designed for hospital intensive care units (ICU), where accurate and robust glucose monitoring is important for patients with and without diabetes. However, because the transition from bedside monitoring to home-use CGM has historically been slow, in this report we focus on systems with more direct implications for the AP.
Major news on the future of CGM was released on June 22, 2011, when the JDRF and Helmsley Charitable Trust announced the launch of a major funding initiative (up to $20 million) aimed at accelerating the development and delivery of advanced continuous glucose monitors. The goal of the initiative will be to develop sensors that are capable of driving closed-loop systems to a glycemic range seen in those without diabetes.
To date, the initiative has announced two industry partnerships to advance CGM technology. On June 1, 2012, Medtronic announced a three-year partnership with JDRF and the Helmsley Charitable Trust, focused on developing a new type of CGM sensor for use in the artificial pancreas. Medtronic will seek to combine two different methods of measuring glucose, creating a so-called “redundant sensor” – in other words, current sensor technology (based on a chemical reaction using glucose oxidase) will be combined with a completely different, future sensor technology (based on fluorescence). The hope is that such a redundant approach will increase the accuracy, reliability, and safety of CGM readings, and thus improve the performance of an artificial pancreas. The partnership is one of the largest collaborations to date by JDRF and the commitments are up to $17 million in funding over three years.
The Helmsley Charitable Trust (HCT) and JDRF also announced a research collaboration in 2012 with BD to accelerate the development of the company’s optical-based CGM technology in development (see above). Payments could total up to $7.5 million over the program’s three-year duration, depending on the successful progress of the research against the plan. As of this writing, the company has two ongoing early-stage studies of the new CGM.
In Summary…
• Accuracy and reliability are the main limitations of current CGMs. Although opinions are divided, many experts believe existing CGM technology is accurate and reliable enough for control-to-range systems.
• We see CGMs continuing to improve in coming years, with innovations from Dexcom and Medtronic in particular poised to make advances in accuracy, convenience, and comfort.
Faster Insulin
Current pumps use so-called “rapid-acting” insulins: Novo Nordisk’s insulin aspart (Novolog), Eli Lilly’s insulin lispro (Humalog), or Sanofi’s insulin glulisine (Apidra). The three rapid-acting analogs are generally similar: they begin working in about 20 minutes and are typically finished lowering blood sugar after three to five hours. However, widespread agreement exists that these insulins are simply not fast acting enough to fully control blood glucose following meals. Blood sugar generally reaches its peak 60 minutes after meals, while the glucose-lowering effect of rapid-acting insulins peaks around 90 minutes after it’s taken. This means that many patients who inject insulin at the start of a meal experience both high blood sugar (because blood glucose rises faster than the insulin can act) and delayed hypoglycemia (if the insulin continues to act even after blood glucose has already fallen).
Right now, endocrinologists emphasize bolusing for carbohydrates up to 30 minutes before a meal begins to obtain optimal after-meal blood sugars. Faster-acting insulin could avoid this inconvenience.
In the AP, even faster-acting insulin would enable tighter control and less risk of hypoglycemia. To control blood glucose, closed-loop systems must currently overcome the major challenge posed by delays in insulin absorption. Many experts have likened this to trying to steer a car that takes over an hour to respond to movement of the steering wheel. Algorithms must consequently walk the fine line between being too aggressive and accidentally dosing what turns out to be too much insulin, or being too conservative and not controlling hyperglycemia. Faster-acting insulin would reduce the time lag between when insulin is given and when blood glucose drops, enabling algorithms to dose more frequently and control blood glucose to a tighter range.
Substantial research efforts are being put toward faster-acting insulin formulations (e.g., Novo Nordisk’s NN1218, Halozyme’s PH20, and Biodel’s BIOD-123). NN1218 is an ultra-rapid version of Novo Nordisk’s current rapid-acting insulin, Novolog. The company completed an initial phase 1 trial in early 2012, and plans to begin phase 3 studies in late 2013.
Halozyme is also attempting to produce faster-acting insulin by adding an enzyme (PH20) that would temporarily degrade connective tissue in the skin, allowing insulin to work faster. A recent 12-week study in 117 patients with type 1 diabetes found that the addition of PH20 to Humalog or Novolog led to an 82% lower blood glucose after meals compared to use of Humalog alone. Additionally, hypoglycemia events (less than 70 mg/dl) were 5% lower when PH20 was used compared to use of Humalog alone [196]. While this study tested the combination of PH20 and insulin (a “coformulation”), Halozyme is also studying the possibility of injecting PH20 by itself prior to inserting an insulin pump infusion set (“preadministration”). Data from a small ongoing study showed encouraging results in 11 patients: on average, the onset of insulin action occurred 21 minutes earlier following preadministration of PH20. This faster absorption resulted in a one-hour post-meal blood glucose of 143 mg/dl when PH20 was preadministered, compared to 184 mg/dl when PH20 was not used [197].The preadministration strategy may also have an advantage over the insulin coformulation strategy, as Halozyme’s PH20 is already FDA approved to speed delivery of other injected drugs. Halozyme plans to conduct further studies of PH20 preadministration and coformulation, though it is not yet clear when either could come to market.
Biodel has been working on the development of a faster-acting insulin for the past decade. Linjeta, the company’s original ultra-rapid-acting insulin candidate, led to quicker reductions in post-meal hyperglycemia and was associated with a lower risk for hypoglycemia relative to current insulins. Linjeta was submitted for FDA approval in 2010, but because of issues with trial design, uncertainty regarding its therapeutic efficacy, and tolerability concerns (injection site pain), the FDA decided not to approve Linjeta’s use in the United States until two new phase 3 trials for the insulin product were completed. Biodel has since decided to drop Linjeta in favor of pursuing BIOD-123, which the company believes will minimize injection site pain while maintaining an ultra-rapid-acting profile similar to Linjeta. A recently completed phase 1 study of BIOD-123 in 12 patients demonstrated 64% faster absorption relative to Humalog and much improved injection site tolerability over Linjeta [198]. Biodel began a 130-patient phase 2 study in the third quarter of 2012, with data expected in about a year. The company is also working on speeding the action of current rapid-acting insulin by adding proprietary chemical substances. Encouraging phase 1 data was reported in early 2013, where Biodel’s compounds demonstrated 35-45% faster absorption than Humalog in a small 12-patient study. We look forward to confirmation in larger studies, especially once glycemic data is collected.
Other methods to provide faster absorption and delivery of insulin are also being explored. For example, BD’s intradermal technology using microneedles would deliver insulin into just the top layer of skin and Insuline’s infusion site warming technology would speed insulin absorption in the body. Both technologies are still in the testing phase and must demonstrate efficacy, clear regulatory hurdles, and prove user-friendly before they will be seen outside the research environment.
Another technology being studied for its ability to speed insulin action is Roche’s Accu-Chek DiaPort. The device consists of a small implantable “port” that connects to standard Roche Accu-Chek insulin pumps to enable insulin delivery directly into the abdominal cavity. This enables a much faster insulin action profile and better exposure at the liver, the primary site of insulin action. A single unit of DiaPort-delivered insulin has a 10-minute absorption peak, as compared to 50-60 minutes for subcutaneous delivery with standard insulin pumps [199]. The DiaPort is available only in Europe, where it is accessible to a very limited patient population. Roche has not announced plans for a US regulatory submission. However, JDRF is supporting a 12-patient trial of the DiaPort in a closed-loop system. Investigators will compare 24 hours of closed-loop control with a traditional insulin pump (Roche’s Accu-Chek Combo) to 24 hours of closed-loop control with insulin delivered through the Accu-Chek DiaPort. The study will be conducted in Montpellier, France using software and algorithms developed at the University of California Santa Barbara [200].
Turning from the liver to the lungs, MannKind has been developing an inhalable insulin called Afrezza for the last several years. In 61 different studies in over 5,600 patients, Afrezza has demonstrated that it is rapidly absorbed into the bloodstream following inhalation, reaching peak levels within 12 to 14 minutes [201]. For reasons related to the inhaler and safety concerns, the FDA chose not to approve Afrezza in March 2010 and January 2011. To address these concerns, MannKind is currently conducting phase 3 studies in patients with type 1 and type 2 diabetes – if the data from the studies is positive and all goes as planned, the company plans to resubmit the drug for FDA approval in late 2013. While the ultra-rapid-acting Afrezza wouldn’t be an automated part of the artificial pancreas, the idea is that it could be used prior to meals to help cut down on the post-meal hyperglycemia typically seen in closed-loop experiments. Researchers at the University of California Santa Barbara/Sansum Diabetes Research Institute are planning to conduct a 24-hour, 12-patient, control-to-range study that will include a Dexcom CGM, an Animas Ping or Insulet OmniPod insulin pump, and 10 units of Afrezza taken before two 50-gram carbohydrate meals. Using an FDA-approved artificial pancreas computer simulator, the addition of Afrezza (over insulin-pump-only closed-loop control) boosted time spent in the range 70-140 mg/dl from 39% to 62% over 24 hours, 2% to 15% four hours after a meal, and 68% to 89% overnight [199]. These early computer simulation results are encouraging. Ultimately, the feasibility of Afrezza for the artificial pancreas rests, of course, not only on demonstrated efficacy in the closed loop, but on the need for it to secure FDA approval.
Yet another approach to making insulin more physiologically responsive is to develop glucose-dependent insulin, a basal insulin that “knows” when blood sugar is high and can increase its own activity in response. Merck is exploring this strategy through its December 2010 acquisition of SmartCells, a small company with a product called SmartInsulin. SmartInsulin is a therapeutic that houses insulin and has certain proteins on its surface that recognize glucose. Insulin is released from the therapeutic only when a certain amount of glucose is present and “sensed.” As of this writing, Merck’s SmartInsulin program is in the early stages of development. Biodel has also conducted preclinical experiments with glucose-dependent insulin, although the company is currently prioritizing its fast-acting insulin analogs instead. Finally, in September 2011, JDRF announced a $100,000 prize for innovative ideas to develop glucose-responsive insulin (GRI). JDRF recently selected several good (albeit theoretical) ideas that merited prize awards, though further research will need to occur to demonstrate their feasibility.
In Summary…
• Despite their name, “rapid-acting” insulins (Novolog, Apidra, Humalog) are quite slow compared to the insulin we naturally produce, significantly impeding tight glucose control.
• Faster-acting insulin formulations from Novo Nordisk, Halozyme, and Biodel as well as faster absorption technologies from MannKind, BD, and Insuline have the potential to markedly improve closed-loop systems.
A Cautious Regulatory Environment
The Food and Drug Administration (FDA) may be one of the biggest hurdles to a closed loop. Many experts in the field worry that the FDA’s conservative stance on device approval and the agency’s commitment to safety will lead to long approval times. In the past few years, stringent FDA requirements have led to new devices typically being approved in Europe before the USA, as we saw with Medtronic’s Veo insulin pump and Enlite sensor, Dexcom’s G4 Platinum CGM, Animas’ Vibe insulin pump, Insulet’s second-generation OmniPod patch pump, and many other devices.
The Medtronic Paradigm Veo, already approved for use in 50 countries outside the United States, features automatic low glucose suspend technology. As noted above, this means that whenever a hypoglycemia alarm goes off and the user doesn’t react, the pump stops delivering insulin for up to two hours unless the user resets it. Designed as a safeguard against nocturnal hypoglycemia and other incapacitating lows, low-glucose suspend is the first step toward a fully closed loop. It’s arguably much safer than current pumps, which will continue to deliver a basal rate when the CGM detects a patient has hypoglycemia. However, the FDA’s call for additional trials and data has led to a four-year regulatory delay in approving the pump in the US. Considering the Veo has been available outside the US since 2009, this device’s journey through the US regulatory process illustrates the agency’s caution compared to other jurisdictions.
For the AP more broadly, the FDA’s Arleen Pinkos, MT (Former Chair of the Interagency Artificial Pancreas Working Group, FDA, Silver Spring, MD) has stated that before any closed-loop system can be approved, it must be shown to work safely for six months and a closed-loop shut-off safety mechanism will likely be necessary to address CGM weaknesses. Additionally, Ms. Pinkos indicated that any closed-loop trial will have to address real-world scenarios and prove that the benefits outweigh the risks [202]. The tasks ahead are significant, but the FDA has highlighted the AP in its Critical Path Initiative and provided significant guidance by explaining the hurdles that manufacturers must clear before a closed-loop system can be approved.
The FDA has reinforced its commitment to AP development in several other ways. In 2007, the FDA formed the Interagency Artificial Pancreas Working Group (IAPWG), a multidisciplinary team of scientists from the FDA and National Institutes of Health (NIH). The IAPWG collaborates with patient groups, industry, private organizations, and other government agencies in order to improve and accelerate closed-loop research and development. The FDA also worked with the NIH and JDRF to lead public workshops on the AP in December 2005, July 2008 and November 2010.
At the most recent public workshop on the AP in 2010, there was broad agreement that a step-wise approach to AP development is key and that the first step will involve successful creation of a low-glucose suspend system. The FDA’s Dr. Patricia Beaston, MD, PhD (Center for Devices and Radiological Health, FDA, Silver Spring, MD) emphasized that the inaccuracy of current CGM (roughly 60% false alarm rate for hypoglycemia) creates a risk that improper shutoffs could lead to hyperglycemia. However, some of the foremost AP researchers argued that the risks of high blood sugar are minor compared to the benefits of reducing nocturnal hypoglycemia, and in some cases, death. Both sides spoke favorably of predictive low-glucose suspend, the “hypoglycemia minimizer” mentioned as the second step toward an AP in Figure 9. Not only do these systems stop hypoglycemia before it starts, they could be more trustworthy, since CGM trend data are generally more accurate than CGM point estimates. An outpatient study is currently evaluating a predictive low glucose suspend system (using an Enlite CGM and Medtronic insulin pump) and is expected to complete in July 2013 [203]. Additionally, Medtronic’s most recent update suggested that it plans to launch a hypoglycemia minimizer system (called the MiniMed 640G) in 2013-2014 in Europe and in 2015-2016 in the United States. Encouragingly, Dr. Charles Zimliki, PhD (Former Chair of the Interagency Artificial Pancreas Working Group) closed the public workshop by saying that an AP in the next five years is “definitely possible” [204]. Separately from the lively debate at this workshop, several patients including Adam Brown, one of the authors of this book, shared their enthusiastic support for an AP (for a transcript of Adam’s testimonial, see Appendix C).
In the past year, the FDA has released two important draft guidance documents (these represent the agency’s current thinking on a particular subject) related to the artificial pancreas. On June 22, 2011, the FDA released a draft guidance document focused on low glucose suspend and predictive low glucose suspend (LGS) systems (steps one and two in Figure 9). The brief offered valuable recommendations for researchers and industry concerning the development and FDA submission of devices like the Medtronic Paradigm Veo. The release of this document was an encouraging sign, as were the accompanying words of Dr. Jeffrey Shuren, MD, JD, director of the FDA’s Center for Devices and Radiological Health: “Our goal is to provide a clear pathway for artificial pancreas development so that people with diabetes can benefit from innovative medical devices. Getting a safe and effective artificial pancreas system to Americans with type 1 diabetes is an FDA priority.”
In December 2011, under immense pressure from JDRF, the FDA released a second draft guidance document on artificial pancreas systems. It is intended to help researchers and manufacturers develop and gain approval for control-to-range and control-to-target artificial pancreas systems (steps three through six in Figure 9). This draft guidance document represented an important step forward from the somewhat controversial LGS draft guidance released earlier in 2011, and encouragingly, the new guidance includes many of the recommendations JDRF called for in the months leading up to its release. The document proposes a three-stage study progression to bring an artificial pancreas to market: early feasibility studies (e.g., in the hospital setting), transitional studies (e.g., at a diabetes camp), and pivotal studies in an unsupervised, home-use environment. FDA believes the first two stages will be small and of short duration, with the pivotal study to approve the final device lasting at least six months. To be cleared by FDA, an AP system must demonstrate that it does not provide inferior glucose control to using a standard insulin pump and CGM – this is a significant improvement over the previous LGS draft guidance, which stipulated that an AP would have to be superior to a patient using a pump and CGM on their own. Overall, the FDA also included many elements that could decrease the time it takes to get an AP system to people with type 1 diabetes.
On November 9, the FDA published the final artificial pancreas guidance on its website, which combines the two aforementioned draft guidances into a single document. On an extremely encouraging note, the finalized guidance addresses much of the feedback on the draft guidance documents—most notably, CGM can now be used to evaluate AP systems and time-in-range will be one of the many acceptable metrics to demonstrate an AP’s safety and effectiveness. The guidance should also help speed the path to market for predictive low glucose suspend systems (PLGS), which now may only show that they significantly decrease hypoglycemia over standard care—highly achievable in our view given the strong performance these systems have demonstrated in computer simulations and outpatient trials thus far.
In Summary…
• The FDA is taking a cautious approach to AP approval but is spending resources on, and signaling its commitment to, helping the process go as fast as possible.
• Approval of an AP will occur in a step-wise fashion after safety mechanisms for both individual components and the system as a whole are put in place and thoroughly tested.
• The agency believes CGM accuracy is a major limitation for low-glucose suspend systems, which are the first step toward an AP.
Increasing Use of Other Hormones
As discussed in Section 4.2, some also believe GLP-1 and other hormones developed for use in type 2 patients have their place with type 1 patients.7 According to Dr. Kowalski, eventually, closed-loop systems will include dosing of other hormones such as glucagon, amylin, and/or leptin, which will help restore normal physiology.
Glucagon is a hormone that triggers the release of glucose from the liver. It doesn’t work as it should in type 1 diabetes, leading to higher blood glucose after meals. It can be used to counter the effect of insulin, leading to very tight control in closed loop experiments using both glucagon and insulin. Current formulations of glucagon are unstable and thus are packaged as a powder and liquid that must be mixed prior to use (Eli Lilly’s Glucagon Emergency Kit and Novo Nordisk’s GlucaGen HypoKit); unfortunately, such formulations are not appropriate for use in the AP. Efforts are ongoing to develop a more stable form of glucagon for use in bi-hormonal pumps. Biodel is developing a stable liquid formulation of glucagon that would not require any mixing prior to use. The company is still working on its formulation and will hopefully provide an update on the timeline soon. Xeris Pharma is also working on a better glucagon formulation and plans to conduct a phase 2 clinical trial in 2013. Other companies working on stabilizing glucagon include Arecor, Latitude, and PhySci. There are also companies like Enject and others working on improved glucagon pens that use the currently approved formulations of glucagon.
Dr. Edward Damiano, PhD (Boston University, Boston, MA) and his team have conducted some of the most impressive closed-loop research using both insulin and glucagon. In the group’s most recently published study, six people with type 1 diabetes participated in two 51-hour closed-loop experiments that included high carbohydrate meals (78 grams per meal on average), exercise, and pumps dosing both insulin and glucagon.8 The system achieved an overall mean blood glucose of 158 mg/dl, with 68% of the time spent in the range 70-180 mg/dl. Notably, just 0.7% of the time was spent in hypoglycemia (<70 mg/dl) [205]. Nighttime control was even better, with an average blood glucose of 123 mg/dl, 93% time in range, and only a single episode of mild hypoglycemia. Dr. Damiano and colleagues have ambitious plans for a mobile AP system and for studies outside the hospital setting – see the section on Important Upcoming Trials for more information.
Closed-loop research is just beginning on amylin, a hormone that slows the a movement of food through the stomach and suppresses the release of glucagon. In the absence of faster insulins, the use of amylin in closed-loop systems may improve post-meal glucose control by slowing the absorption of glucose into the blood. Promisingly, at a recent diabetes technology meeting, Dr. Stuart Weinzimer, MD (Yale University, New Haven, CT) presented results from a small closed-loop study in which an amylin analog pramlintide (Symlin, Amylin Pharmaceuticals), when given before a meal, improved postprandial closed-loop control by delaying and lowering peak post-meal glucose levels [206]. JDRF and Amylin also recently announced a collaboration to study coformulating insulin and pramlintide so that the two hormones can be taken in a single injection. However, just the first round of clinical feasibility studies could take up to four years, so at best it will be several years more until insulin and pramlintide are available in a single vial (let alone in a single-chambered pump).
Finally, leptin, a hormone involved in the regulation of body weight and metabolism, has shown potential to treat type 1 diabetes in mice. Currently, JDRF, Amylin, and the University of Texas Southwestern Medical Center are recruiting for a 15-patient phase 1 study that will examine the effects of metreleptin (a leptin analog) on blood glucose control, insulin dose requirements, and blood glucose variability in people with type 1 diabetes [207].
It should be noted that for such hormones to be incorporated into the AP in a way that is not excessively burdensome to patients, dual-chamber pumps would also need to be researched and developed. According to Dr. Steven Russell, MD, PhD (Massachusetts General Hospital, Boston, MA), Tandem Diabetes Care believes it can create a device with the same form factor and size as its current t:slim insulin pump, but capable of holding 300 units of insulin and 100 units of glucagon [208].
In Summary…
• Future closed-loop systems will likely include multiple hormones in addition to insulin, making them better able to mimic physiological glucose control.
• Research is being conducted into the use of glucagon (probably the most logical option), amylin, and leptin in the multi-hormone AP.
Important Upcoming Trials
The many past and upcoming studies on the AP signal its medical and commercial importance [209, 210]. Recent studies have demonstrated that current technology is capable of handling overnight glucose control; however, as noted in Section 5.2, because blood glucose changes rapidly during meals and exercise, these activities present additional challenges for the AP. Future studies addressing these daytime fluctuations will be crucial in the development and approval of an AP. Tremendous excitement surrounds JDRF’s initiation of a Multi-Center Trial of Control-to-Range in seven centers worldwide. This trial will focus on keeping glucose values within a targeted range following meals and exercise. At a recent diabetes conference, data from a pilot version of the trial was presented. Eight subjects using the control-to-range system spent an impressive 93% of a 24-hour period between 70-180 mg/dl, compared to just 77% for those on standard pump therapy [211]. Everyone in the field is looking forward to seeing more data soon.
A major achievement in the history of the artificial pancreas occurred in late 2011: the first studies to occur outside the hospital setting. The DREAM 3 trial tested an overnight closed-loop system in 56 patients at three diabetes camps in Europe. Patients wore an insulin pump and a CGM, and they slept with a bedside laptop running a control algorithm to direct insulin delivery. Impressively, all patients at each camp were simultaneously monitored from a central command and control center. Encouragingly, the system reduced the frequency and duration of hypoglycemia and increased time spent in the range of 70-140 mg/dl [212]. The other landmark outpatient study of 2011 also occurred in Europe in Montpellier, France and Padova, Italy. The study utilized a mobile-phone-based artificial pancreas system called the Diabetes Assistant, developed at the University of Virginia – it includes an Android cell phone running a hypoglycemia-hyperglycemia minimizer algorithm and communicating wirelessly with an insulin pump and CGM sensor. In 18 hours of outpatient closed-loop therapy, which included a meal at a restaurant, sleeping overnight in a hotel, and breakfast the following morning, patients experienced no hypoglycemia (<70 mg/dl) and comparable time in range to open-loop therapy [213].
A key element in these early outpatient trials was the movement toward more portable and mobile artificial pancreas systems. These not only allow for more real world testing of the devices outside the confines of a hospital room, but they also open the door to remote monitoring of patients and alarms. This trend will continue in the coming years, evident in the vast number of outpatient studies that will use similar portable systems.
Dr. Edward Damiano, PhD (Boston University, Boston, MA) and his team plan to conduct a study featuring five-day closed-loop experiments at the Massachusetts General Hospital Campus, hopefully starting in early 2013. A mobile system will be used, which includes an iPhone 4s, two Tandem t:slim pumps delivering insulin and glucagon, and either an Abbott FreeStyle Navigator or Dexcom G4 CGM. The iPhone controller will wirelessly communicate with both t:slim pumps and display CGM readings and insulin/glucagon delivery data. The system can operate in a fully closed-loop mode, or allow patients to enter the approximate size of their meal (for pre-meal boluses). The system also allows the option to revert to manual open-loop bolus control with automated basal control. Most notably, patients wearing the closed-loop system will be allowed to roam freely around the hospital campus during the day (accompanied by a chaperone), with unrestricted eating and exercise. This study will represent one of the longest and most real-world AP studies to date. Even more ambitious is a two-week study that Dr. Damiano has planned for the summer of 2013 at a diabetes camp. Participants wearing the closed-loop system will engage in typical summer camp activities, eat a normal diet, and be monitored by on-site nurses using wireless telemetry. Lastly, a two-week hospital staff study is planned to begin in 2014 – participants with type 1 diabetes will wear the closed-loop system during their workday at the hospital and will sleep at home with the system at night. Dr. Damiano hopes a pivotal study using the final device to be commercialized could be initiated in 2015 [214].
Dr. Roman Hovorka, PhD (University of Cambridge, UK) and his team have been developing the “FlorenceD” home closed-loop prototype. The system consists of the Abbott FreeStyle Navigator CGM, a small laptop running a control algorithm, a Dana R Diabecare pump, and the Companion (a device to assist communication with the Navigator). In May 2012, the team received approval from UK regulatory authorities to conduct overnight trials in patients’ homes [215]. The study includes three weeks of overnight closed-loop control, and as of this writing, six adolescents (12-18 years old) have been recruited for the study out of a planned 16. Dr. Hovorka and his team have been pioneers in overnight closed-loop research and there is much excitement as the team begins moving studies outside the hospital.
Significant research is also taking place into optimal predictive alarms and pump suspension in the face of hypoglycemia. Dr. Bruce Buckingham and colleagues recently published a study where a statistical prediction algorithm was able to prevent hypoglycemia 84% of the time [216]. Along with collaborators at three other institutions, Dr. Buckingham has been testing a predictive low glucose suspend system in overnight home experiments. The trial has FDA approval for 1,600 in-home nights with 44 patients, and has already amassed 250 nights of data with 20 participants. Participants sleep with a laptop at their bedside, a Medtronic insulin pump, and a CGM sensor. The early data shows that the system has already halved the number of nights at which patients go below 60 mg/dl [182].
Other important artificial pancreas research centers are also planning outpatient trials using more mobile systems. The University of Virginia, developer of the aforementioned Diabetes Assistant, plans to use the device along with the Dexcom G4 sensor and t:slim insulin pump in upcoming studies [217]. The team at UCSB/Sansum has developed a version of its widely used Artificial Pancreas System that can be run on tablets as well as ultra-portable devices slightly larger than cell phones (~1.2 lbs). These mobile devices will be used in upcoming outpatient studies. Finally, JDRF is sponsoring a Closed-Loop Control at Home study that is expected to enroll 80 patients at four institutions across the globe (University of Virginia; University of Padua, Italy; University of California Santa Barbara; Montpellier University, France) [218].
Studies are also beginning to look at the therapeutic benefits of short-term closed-loop control in people at the onset of diagnosis. The ultimate hope is that such treatment can preserve beta cell function and improve future glycemic control. In a recent presentation, Dr. Stuart Weinzimer shared early results from a TrialNet and DirecNet study that puts patients on three to four days of inpatient hybrid closed-loop control within one week of diagnosis. Patients will be followed for the next two years, but preliminary results show that those receiving closed-loop control within one week of diagnosis spent 85% of time within the target blood glucose range (71-180 mg/dl) and achieved mean glucose levels of 138 mg/dl by day three of the study. Case studies of patients also demonstrate that glycemic control is still excellent even months after hybrid closed-loop therapy [219]. If the study indeed turns out to preserve beta cell function, it may change the treatment of new-onset type 1 diabetes.
“Kelly’s Take”

In terms of all the therapies in this report aimed to cure type 1 diabetes, this is the one we are most excited about. Although there are some constraints associated with both the hybrid and the longer-term artificial pancreas (insulin isn’t fast enough, sensors not quite developed enough, etc.), we are so enthusiastic about this work because of its benefits in the “here and now.” Many of the therapies in this report will be all-or-nothing—either they work or they don’t. However, the AP will be based on therapies that already work and that people are already using. That means every step toward an AP will involve tangible improvements in diabetes care, with a truly game-changing therapy at the end of the road.
5.4
Overall Potential
In our view, the AP, while not changing the fundamental biology of type 1 diabetes, is the most likely out of all the therapies discussed in this report to become a “cure.” Because incremental and steady improvements have been made in all of its components over the years, and patients have taken up new technologies as they’ve become available, the AP as a whole is moving forward. This area has great momentum, and as of 2012, current closed-loop components are as close as we’ve gotten to giving people with diabetes proper, daily glycemic control without unmanageable side effects. As improvements in CGM, algorithms, insulin and use of other hormones (i.e., glucagon, amylin, leptin) become available, people with diabetes will get closer and closer to full restoration of normal physiology and freedom from counting carbohydrates, taking injections, and fighting hypo—and hyperglycemia.
5.5
Where to Learn More
Background
Artificial Pancreas Project—JDRF
Using Mathematics to Treat Diabetes—ArtificialPancreas.org
Current State of Closed Loop Components
Insulin Pump Overview—The American Diabetes Association
Paradigm REAL-TIME Revel—Medtronic
The Path Ahead
Medtronic Paradigm Veo Insulin Pump
The Sansum Diabetes Research Institute
Medtronic Enlite Sensors and the Animas Vibe—Diabetes Mine
The Artificial Pancreas Workshop—diaTribe
JDRF and Amylin Partner to Investigate Co-Formulating
Behind the Scenes: An Artificial Pancreas Clinical Trial
Conclusion
From Kelly Close, Editor in Chief, diaTribe
The work discussed in this book represents many of the latest efforts of the scientific and medical communities to develop a cure for type 1 diabetes. Immune therapeutics, transplantations, beta cell regeneration and survival agents, and the artificial pancreas (AP) all tackle the idea of a “cure” in a different way and each possesses its own unique potential. But cure-targeted research is by no means limited to just these approaches, and notable progress has been made on a number of other fronts. Much of this work, including that of the late Dr. George Eisenbarth of the University of Colorado and Dr. Jeffrey Bluestone of the University of California at San Francisco, remains in early stages, but also holds real promise to improve the lives of people with diabetes.
After nearly 25 years of type 1 diabetes, I’ve been through a great deal. Tens of thousands of fingersticks. Dozens of different blood glucose meters. Six insulin pumps. Countless times feeling like I want to eat our refrigerator just to treat another (severe bout) of hypoglycemia. Getting searched at the airport. Losing my temper during hyperglycemia. Diabetes and pregnancy (three times). Hospitalizations (24) due to hypoglycemia (though just one in the last decade). I’ve never had a break, never taken a day off, never been able to say, “I quit this whole diabetes thing.”
And still . . . I’ve been fortunate to witness so much progress in the field. Back when I was diagnosed, my treatment consisted of just a fixed dose of insulin in the morning and a fixed dose at night. I took the same amount of insulin irrespective of food, exercise, or stress! There was no notion of personal dosing back then, and we certainly didn’t yet know about the importance of tight glycemic control.
As it happens, my insulin dosing was no more sophisticated than my understanding of the cure. I knew nearly nothing about the complexity of type 1 diabetes, and as a result, I was hopeful that a cure was coming soon and my pancreas would eventually be as good as new. Like everyone who has had diabetes for some time, I got used to hearing that “a cure might be just around the corner” or “coming in the next five years…” or “…ten years…” Of course, five years later, similar voices uttered eerily similar predictions. I eventually grew disenchanted, as many patients have, and when the DCCT results came out in 1993, I focused my time on what I could control: managing my diabetes to the best of my abilities.
However, over the past few years, as I’ve learned more and more about the search for the cure, I’m now nearly as (and in some ways more) hopeful than I was when I was first diagnosed. I know from attending over a hundred diabetes conferences since 2002 and from writing so many words on diabetes (over 2 million last year alone with my team that pens diaTribe and Closer Look) that our understanding of type 1 diabetes has improved by leaps and bounds. Technology, while imperfect, is definitely better than ever. And most important, there are so many brilliant, remarkably committed people working toward a cure. The work being done by these researchers is profoundly moving for even the most pessimistic patient, and having seen a great deal of progress in the last year, I’m once again excited and hopeful about a number of the cure-based therapies discussed in this report.
Of course, a cure means different things to people at different stages of type 1 diabetes. To me, a “cure” would mean I would never have to think about it. No more days when I do everything right and my blood sugar seems to do everything wrong. No more counting carbohydrates, dosing insulin, and fearing hypoglycemia. And no more haunting concerns that I might be diagnosed with retinopathy when I visit the ophthalmologist (I go every six months rather than every 12 just because I’m so worried about this happening).
But to think that a cure would affect only me would be a spectacular understatement; diabetes affects those around me as well. My husband has been an integral part of my diabetes management in every way, from helping me download my CGM to recognizing that I’m hypoglycemic. Before I got my CGM (which he can look at anytime), he had about 62 ways of asking me if I thought I should check my blood sugar. “Wow, should we check your blood sugar?” “You seem frustrated. Want to check your blood sugar?” “This seems like it was a hard meal to dose. Let’s just take a second to check your blood sugar!” And on and on. My children think about diabetes every time I check my blood sugar, eat dinner, or drive my car. And lots of other times too. Recently, I spoke sharply to my six-year-old, and I heard her whisper to her friend, apropos of nothing (and everything), “She has diabetes.” That one really made me pause. And of course, diabetes especially affects my relationships with friends. They know what a low is, they know when I’m exhausted, and they know to frown at anyone who says, “Wow, should you be eating that?” Ultimately, when I really think about it, a cure would not just liberate me from diabetes. It would liberate those who I love the most, my friends and my family.
I admit that my definition of a cure—to be free of all glycemic burdens and worries—is very demanding, but that’s not to say I’ll be unhappy if this pinnacle is never reached. On the contrary, innovations like insulin pumps, faster-acting insulin, and better blood glucose monitoring have been huge bright spots in my diabetes management. And just five years ago, CGM revolutionized my ability control my blood sugar. I’ve not only achieved more time in target and a lower A1c, but CGM has often helped me recognize hypoglycemia.
As I look toward the future, I think about all the stellar researchers working on the cure, and I can’t help but be optimistic about many of the cures discussed in this report. The artificial pancreas really will change the lives of people with type 1 diabetes—and I believe it is coming soon. It won’t be perfect, but it’s moving us in the right direction. No doubt, the challenges ahead are significant. We need more scientific knowledge, a more responsive FDA, better-resourced regulatory agencies globally, and continued commitment from companies and researchers to finding a cure. But it can be done.
I’ve always said that having type 1 diabetes is like being on a precipice. Twenty-five years ago, it was pretty much impossible to stand confidently on that precipice. Today, it’s quite a bit easier, but I still fall off too often. In the future, I hope to walk up to that precipice and stand on it without thinking. It seems pretty far away in the distance, but with the right people working hard, I really believe we’ll get there.
I hope you have enjoyed this report. Thanks so much to our crackerjack diaTribe team that wrote it. My hope and prayer is that some day down the road, no such report will be necessary. Hope with me, please.
Very best,
Kelly L. Close
Acknowledgments
We would like to thank the many people who made this book possible. First, we would like to acknowledge all of the researchers, including Dr. David Harlan, Dr. Peter Gottlieb, Dr. David Sutherland, and Dr. Susan Bonner-Weir, who took time out of their busy schedules to share the details of their work with us. We thank Dr. Satish Garg, whose invitation to the 2009 conference “Practical Ways to Achieve Targets in Diabetes Care” deepened our engagement with many of the topics in this book and whose enthusiasm for this project conceptually meant a lot to us.
Throughout this process, numerous people at JDRF contributed significant knowledge to our effort, including Dr. Dick Insel, Dr. Aaron Kowalski, Dr. Albert Hwa, Jeffrey Brewer, Dr. Pat Kilian, Dr. Sanjoy Dutta and Rachel Steinhardt. The guidance of Dr. Cherie Stabler of the Diabetes Research Institute was also invaluable in the creation of the book’s section on the “Bioartificial Pancreas”.
Mighty thanks to the leaders of many companies in the diabetes arena—in particular, we are incredibly appreciative of the work of Terry Gregg, Dr. Francine Kaufman, Dr. Allen Moses, Chris O’Connell, Doug Ringler, and Katie Szyman, to move us closer to a cure.
Closer to home, we owe a major debt to our team at diaTribe. Joe Shivers played an integral part in editing every section of this report, and in particular lent his expertise to expanding the section on the artificial pancreas. Kira Maker also devoted her time and insight to ensuring the strength and quality of the artificial pancreas section. Along with Joe and Kira, Sanjay Trehan and Vincent Wu spent countless extra hours late at night and on weekends making sure deadlines (inside and outside diaTribe) were met. Additionally, we extend our warmest thanks to Gavin Front and Margaret Nguyen who helped get this project over the finish line. Ellen Ullman devoted a great deal of time to reading the book and advising us, and we are most grateful for her insights. Jim Hirsch, another steady hand at the editorial wheel, made sure we always kept in mind the families affected by type 1 diabetes; for a book of this length, Jim’s background as an author helped us to bring the shape of the document into focus. John Close honed the manuscript with his inimitable editing style, pushed us to make this book ever more accessible, and ensured that we constantly strove to improve the scope and depth of our writing.
Eleni Konstantinopoulos designed the cover and many of the figures in the book—we very much appreciate her remarkable enthusiasm for every step of the project. Likewise, we cannot thank the Varon family enough, particularly Lilly, for being open to being photographed for our cover. Lilly’s joy and energy during the photo shoot came across vibrantly in the images of that day. Thanks as well to our wonderful photographer Julia Sperling who came in to this project quickly and yet made it all look effortless.
Numerous members of our diaTribe advisory board have been very generous with their time, particularly Dr. Irl Hirsch, Dr. Jay Skyler, Dr. Howard Zisser, Jeff Halpern, and Jeff Hitchcock.
We owe particular gratitude to Victor van Beuren, who believed in this book from the start and who helped us enormously as we worked through swaths of constantly-changing material. Victor and his team at ADA Books, especially Greg Guthrie, have skillfully guided us through the publication of Targeting A Cure, and we are truly thankful not only for their keen attention and dedication to publishing valuable material about diabetes, but also for their choosing to put ADA’s weight behind sharing this material with so many healthcare providers, patients, and families.
Finally, we thank the readers whose passion for this subject—and whose insistence that a cure can and will be found—renews our spirits every day.
Sincerely,
Lisa, Ben, Adam, Mike, Hannah, and Kelly
Appendices
Appendix A
Targeting a Cure: The Takeaways
In Dan Pink’s latest book Drive, he impressed us with various syntheses of his book. We have borrowed the approach—we have a two-line and a 10-plus line summary of Targeting a Cure for Type 1 Diabetes below, plus a chapter by chapter summary. We hope this helps deepen your knowledge of where we stand on the path to a cure for type 1 diabetes.
Twitter Summary [220]
Will T1D ever be cured? New book offers the most current, thorough + readable assessment of exciting breakthroughs + obstacles ahead.
Cocktail Party Summary [220]
Targeting a Cure for Type 1 Diabetes is a thorough assessment of the diverse efforts now underway to cure, eliminate, or better manage this ancient disease. Highlighting the opportunities as well as the obstacles, the book focuses on the most promising research in immune therapeutics, islet and pancreas transplantation, beta cell regeneration and survival agents, and the artificial pancreas. The document is rich in detail, but it’s also made highly readable for patients and their families through the pull-out of key message points and the graphic display of essential information. Written by the editors and reporters at diaTribe (www.diaTribe.org), a free online newsletter about diabetes, the book includes an introduction from Dr. Aaron Kowalski, the Assistant Vice President for JDRF who was diagnosed with type 1 diabetes at age 13. As his words remind us, the search for the cure is ultimately about the patients, and this document helps reveal where we are today and where we are headed.
Chapter 1: The Basics
Glucose, Insulin, and Type 1 Diabetes
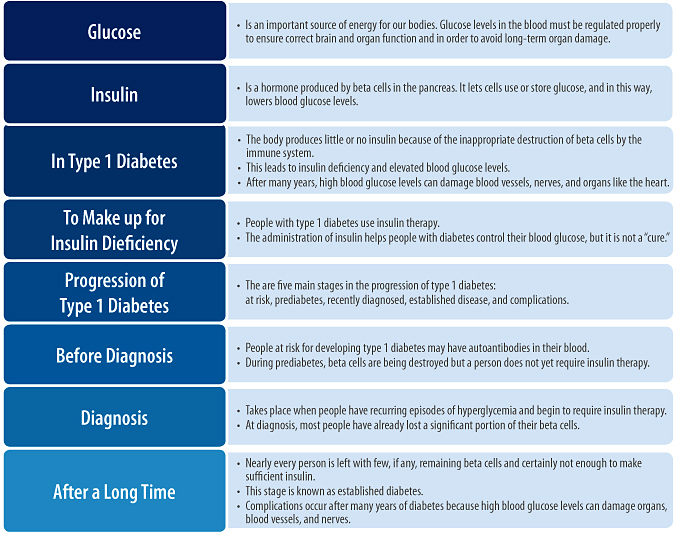
Targeting a Cure
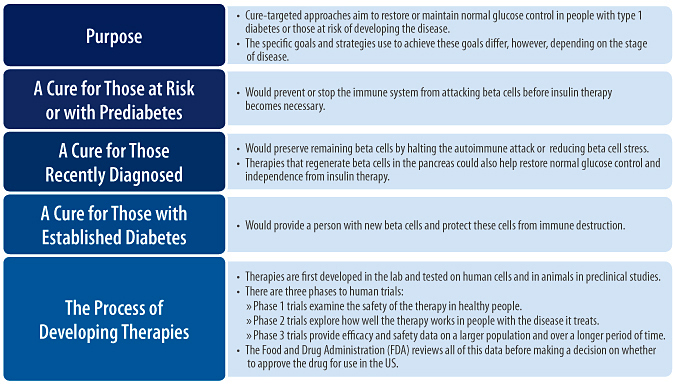
Cure-Targeted Therapies
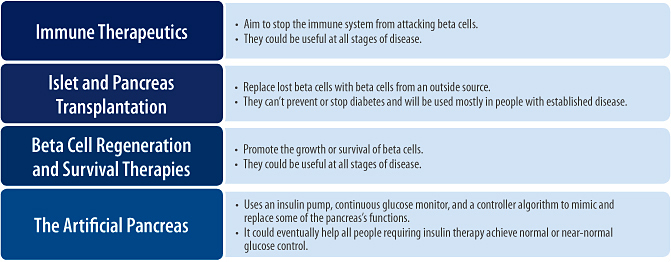
Chapter 2: Immune Therapeutics
Overview
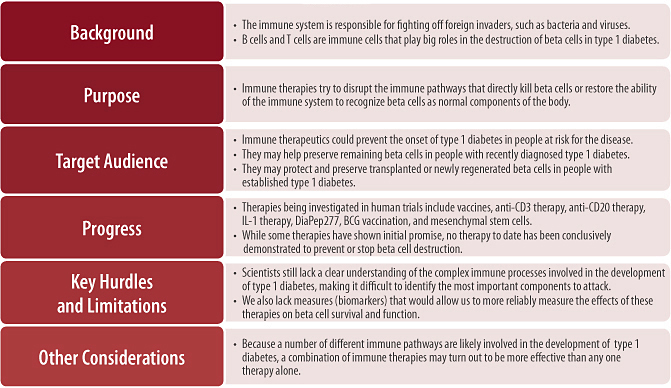
Diabetes Vaccines
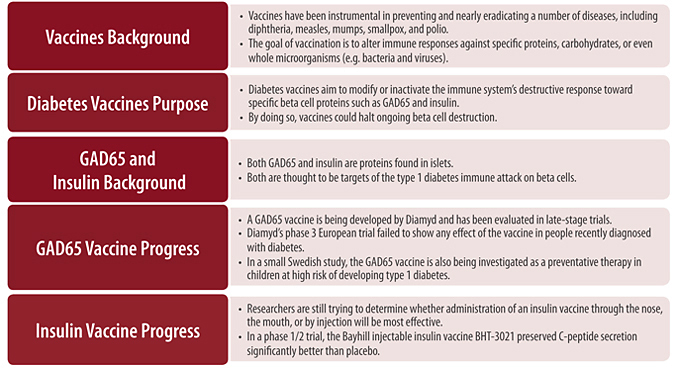
Anti-CD3 Therapy
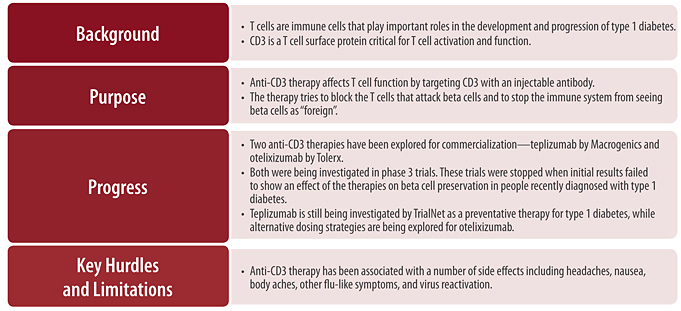
Anti-CD20 Therapy
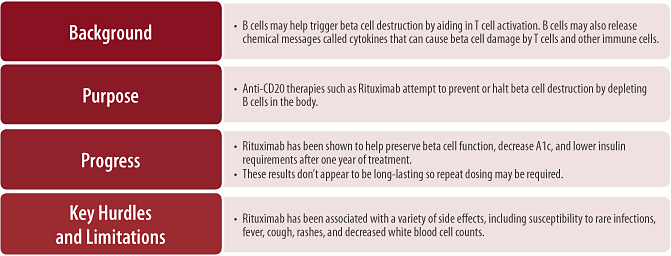
Abatacept
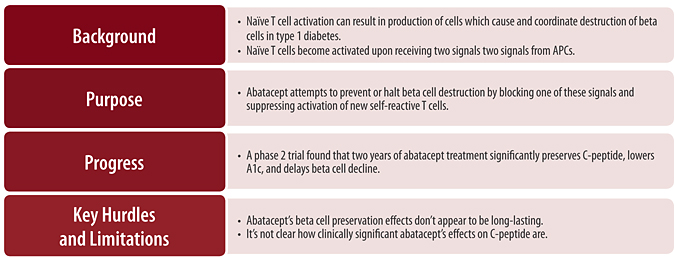
IL-1 Therapies
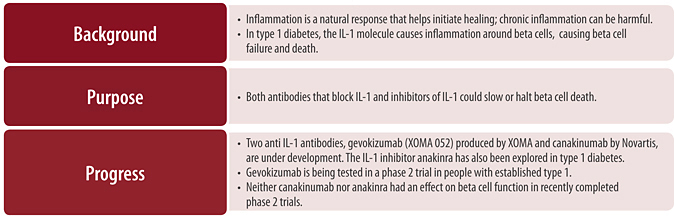
DiaPep277
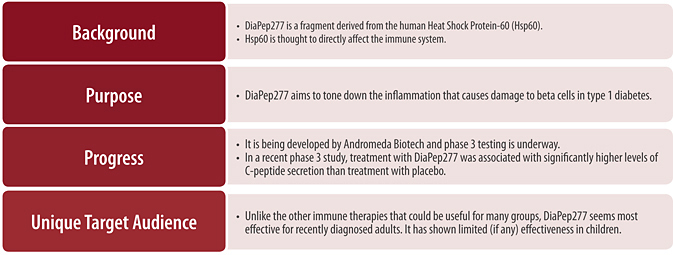
BCG Vaccine
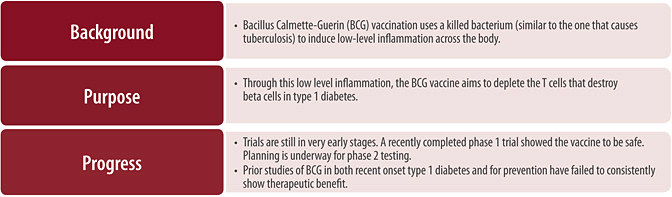
Mesenchymal Stem Cells
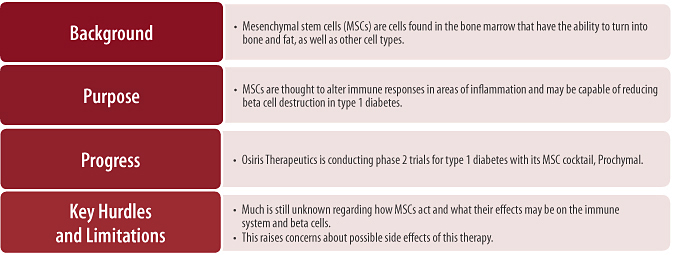
Chapter 3: Islet and Pancreas Transplantation
Overview
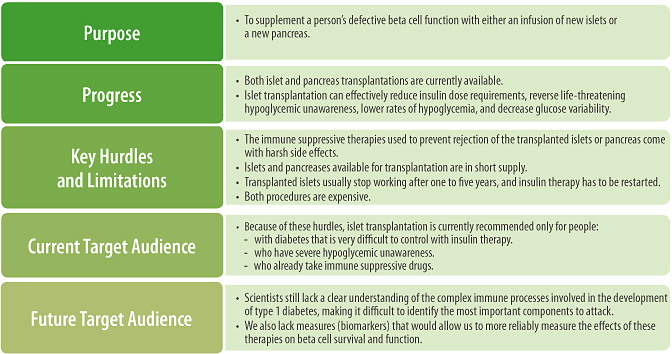
New Source of Islets
Creating Beta Cells From Stem Cells
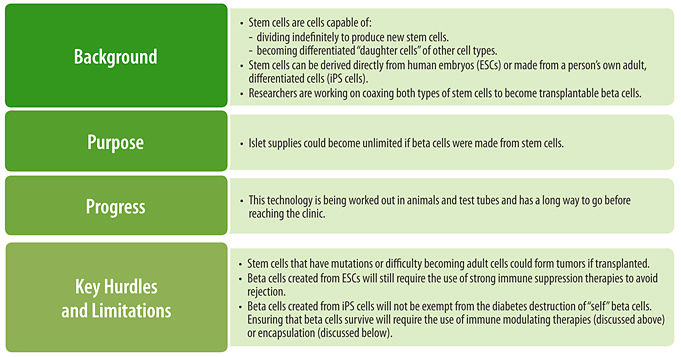
Animal Cell Transplants
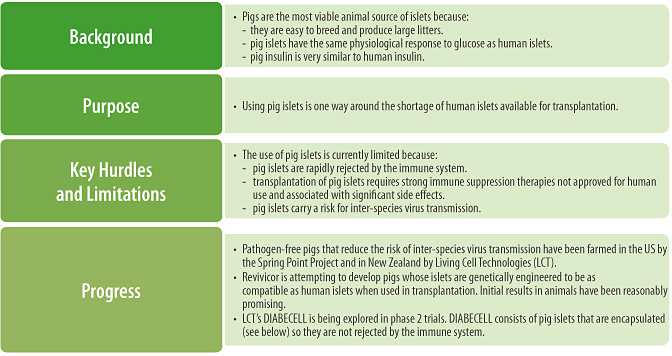
The Bioartificial Pancreas
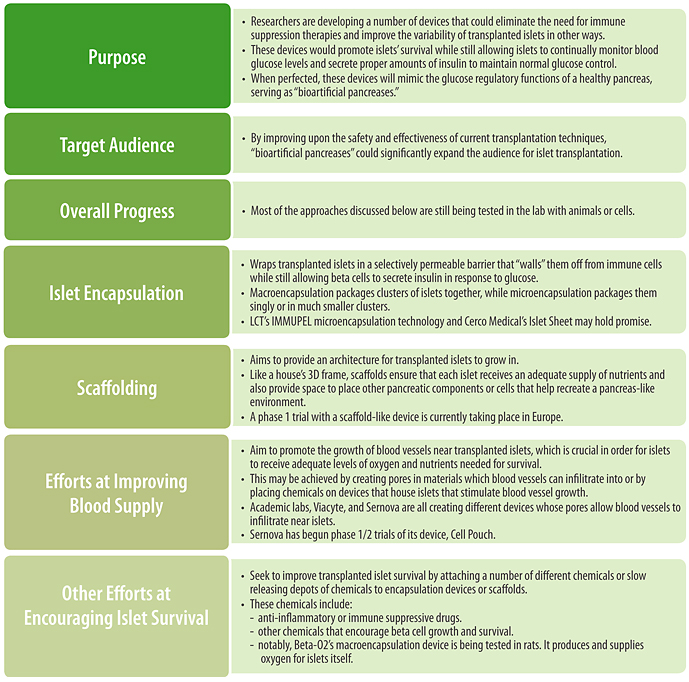
Chapter 4: Beta Cell Regeneration and Survival Therapies
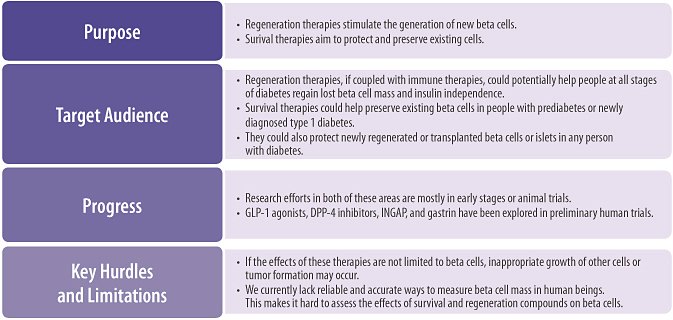
Chapter 5: The Artificial Pancreas
Overview
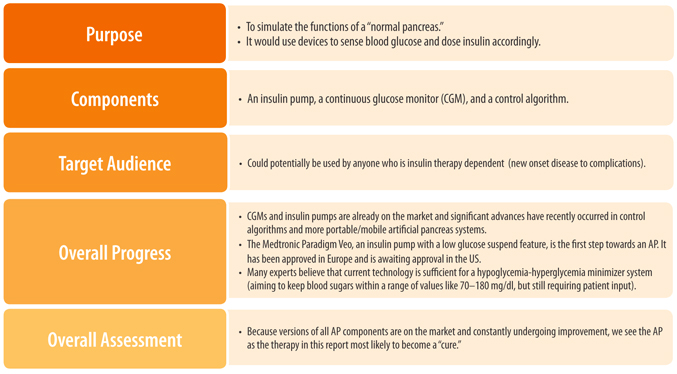
The Current State of AP Components
Insulin Pumps

Continuous Glucose Monitors
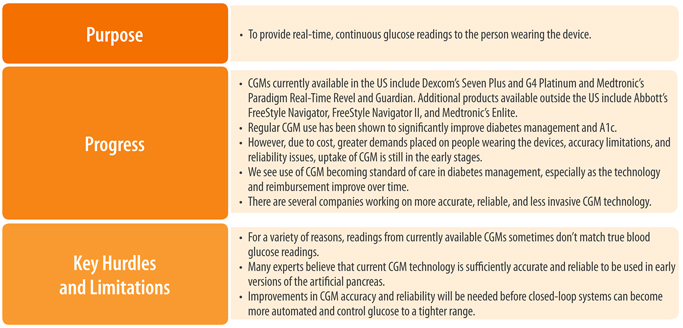
Control Algorithms

The Path Ahead

Conclusion

Appendix B
The Late Breaker Website
We are happy to say that the search for a type 1 diabetes cure has progressed faster than the book can be published. Therefore, the information in this edition is an accurate representation of the field when we finished writing it in the fall of 2012. We will continue to follow the field and publish updates—and one day a report on the cure itself—on www.diatribe.org/latebreakers. Please visit this open access website for the latest news in the hunt for a cure.
Appendix C
Adam Brown’s Testimonial at the FDA/NIH Public Workshop on the Artificial Pancreas
(November 2010)
Good afternoon, Mr. Chairman and members of the Committee. Thank you for the opportunity to comment. My name is Adam Brown, and I’ve had type 1 diabetes since 2000, when I was just 12 years old. I am an editor of diaTribe, a free online newsletter for people with diabetes, and I am speaking for myself, for diaTribe’s 20,000 subscribers, and for Kelly Close, our type 1 editor in chief.
Our main request today is to ask FDA to move faster in approving technologies that could help people with diabetes and to renew its commitment in promoting medical innovation—as your Mission Statement promises. It is clear that the regulatory climate is choking investments for new products in diabetes. I attend the Wharton School, and I know investment firms that now include as a point of pride in their literature that they do not invest in diabetes.
Given our needs, this is a travesty.
As you know, the most harrowing risk about type 1 diabetes is hypoglycemia. It can strike at any time, it strikes suddenly, and it kills. I am aware of three teenagers who this year alone have died of “dead in bed”—they never woke up because of severe low blood sugar. Those three teenagers deserved better.
Hypoglycemia also affects—and kills—people with type 2 diabetes. A recent study in the United Kingdom concluded that 40 elderly people each year die of hypoglycemia from sulfonylureas.
No diabetic therapy is perfect, and we don’t expect perfection. But we know that low glucose suspend pumps and the artificial pancreas can reduce hypoglycemia, and their increased use will save greater numbers of patients from death.
The FDA’s inaction in this area has resulted in real patient loss—more uncertainty, more ER visits, and more deaths.
We ask the FDA to consider conditional approvals. You may feel you are saving patients from products that aren’t perfect, but for people with diabetes, the price of perfection is too high. We just want better.
Thank you very much for your consideration.
Glossary
The adaptive immune system plays a large role in the elimination of invading organisms that have evaded destruction by the innate immune system, and is the major target of immune therapeutics for type 1 diabetes. The adaptive immune system is highly specific: cells of this system recognize and remember particular foreign agents. This ability allows the adaptive immune system to mount a powerful attack against certain viruses or bacteria, while ignoring others. The adaptive immune system also has a form of memory, allowing the body to quickly eliminate an invading organism if it is encountered again.
Alpha cells are the body’s glucagon (see glucagon entry) producing cells found in the pancreas.
Amylin is a hormone (see hormones entry) that slows the movement of food through the stomach and suppresses the release of glucagon. In the absence of faster insulins, the use of amylin in closed-loop systems (see closed-loop system entry) may improve post-meal glucose control by slowing the absorption of glucose into the blood.
Anakinra is a drug derived from the natural IL-1 inhibitor that was developed by Amgen to treat diseases caused by inflammation in which IL-1 plays an important role (see IL-1 and Inflammation entries). Researchers are currently examining whether anakinra is able to help preserve beta cells in people with type 1 diabetes. In a recently completed phase 2 trial, anakinra was not found to preserve C-peptide secretion better than a placebo.
Analogs have a function similar to the original molecule but are chemically different.
Anti-CD20 antibody therapy is a type of immune therapy that targets CD20, a protein found on the surface of most B cells (see B cell entry). Binding of anti-CD20 antibodies to the outside of the B cells leads to decreased B cell numbers in the body. Rituximab is an anti-CD20 antibody that has been investigated as a therapy for type 1 diabetes.
Anti-CD3 therapy is a type of immune therapeutic that targets T cells (see T cell entry) with an injectable synthetic antibody against the T cell surface protein CD3. CD3 plays a critical role in T cell activation and function, and binding to it with an antibody induces several effects on T cells. The goals of anti-CD3 therapy are to (1) interrupt the T cells that directly attack beta cells and (2) to initiate natural regulatory responses that restore the ability of the immune system to properly recognize beta cells as self-cells, further inhibiting T cell attacks on the pancreas. Two anti-CD3 therapies that have been explored for type 1 diabetes are Macrogenics’s teplizumab and Tolerx’s otelixizumab (see individual entries on these antibodies).
Antibodies are a type of protein produced by the immune system that can bind to foreign objects, marking them for attack or ingestion by other cells of the body.
Antigens are the small parts of specific foreign agents that are recognized by the adaptive immune system (see adaptive immune system entry). Each adaptive immune cell recognizes a single antigen; the ability of the immune system to recognize many antigens comes from the wide variety of antigen specificities among the many adaptive immune cells in any person’s body. When an adaptive immune cell comes into contact with the antigen it recognizes, it becomes activated and begins performing a variety of functions that helps contain or eliminate the invading foreign agent.
The artificial pancreas (AP) uses mechanical devices to mimic the functions of a normal pancreas. The goal of the AP is to detect changes in blood glucose (via a continuous glucose monitor) and regulate insulin delivery (via a control algorithm and an insulin pump) to maintain blood glucose within a normal range, reducing the risks for long-term complications and easing the daily management of diabetes by eliminating nearly all patient involvement.
Autoantibodies are antibodies that can recognize components of a person’s own cells. Four main autoantibodies are routinely measured to assess type 1 diabetes risk or to assist in diabetes diagnosis: insulin (IAA), glutamic acid decarboxylase (GAD), insulinoma associated-2 (IA-2), and zinc transporter (ZnT8) autoantibodies. The risk for type 1 diabetes has been shown to increase with the number of autoantibodies detected. The role of autoantibodies in the development and progression of type 1 diabetes remains unclear.
Autoimmune disease (or autoimmunity) refers to a process in which damage to any tissue, organ or cell of the body is caused by the inappropriate activity of a person’s own immune system. In type 1 diabetes, this process specifically destroys beta cells.
B cells are cells of the adaptive immune system. B cells function primarily as a source of antibody production. B cells can also produce some cytokines and can have a role in activating T cells (see T cell entry). Although B cells produce the autoantibodies associated with the development of type 1 diabetes, these autoantibodies are not thought to play a direct role in the autoimmune attack against beta cells. Instead, in type 1 diabetes, B cells may largely function as facilitators of T cell activation, and may also influence ongoing anti-beta cell immune responses through cytokine production.
Bacillus Calmette-Guerin (BCG) vaccination uses a killed bacterium (similar to the one that causes tuberculosis) to induce low-level inflammation across the body. In doing so, BCG vaccination is hypothesized to deplete T cells (see T cell entry) involved in the destruction of beta cells, and may help stop the progression of beta cell death in type 1 diabetes. A recently completed phase 1 trial showed the vaccine to be safe. Prior studies of BCG in both recent onset type 1 diabetes and for the prevention of type 1 diabetes, however, have failed to show consistent therapeutic benefit.
Beta cell regeneration therapies encourage the growth of new beta cells inside the body.
Beta cell survival therapies are drugs that help block beta cell loss or death. In contrast to immune therapeutics, which aim to preserve and protect beta cells by modifying the immune system, survival therapies generally have little impact on the immune system but instead focus on survival mechanisms intrinsic to the beta cells themselves.
Beta cells are the body’s insulin producing cells found in the pancreas. Beta cells are attacked by the immune system in type 1 diabetes, leading to insulin production (see insulin entry) that is insufficient to control blood glucose levels.
BHT-3021 is an insulin vaccine (see vaccines entry) being developed by Bayhill Therapeutics as a method for potentially halting the immune attack against pancreatic beta cells that causes type 1 diabetes. In a phase 1/2 trial, BHT-3021 significantly preserved C-peptide secretion compared to placebo and was shown to be safe and tolerable. Planning for phase 2 testing of the vaccine is underway.
Bioartificial pancreas is a term used to describe a device that aims to promote the survival of transplanted islets. Ideally, when perfected, a bioartificial pancreas will mimic the glucose regulatory functions of a healthy pancreas and eliminate the need for the broad immune suppression therapies currently required with islet transplantation procedures. At the moment, a variety of different devices are being explored for this purpose. Generally, these devices use biomaterials to create a protective or supportive unit around the transplanted islets. Such a unit promotes the survival of the islets while still allowing them to continually monitor blood glucose levels and secrete proper amounts of insulin to restore and maintain normal glucose control. Examples of these devices include micro—and macrocapsules and scaffolds. A device considered a true bioartificial pancreas will also likely have presented on it chemicals that encourage beta cell survival and ward off immune attack. The bioartificial pancreas is a separate concept from the artificial pancreas (AP) (see AP entry), which exclusively uses mechanical devices to mimic the glucose regulatory functions of the pancreas.
βO2 is a macroencapsulation device (see encapsulation entry) developed by Beta-O2 that is capable of producing and supplying oxygen to the encapsulated islets itself.
Bydureon (exenatide ER) is a once-weekly GLP-1 analog used for the treatment of type 2 diabetes (see GLP-1 entry). It is sold by Bristol-Myers Squibb, AstraZeneca, and Amylin.
Byetta (exenatide) is a GLP-1 analog used for the treatment of type 2 diabetes (see GLP-1 entry). It is sold by Bristol-Myers Squibb, AstraZeneca, and Amylin.
C-peptide is a fragment of natural insulin that is cleaved off prior to insulin secretion. C-peptide is commonly used as a measure of insulin secreted by the pancreas since it is not included as part of any injectable insulin therapy.
Canakinumab is an IL-1 therapy (see IL-1 entry) developed by Novartis. In a phase 2 study conducted by TrialNet, the drug did not preserve beta cell function better than placebo in people recently diagnosed with type 1 diabetes.
Carbohydrates are the most important energy source for our bodies. Carbohydrates are found in high quantities in foods such as rice, pasta, fruit, potatoes, and bread, and include simple sugars as well as long chains of sugars (referred to as starches). As they pass through the stomach and into the intestines, all digestible carbohydrates are eventually broken down into the sugar glucose (see glucose entry).
CD4 T cells are a subset of T cells (see T cell entry) that are also referred to as helper T cells. CD4 T cells carry out most of the immune coordinating function of T cells. These T cells have the ability to modify the function of many other immune cells including B cells and other T cells. Much of this function is carried out through the secretion of cytokines.
CD8 T cells are a subset of T cells (see T cell entry) that are also referred to as killer or “cytotoxic” T cells. These T cells have the ability to kill infected or damaged cells. Because type 1 diabetes ultimately involves the death of beta cells, CD8 T cells are also hypothesized to play a central role in type 1 diabetes.
Cell Pouch is a porous scaffold device (see scaffold entry) being developed by Sernova that has been shown to improve the survival of transplanted islets in animals by quickly promoting the growth of blood vessels into the device. It entered phase 1/2 testing in August 2012.
Clinical trials are studies that examine the effectiveness and safety of a drug in human beings. There are three phases of clinical trials (phase 1, 2, and 3). Phase 1 trials are the first human trials conducted, and they investigate the safety of different doses of the drug, typically in healthy people. Phase 2 trials are typically larger than phase 1 trials and explore how well the drug works in people with the disease it treats; although some safety and efficacy data are collected in Phase 2, the primary purpose is generally to determine drug dosing for Phase 3. Phase 3 trials are the last stage of human studies; these trials help provide efficacy and side effect data on a larger population and over a longer period of time.
Closed-loop system is a commonly used term that refers to an artificial pancreas (AP).
Continuous glucose monitors (CGM) provide real-time, continuous glucose readings to the person wearing the device. CGM is comprised of a sensor that is placed just under the skin that detects glucose from interstitial fluid, a transmitter that wirelessly sends the values, and a receiver that displays real-time glucose values, trends, and graphs. There are three currently available CGMs in the US: Dexcom’s G4 Platinum, Dexcom’s Seven Plus, and Medtronic’s Paradigm Real-Time Revel and Guardian. Outside the US, Abbott’s FreeStyle Navigator, FreeStyle Navigator II, and Medtronic’s Enlite are also available.
Control algorithms form an important part of artificial pancreases (see AP entry). Control algorithms take readings from a continuous glucose monitor, interpret them, and instruct insulin pump dosing accordingly (see CGM and insulin pump entries). The three major algorithms currently being studied are Model Predictive Control (MPC), Proportional, Integrative, Derivative Control (PID), and MD-Logic Control.
Cytokines are communication factors released by cells that can influence the activity of other cells. Cytokines typically act over a relatively short distance, and have a short duration of action.
DIABECELL is a pig islet encapsulation (see encapsulation entry) therapy for the treatment of type 1 diabetes under development by Living Cell Technologies. The company has already received approval to sell DIABECELL in Russia, but LCT is unlikely to receive approval in many other countries, including the United States, until longer studies are conducted. DIABECELL is being investigated in phase 2 trials in New Zealand and Argentina.
Diabetes is a chronic disease characterized by elevated blood glucose levels. Diabetes develops when the body is unable to produce sufficient quantities of insulin to meet its needs. In type 1 diabetes, this occurs because of the inappropriate destruction of the insulin producing beta cells (see beta cells entry) by the immune system. In type 2 diabetes, insulin deficiency is caused by the inability of cells to use insulin properly. This condition is referred to as insulin resistance. The pancreas tries to compensate for insulin resistance by producing more insulin; however, the pancreas can wear out over time, leading to progressively declining insulin production and elevated blood glucose levels.
Diamyd is a GAD65 vaccine (see vaccines entry) currently under development by the company Diamyd as a method for potentially halting immune attack against pancreatic beta cells in type 1 diabetes. Diamyd failed to show indications of beta cell preservation in a phase 3 study in Europe in people with recently diagnosed type 1 diabetes. Following announcement of this result, dosing was suspended in a US phase 3. A prevention study with Diamyd is currently ongoing in Sweden.
DiaPep277 is a fragment derived from the human Heat Shock Protein-60 (Hsp60), which is currently in phase 3 testing for its ability to prevent or slow the progression of type 1 diabetes. In contrast to other vaccination methods (see vaccines entry), where the actual function of the protein in the vaccine is not important for the vaccine’s therapeutic effect, Hsp60 itself is thought to have a direct immune modulating function. In a recently completed phase 3 trial, treatment with DiaPep277 was shown to be associated with greater C-peptide secretion than treatment with placebo.
DPP-4 inhibitors act to block the break-down of GLP-1 in the body. By increasing the concentration of GLP-1 in the blood, DPP-4 inhibitors stimulate increased insulin secretion from beta cells when blood glucose levels are elevated. Additionally, studies in animals and early trials in humans have suggested that DPP-4 inhibitors may have positive effects on beta cell mass, proliferation, and survival. Januvia (sitagliptin), Nesina (alogliptin), Onglyza (saxagliptin), and Tradjenta (linagliptin) are the four DPP-4 inhibitors currently approved for sale in the United States; these drugs are approved as treatments for type 2 diabetes.
Embryonic stem cells (ESC) are stem cells (see stem cell entry) obtained from embryos that have the potential to differentiate into all cell types of the body, including beta cells. Researchers are attempting to drive ESCs to become beta cells by delivering particular molecular messages to the ESCs that encourage beta cell development. Because an embryo must be destroyed in order to obtain ESCs, their use continues to be controversial today.
Encapsulation aims to “wall off” transplanted islets from immune rejection while still allowing beta cells to secrete insulin in response to glucose. The goal is to create a selectively permeable barrier, a capsule, that allows blood, oxygen, glucose, and insulin to move in and out, but that also blocks the infiltration of cells or large molecules from the immune system. Macroencapsulation is an islet encapsulation strategy that packages a large cluster of cells into a single device. Alternatively, microencapsulation coats single cells or small groups of cells to obtain a smaller capsule than in macroencapsulation.
Encaptra is a macroencapsulation product (see encapsulation entry) developed by ViaCyte. Encaptra features a layer of mesh that serves to facilitate the growth of blood vessels near the transplanted islets.
Established diabetes is a term that refers to the stage of type 1 diabetes which occurs a good while after diagnosis. During this stage, beta cells are still under attack by the immune system and patients are thought to have lost a significant portion of their beta cells.
GAD65 is a protein found in pancreatic islet cells. People with type 1 diabetes typically have antibodies to GAD65, and the measurement of these antibodies is often used in diagnosis. GAD65 is believed to be an important target of the immune system in type 1 diabetes, leading to beta cell destruction. A GAD65 vaccine is currently under development by the company Diamyd as a method for potentially halting immune attack against pancreatic beta cells; like the company, this vaccine is named Diamyd (see Diamyd entry).
Gastrin is a digestive hormone secreted in the stomach. Analogs of gastrin are under investigation as possible beta cell regeneration agents.
Gevokizumab (formerly XOMA 052) is an IL-1 therapy (see IL-1 entry) under development by XOMA Pharmaceuticals. Gevokizumab is currently in a phase 2 trial in people with established type 1 diabetes that will examine the ability of the drug to prevent further beta cell destruction.
GLP-1 (Glucagon Like Peptide-1) is an incretin hormone that stimulates insulin secretion from beta cells when blood glucose levels are elevated, causes weight loss, suppresses glucagon secretion, and slows the rate at which nutrients from the food we eat enter the blood. GLP-1 and its analogs have also been shown to increase islet neogenesis, beta cell proliferation, and beta cell survival in animals. Whether GLP-1 has similar regenerative and survival effects on human beta cells is currently unclear. Three GLP-1 analogs, Byetta (exenatide), Bydureon (exenatide ER), and Victoza (liraglutide), are currently approved as treatments for type 2 diabetes in the United States.
Glucagon is a hormone produced in the pancreas that triggers the release of glucose from the liver when blood glucose levels become too low. Glucagon doesn’t work as it should in type 1 diabetes, leading to higher blood glucose after meals. Glucagon can be used to counter the effect of insulin, potentially leading to very tight control of blood glucose in closed loop experiments that use both glucagon and insulin.
Glucose is a sugar that is the principal source of energy for cells in the body. Glucose is derived from the carbohydrates (see carbohydrates entry) found in food. Many cells require the presence of insulin (see insulin entry) to remove glucose from the blood. Once inside the cell, glucose can be used immediately to satisfy energy requirements or it can be stored as a long chain of glucose molecules called glycogen (see glycogen entry). Glucose levels in the blood must be regulated properly for correct brain function and in order to avoid long-term organ damage.
Glycogen is a long chain of glucose molecules (see glucose entry) that can form in muscle and liver cells. It acts as a storage depot for extra glucose and can be broken down and released back into the blood stream if blood glucose levels drop, such as between meals or during sleep.
Hormones are “long range” chemical messengers. Hormones secreted from one part of the body travel through the bloodstream to deliver messages or signals to other distant cells. Insulin is a hormone as are glucagon, amylin, and leptin.
Human proIslet Peptide (HIP) is also referred to as Pancreate and is a beta cell regeneration factor under development by CureDM.
Hyperglycemia occurs when blood glucose levels become too high, such as when too little insulin is secreted from beta cells. When uncontrolled for years, hyperglycemia causes serious damage to blood vessels, nerves, and the kidneys. Overall, maintaining normal blood glucose levels (between the ranges of 70 to 140 mg/dl) is extremely important for long-term health, and insulin plays a dominant role in blood glucose regulation.
Hypoglycemia occurs when blood glucose levels become too low, such as when too much insulin is secreted from beta cells. Hypoglycemia can lead a person to experience lethargy, convulsions, a coma, brain damage, or even death. While no specific level of blood glucose strictly defines hypoglycemia, symptoms rarely develop above 70 mg/dl.
IL-1 is a protein that has an important role in initiating inflammation (see inflammation entry), as well as the production of fevers. Because inflammation is believed to contribute to beta cell failure in type 1 diabetes, therapies that block IL-1 are under investigation as possible beta cell preserving treatments. These therapies include Amgen’s anakinra, XOMA Pharmaceuticals’s XOMA 052, and Novartis’s canakinumab.
Immune therapeutics for type 1 diabetes are a group of cure-targeted therapies that aim to stop the immune system from destroying beta cells. By doing so, they could potentially halt disease progression and enable beta cell recovery. These therapies either try to disrupt the pieces of the immune system involved in beta cell destruction, or attempt to restore the immune system’s ability to recognize beta cells in a non-harmful manner.
IMMUPEL is a microencapsulation method (see encapsulation entry) under development by Living Cell Technologies. IMMUPEL is used in the company’s encapsulated pig islet therapy DIABECELL (see DIABECELL entry), which is currently approved in Russia and is in phase 2 studies in New Zealand and Argentina.
Incretins, including GLP-1 (Glucagon Like Peptide-1) (see GLP-1 entry), are hormones that increase insulin secretion only when glucose levels in the blood are increased.
Induced pluripotent stem cells (iPSCs) are stem cells formed from adult cells (such as skin cells) (see pluripotent stem cell, and stem cell entries). In principle, these iPS cells could be coaxed into becoming beta cells. iPS cell technology would allow scientists to generate perfectly matched beta cells for any person who elected to undergo islet transplantation, eliminating the need for the harsh immune suppressive regimens used alongside islet transplantation procedures today. However, the use of iPS cell-derived beta cells will still not avoid the autoimmune destruction of “self” beta cells characteristic of type 1 diabetes. Consequently, immune modulating therapies or encapsulation devices will likely remain necessary to promote long-term transplant survival.
Inflammation is a natural response to infections, damaged tissue and cells, or irritants. Through acute inflammation, the body attempts to remove the harmful stimuli and start the healing process. But inflammation that continues for a long period of time (chronic inflammation) can sometimes have harmful effects. Chronic inflammation is believed to contribute to beta cell destruction in type 1 diabetes.
INGAP (islet neogenesis associated protein) is a peptide under investigation as a beta cell regeneration agent.
Innate immune system is our first line of defense against foreign invaders. This system mounts an immediate attack on foreign material (viruses, bacteria, or fungus) by its ability to recognize a variety of molecules typically contained within or displayed on the surfaces of microorganisms.
Insulin is a hormone produced by beta cells in the pancreas that allows cells to use or store glucose, lowering blood glucose levels. Additionally, insulin promotes the synthesis and storage of proteins and fats in cells, which can help facilitate muscle growth and weight gain.
Insulin pumps are devices that deliver designated doses of insulin subcutaneously and can reduce A1c as well as hypoglycemia. Two types of pumps are currently available: standard pumps (e.g. Medtronic Paradigm Revel) and disposable patch pumps (e.g. Insulet OmniPod). Standard pump systems consist of the pump and insulin reservoir attached via tubing to the infusion site. The more recently invented patch pumps are tubeless, using a remote wireless controller and a wearable unit filled with insulin.
Interstitial fluid is the fluid that bathes and surrounds cells throughout the body. This fluid helps deliver nutrients from the blood to the cells and helps move waste away from cells.
Islet and pancreas transplantations are a group of cure-targeted therapies that attempt to replace lost beta cells directly by transplanting them into the body from a cadaveric donor.
Islet Sheet is a macroencapsulation device (see encapsulation entry) under development by Cerco Medical. It is currently being examined in animal studies.
Islets of Langerhans are collections of cells found in the pancreas that play important roles in regulating blood glucose levels. Both beta cells and alpha cells (see beta cell and alpha cell entries) are located in islets of Langerhans.
Januvia (sitagliptin) is a DPP-4 inhibitor made by Merck & Co. that is currently approved as a treatment for type 2 diabetes (see DPP-4 inhibitors entry).
Leptin is a hormone (see hormones entry) involved in the regulation of body weight and metabolism and has shown potential for treating type 1 diabetes in mice. A phase 1 study is currently underway that will examine the effects of metreleptin (a leptin analog) on blood glucose control, insulin dose requirements, and blood glucose variability in people with type 1 diabetes.
Macroencapsulation (see encapsulation entry).
Mesenchymal stem cells (MSCs) are cells found in the bone marrow that have the ability to turn into bone and fat, as well as other cell types. These cells appear to be able to travel to sites of inflammation and may be able to reduce or alter the inflammation (see T cell entry) associated with type 1 diabetes, ultimately reducing beta cell destruction.
Microencapsulation (see encapsulation entry).
Multipotent stem cells have the potential to differentiate into some, but not all other cell types in the body (see stem cell entry).
Neogenesis refers to the generation of completely new cells of a particular cell type from stem cells or other precursor cells. Neogenesis is thought to play a role in the generation of new beta cells in mice; the role of neogenesis in human beta cell expansion is not well understood.
Nesina (alogliptin) is a DPP-4 inhibitor sold by Takeda and is currently approved for the treatment for type 2 diabetes (see DPP-4 inhibitors entry).
Nutrients provide the fuel necessary for our cells to grow and carry out activities that allow us to function properly. There are three basic nutrients: carbohydrates (see carbohydrates entry), proteins, and fat.
Onglyza (saxagliptin) is a DPP-4 inhibitor sold by Bristol-Myers Squibb and AstraZeneca and is currently approved for the treatment for type 2 diabetes (see DPP-4 inhibitors entry).
Otelixizumab is an anti-CD3 therapy (see anti-CD3 entry) that was previously being developed by Tolerx in partnership with GlaxoSmithKline. A phase 3 study for otelixizumab failed to show that it was capable of preserving beta cell function in people with recently diagnosed type 1 diabetes.
Phagocytes are cells of the immune system that specialize in swallowing foreign material, digesting it, and presenting fragments or “antigens” (see antigens entry) derived from this material to T cells. This antigen presenting step is critical for immune responses to viruses and bacteria, and in type 1 diabetes, likely plays an important role in the initiation of beta cell destruction.
Pluripotent stem cells have the potential to differentiate into all cell types of the body. Embryonic stem cells (ESCs) are a type of pluripotent stem cell (see stem cell and ESC entries).
Preclinical studies are the initial studies that examine the effectiveness and safety of a drug in animals, cells grown in culture dishes, and test tubes before the drug is moved into human trials.
Prediabetes (type 1 definition) is a term that refers to the stage of disease during which beta cells are under attack by the immune system, but when people do not currently need insulin therapy. Many of the people with prediabetes will eventually develop type 1 diabetes. The type 2 definition of prediabetes is more commonly used and refers to people who are at high risk for developing type 2 diabetes (this latter definition is not used in this report).
Prochymal is a mesenchymal stem cell cocktail (see MSC entry) being developed by Osiris Therapeutics for the treatment of type 1 diabetes and a variety of other inflammatory diseases. A phase 2 trial in people with recently diagnosed type 1 diabetes is currently underway. Preliminary results from this trial suggested that Prochymal is safe and tolerable, but doesn’t preserve C-peptide secretion. Prochymal has been approved in Canada and New Zealand for treatment of pediatric graft-versus-host disease (GVHD) and has also been explored for other conditions.
Regulatory T cells are a specific type of CD4 T cells (see CD4 T cell entry) that function to suppress the activation of the immune system; these cells help the immune system distinguish between a body’s healthy cells and foreign material, protecting normal cells from inappropriate destruction.
Replication refers to the division of cells to create additional cells of the same type. Replication is believed be the most important mechanism for generating new beta cells in adult animals; however, the role of replication in beta cell expansion in humans remains unclear.
Rituximab is an anti-CD20 antibody (see anti-CD20 antibody entry) that has been investigated as an immune therapy for the treatment of type 1 diabetes. Rituximab has been shown to increase C-peptide levels, lower A1c, and lower insulin requirements in patients with newly diagnosed type 1 diabetes for approximately one year after treatment. The therapy has also been associated with notable side effects, however, including increased risks for infections.
Scaffolds are devices that aim to improve the survival of transplanted islets. Scaffolds can be compared to the frame of a house before it is built, where wood beams provide the framework for the three dimensional structure. Instead of wood, scaffolds are made of biomaterials that form compartments for transplanted islets to reside within. This compartmentalized structure helps provide support for the transplanted islets, protection against damaging physical forces, and proper spacing between each islet. Proper spacing ensures that each islet receives an adequate supply of nutrients, and provides surfaces within which to place other pancreatic components or cells. This design helps to recreate a pancreas-like environment.
Stem cells are cells that are uniquely capable of both replicating indefinitely without changing cell type (i.e. stem cells can continue to divide to produce a continuous supply of identical stem cells) and differentiating into or becoming “daughter cells” of other cell types. Researchers are attempting to use stem cells to create beta cells that could be used for transplantation in people with diabetes.
T cells are cells of the adaptive immune system. T cells function both as coordinators of immune responses and as potent inducers of cell death. T cells are believed to play an important role in beta cell destruction in type 1 diabetes. Several different types of T cells exist, including CD4 and CD8 T cells, each of which carries out different immune functions.
Teplizumab is an anti-CD3 therapy (see anti-CD3 entry) under development by Macrogenics. In a phase 3 study that enrolled people with type 1 diabetes, teplizumab failed to achieve its primary target outcome (a combination of insulin usage and A1c levels) after one year. TrialNet has recently launched a trial that will investigate the ability of teplizumab to prevent or delay the onset of type 1 diabetes in people who are at high risk of developing the disease.
Tradjenta (linagliptin) is a DPP-4 inhibitor sold by Eli Lilly and Boehringer Ingelheim and is currently approved for the treatment for type 2 diabetes (see DPP-4 inhibitors entry).
Transdifferentiation refers to the transformation of an adult cell into another cell type. Transdifferentiation has been found to help generate new beta cells in mice; whether transdifferentiation plays a role in the expansion of beta cells in humans is still unclear.
Vaccines are therapies that aim to alter immune responses against specific proteins, carbohydrates, or even whole microorganisms such as bacteria and viruses. In type 1 diabetes vaccination, people are given certain beta cell and islet proteins that are thought to be targeted by the immune system with the goal of preventing harmful immune responses against those proteins and, therefore beta cells, in the future. In general, type 1 diabetes vaccines aim to both inactivate the T cells responsible for beta cell attack and to restore the ability of the immune system to properly recognize and respond to beta cells as normal self cells.
Victoza (liraglutide) is a GLP-1 analog developed by Novo Nordisk that is approved for the treatment of type 2 diabetes (see GLP-1 entry).
Xenotransplantation is the transplantation of cells, tissues, or organs from one species to another. The most active area of diabetes-focused xenotransplantation investigation involves transplanting pig organs into primates. Hopefully, this investigation will pave the way for eventual transplantation of pig organs into humans.
References
1. Leoni, L. and B.B. Roman, MR imaging of pancreatic islets: Tracking isolation, transplantation and function. Curr Pharm Des, 2010. 16(14): p. 1582-94.
2. Beaser, R.S. and A.P. Campbell, The Joslin Guide to Diabetes. 2005, New York, New York: Fireside Books.
3. Eisenbarth, G.S., Banting lecture 2009: An unfinished journey: molecular pathogenesis to prevention of type 1A diabetes. Diabetes, 2010. 59(4): p. 759-74.
4. Eisenbarth, G., Natural history to molecular pathogenesis to preventive trials. J Med Sci, 2010. 3(3): p. 136-41.
5. Wenzlau, J.M., et al., The cation efflux transporter ZnT8 (Slc30A8) is a major autoantigen in human type 1 diabetes. Proc Natl Acad Sci USA, 2007. 104(43): p. 17040-5.
6. Burn, P., Type 1 diabetes. Nat Rev Drug Discov, 2010. 9(3): p. 187-8.
7. Hernandez-Alavarez, M.I., et al., Subjects with early-onset type 2 diabetes show defective activation of the skeletal muscle PGC-1{alpha}/Mitofusion-2 regulatory pathway in response to physical activity. Diabetes Care, 2010. 33(3): p. 645-51.
8. Ryan, A., et al., Diabetes mellitus and apoptosis: inflammatory cells. Apoptosis, 2009. 14(12): p. 1435-50.
9. Daneman, D., Type 1 diabetes. Lancet, 2006. 367(9513): p. 847-58.
10. Ablamunits, V., et al., Autoimmunity and beta cell regeneration in mouse and human type 1 diabetes—The peace is not enough. Ann N Y Acad Sci 2007. 1103: p. 19-32.
11. Staeva-Vieira, T., M. Peakman, and M. von Herrath, Translational mini-review series on type 1 diabetes: Immune-based therapeutic approaches for type 1 diabetes. Clin Exp Immunol, 2007. 148(1): p. 17-31.
12. Luo, X., K.C. Herold, and S.D. Miller, Immunotherapy of type 1 diabetes: where are we and where should we be going? Immunity, 2010. 32(4): p. 488-99.
13. Faustman, D.L. and M. Davis, The primacy of CD8 T lymphocytes in type 1 diabetes and implications for therapies. J Mol Med-Jmm, 2009. 87(12): p. 1173-8.
14. Bluestone, J.A., K. Herold, and G. Eisenbarth, Genetics, pathogenesis and clinical interventions in type 1 diabetes. Nature, 2010. 464(7293): p. 1293-300.
15. Bour-Jordan, H. and J.A. Bluestone, B cell depletion: a novel therapy for autoimmune diabetes? J Clin Invest, 2007. 117(12): p. 3642-45.
16. Pescovitz, M.D., et al., Rituximab, B-Lymphocyte Depletion, and Preservation of Beta-Cell Function. N Eng J Med, 2009. 361(22): p. 2143-52.
17. Hu, C.Y., et al., Treatment with CD20-specific antibody prevents and reverses autoimmune diabetes in mice. J Clin Invest, 2007. 117(12): p. 3857-67.
18. Harrison, L.C., Vaccination against self to prevent autoimmune disease: the type 1 diabetes model. Immunol Cell Biol, 2008. 86(2): p. 139-45.
19. Rasche, S., R.Y. Busick, and A. Quinn, GAD65-Specific Cytotoxic T Lymphocytes Mediate Beta-Cell Death and Loss of Function. Rev Diabet Stud, 2009. 6(1): p. 43-53.
20. Islam, S., ed. The Islets of Langerhans. Advances in Experimental Medicine and Biology. Vol. 654. 2010, Springer Dordrecht Heidelberg: New York, NY. p. 798.
21. Thivolet, C., et al., Glutamic-acid decarboxylase (GAD) autoantibodies are additional predictive markers of type-1 (insulin-dependent) diabetes-mellitus in high-risk individuals. Diabetologia, 1992. 35(6): p. 570-6.
22. Waldrop, M.A., et al., Release of glutamate decarboxylase-65 into the circulation by injured pancreatic islet ss-cells. Endocrinology, 2007. 148(10): p. 4572-8.
23. Ludvigsson, J., et al., GAD treatment and insulin secretion in recent-onset type 1 diabetes. N Engl J Med, 2008. 359(18): p. 1909-20.
24. Ludvigsson, J., et al., GAD65 antigen therapy in recently diagnosed type 1 diabetes mellitus. N Engl J Med, 2012. 366(5): p. 433-42.
25. Fourlanos, S., et al., Evidence that nasal insulin induces immune tolerance to insulin in adults with autoimmune diabetes. Diabetes, 2011. 60(4): p. 1237-45.
26. Skyler, J.S., et al., Effects of oral insulin in relatives of patients with type 1 diabetes: The Diabetes Prevention Trial—Type 1. Diabetes Care, 2005. 28(5): p. 1068-76.
27. Miller, S.D., D.M. Turley, and J.R. Podojil, Antigen-specific tolerance strategies for the prevention and treatment of autoimmune disease. Nat Rev Immunol, 2007. 7(9): p. 665-77.
28. Vehik, K., et al., Long-term outcome of individuals treated with oral insulin: Diabetes Prevention Trial–Type 1 (DPT-1) oral insulin trial. Diabetes Care, 2011. 34(7): p. 1585-90.
29. Solvason, N., et al., Improved efficacy of a tolerizing DNA vaccine for reversal of hyperglycemia through enhancement of gene expression and localization to intracellular sites. J Immunol, 2008. 181(12): p. 8298-307.
30. Utz, P., Proinsulin DNA vaccine therapy in type 1 diabetes. 12th Annual Rachmiel Levine Diabetes and Obesity Symposium. 2012. Pasadena, CA: City of Hope.
31. Gottlieb, P., et al., One-year results from a phase 1/2 clinical trial of BHT-3021, a DNA plasmid vaccine for type 1 diabetes (T1D). Diabetes, 2010. 59: p. A18.
32. Thrower, S.L., et al., Proinsulin peptide immunotherapy in type 1 diabetes: Report of a first-in-man phase I safety study. Clin Exp Immunol, 2009. 155(2): p. 156-65.
33. Pozzilli, P. and R.D. Leslie, New prospects for immunotherapy at diagnosis of type 1 diabetes. Diabetes Metab Res Rev, 2009. 25(4): p. 299-301.
34. Macrogenics and Eli Lilly announce pivotal clinical trial of teplizumab did not meet primary efficacy endpoint. 2010 October 20 [cited 2010; Available from: http://www.fiercebiotech.com/press-releases/macrogenics-and-lilly-announce-pivotal-clinical-trial-teplizumab-did-not-meet-primary.
35. Sherry, N., et al., Teplizumab for treatment of type 1 diabetes (Protégé study): 1-year results from a randomised, placebo controlled trial. Lancet, 2011. 378(9790): p. 487-97.
36. Herold, K.C., et al., A single course of anti-CD3 monoclonal antibody hOKT3 gamma 1(Ala-Ala) results in improvement in C-peptide responses and clinical parameters for at least 2 years after onset of type 1 diabetes. Diabetes, 2005. 54(6): p. 1763-9.
37. Herold, K.C., et al., Treatment of patients with new onset type 1 diabetes with a single course of anti-CD3 mAb teplizumab preserves insulin production for up to 5 years. Clin Immunol, 2009. 132(2): p. 166-73.
38. Gitelman, S.E., AbATE (Anti-CD3 MAB). American Diabetes Association’s 71st Scientific Sessions. 2011. San Diego, CA.
39. Herold, K.C., et al., Teplizumab preserves insulin production in type 1 diabetes after the new onset period (85-OR). American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
40. Tsalikian, E., et al., Prevention of hypoglycemia during exercise in children with type 1 diabetes by suspending basal insulin. Diabetes Care, 2006. 29(10): p. 2200-4.
41. Gottlieb, P., Pozzilli, P., DEFEND. American Diabetes Association’s 71st Scientific Sessions. 2011. San Diego, CA.
42. Keymeulen, B., et al., Insulin needs after CD3-antibody therapy in new-onset type 1 diabetes. N Eng J Med, 2005. 352(25): p. 2598-608.
43. Keymeulen, B., et al., Four-year metabolic outcome of a randomised controlled CD3-antibody trial in recent-onset type 1 diabetic patients depends on their age and baseline residual beta cell mass. Diabetologia, 2010. 53(4): p. 614-23.
44. Ochi, H., et al., New immunosuppressive approaches: Oral administration of CD3-specific antibody to treat autoimmunity. J Neurol Sci, 2008. 274(1-2): p. 9-12.
45. You, S., et al., CD3 antibodies as unique tools to restore self-tolerance in established autoimmunity: Their mode of action and clinical application in type 1 diabetes. Adv Immunol, Vol 100, 2008. 100: p. 13-37.
46. Wong, F.S., et al., To B or not to B—pathogenic and regulatory B cells in autoimmune diabetes. Curr Opin Immunol, 2010. 22(6): p. 723-31.
47. Pescovitz, M.E., et al., In vivo immune responses in type 1 diabetes following treatment with rituximab. Diabetes, 2009. 58: p. A51.
48. Cattaneo, C., et al., Delayed-onset peripheral blood cytopenia after rituximab: Frequency and risk factor assessment in a consecutive series of 77 treatments. Leukemia Lymphoma, 2006. 47(6): p. 1013-7.
49. Orban, T., et al., Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: A randomised, double-blind, placebo-controlled trial. Lancet, 2011. 378(9789): p. 412-9.
50. Mease, P., et al., Abatacept in the treatment of patients with psoriatic arthritis: Results of a six-month, multicenter, randomized, double-blind, placebo-controlled, phase II trial. Arthritis Rheum, 2011. 63(4): p. 939-48.
51. Linsley, P.S. and S.G. Nadler, The clinical utility of inhibiting CD28-mediated costimulation. Immunol Rev, 2009. 229(1): p. 307-21.
52. Khraishi, M., A. Russell, and W.P. Olszynski, Safety profile of abatacept in rheumatoid arthritis: A review. Clin Ther, 2010. 32(11): p. 1855-70.
53. Maxwell, L.J. and J.A. Singh, Abatacept for rheumatoid arthritis: a Cochrane systematic review. J Rheumatol, 2010. 37(2): p. 234-45.
54. Orban, T., Co-stimulation modulation with abatacept in patients with recent-onset type 1 diabetes: A follow-up study at 3 years. American Diabetes Association’s 72nd Scientific Sessions, 2012: Philadelphia, PA.
55. Tooley, J.E., F. Waldron-Lynch, and K.C. Herold, New and future immunomodulatory therapy in type 1 diabetes. Trends Mol Med, 2012. 18(3): p. 173-81.
56. Bristol Myers Squibb, Highlights of prescribing information: Orencia. 2012 [cited 2012 2 September]; Available from: http://packageinserts.bms.com/pi/pi_orencia.pdf.
57. Chatenoud, L., K. Warncke, and A.G. Ziegler, Clinical immunologic interventions for the treatment of type 1 diabetes. Cold Spring Harb Perspect Med, 2012. 2(8).
58. Gallagher, M.P., R.S. Goland, and C.J. Greenbaum, Making progress: Preserving beta cells in type 1 diabetes. Ann N Y Acad Sci, 2011. 1243: p. 119-34.
59. Matthews, J.B., et al., Developing combination immunotherapies for type 1 diabetes: Recommendations from the ITN-JDRF Type 1 Diabetes Combination Therapy Assessment Group. Clin Exp Immunol, 2010. 160(2): p. 176-84.
60. Vergani, A., et al., A novel clinically relevant strategy to abrogate autoimmunity and regulate alloimmunity in NOD mice. Diabetes, 2010. 59(9): p. 2253-64.
61. Schneider, D.A., G. Sarikonda, and M.G. von Herrath, Combination therapy with InsB9-23 peptide immunization and CTLA4-IgG does not reverse diabetes in NOD mice. Clin Immunol, 2012. 142(3): p. 402-3.
62. Lundquist L.M., S.W. Cole, and J.M. Augustine, Critical appraisal of efficacy and safety of abatacept in the treatment of refractory rheumatoid arthritis. Open Access Rheum Res Rev, 2012. 4: p. 9-19.
63. Donath, M.Y., et al., Islet inflammation in type 2 diabetes: From metabolic stress to therapy. Diabetes Care, 2008. 31 Suppl 2: p. S161-4.
64. Mandrup-Poulsen, T., IL-1 receptor antagonist in recent onset type 1 diabetes—A multicenter randomized, placebo-controlled trial. American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
65. Moran, A., Canakinumab, an anti-IL-1 monoclonal antibody in recent-onset type 1 diabetes. American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
66. Eldor, R., S. Kassem, and I. Raz, Immune modulation in type 1 diabetes mellitus using DiaPep277: A short review and update of recent clinical trial results. Diabetes Metab Res Rev, 2009. 25(4): p. 316-20.
67. Cnop, M., et al., Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: Many differences, few similarities. Diabetes, 2005. 54 Suppl 2: p. S97-107.
68. Raz, I., et al., Treatment of new-onset type 1 diabetes with peptide DiaPep277 is safe and associated with preserved beta cell function: Extension of a randomized, double-blind, phase II trial. Diabetes Metab Res Rev, 2007. 23(4): p. 292-8.
69. Huurman, V.A., et al., Immunological efficacy of heat shock protein 60 peptide DiaPep277 therapy in clinical type I diabetes. Clin Exp Immunol, 2008. 152(3): p. 488-97.
70. Schloot, N.C., et al., Effect of heat shock protein peptide DiaPep277 on beta-cell function in pediatric and adult patients with recent-onset diabetes mellitus type 1: two prospective, randomized, double-blind phase II trials. Diabetes Metab Res Rev, 2007. 23(4): p. 276-85.
71. Pozzilli, P. Recent data from DIA-AID 1, a global phase III clinical study in newly diagnosed type 1 diabetes patients (292-OR). American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
72. Lazar, L., et al., Heat-shock protein peptide DiaPep277 treatment in children with newly diagnosed type 1 diabetes: A randomised, double-blind phase II study. Diabetes Metab Res Rev, 2007. 23(4): p. 286-91.
73. Rousseau, M.C., M.E. Parent, and Y. St-Pierre, Potential health effects from non-specific stimulation of the immune function in early age: The example of BCG vaccination. Pediatr Allergy Immu, 2008. 19(5): p. 438-48.
74. Faustman, D.L., et al., Proof-of-concept, randomized, controlled clinical trial of bacillus-calmette-guerin for treatment of long-term type 1 diabetes. PLoS One, 2012. 7(8): p. 1-16.
75. Huppmann, M., et al., Neonatal bacille calmette-guerin vaccination and type 1 diabetes. Diabetes Care, 2005. 28(5): p. 1204-6.
76. Elliott, J.F., K.L. Marlin, and R.M. Couch, Effect of bacille calmette-guerin vaccination on C-peptide secretion in children newly diagnosed with IDDM. Diabetes Care, 1998. 21(10): p. 1691-3.
77. Herr, H.W. and A. Morales, History of bacillus calmette-guerin and bladder cancer: An immunotherapy success story. J Urol, 2008. 179(1): p. 53-6.
78. Andersen, P. and T.M. Doherty, The success and failure of BCG—implications for a novel tuberculosis vaccine. Nat Rev Microbiol, 2005. 3(8): p. 656-62.
79. Chamberlain, G., et al., Concise review: Mesenchymal stem cells: Their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells, 2007. 25(11): p. 2739-49.
80. Abdi, R., et al., Immunomodulation by mesenchymal stem cells—A potential therapeutic strategy for type 1 diabetes. Diabetes, 2008. 57(7): p. 1759-67.
81. Newman, R.E., et al., Treatment of inflammatory diseases with mesenchymal stem cells. Inflamm Allergy Drug Targets, 2009. 8(2): p. 110-23.
82. Tyndall, A., et al., Immunomodulatory properties of mesenchymal stem cells: A review based on an interdisciplinary meeting held at the Kennedy Institute of Rheumatology Division, London, UK, 31 October 2005. Arthritis Res Ther, 2007. 9(1): p. 301.
83. Nauta, A.J. and W.E. Fibbe, Immunomodulatory properties of mesenchymal stromal cells. Blood, 2007. 110(10): p. 3499-506.
84. Osiris Therapeutics. Osiris Therapeutics provides update on groundbreaking stem cell trial for type 1 diabetes. 2012 [cited 2012 August 27]; Available from: http://investor.osiris.com/releasedetail.cfm?ReleaseID=636520.
85. Osiris Therapeutics announces preliminary results for Prochymal phase III GvHD trials. 2009 [cited 2010 July]; Available from: http://www.fiercebiotech.com/press-releases/osiris-therapeutics-announces-preliminary-results-prochymal-phase-iii-gvhd-trials.
86. Osiris Therapeutics. New study demonstrates effectiveness of Prochymal as a rescue therapy for pediatric patients with severe graft vs. host disease. 2010 [cited 2012 August 27]; Available from: http://files.shareholder.com/downloads/OSIR/1967801628x0x352372/f5d5d277-5599-4e45-b0a6-7c122e6371ed/OSIR_News_2010_2_24_General.pdf.
87. Osiris Therapeutics. World’s first approved stem cell drug; Osiris receives marketing clearance from Health Canada for Prochymal. 2012 [cited 2012 August 27]; Available from: http://investor.osiris.com/releasedetail.cfm?ReleaseID=674658.
88. Osiris Therapeutics. Osiris receives second approval for life-saving stem cell drug; Prochymal granted marketing consent by New Zealand. 2012 [cited 2012 August 27]; Available from: http://investor.osiris.com/releasedetail.cfm?ReleaseID=683073.
89. Osiris Therapeutics. Osiris resumes enrollment in stem cell trial for Crohn’s disease following positive interim analysis. 2010 [cited 2011 29 May]; Available from: http://osir.client.shareholder.com/releasedetail.cfm?releaseID=466950.
90. Ryan, E.A., D. Bigam, and A.M. Shapiro, Current indications for pancreas or islet transplant. Diabetes Obes Metab, 2006. 8(1): p. 1-7.
91. Khan, M.H. and D.M. Harlan, Counterpoint: Clinical islet transplantation: Not ready for prime time. Diabetes Care, 2009. 32(8): p. 1570-4.
92. National Institute of Diabetes and Digestive and Kidney Diseases. Pancreas transplant. 2009 [cited 2011 23 June]; Available from: http://www.kidney.org/atoz/content/pancreastx.cfm.
93. Hering, B.J., et al., Single-donor, marginal-dose islet transplantation in patients with type 1 diabetes. JAMA, 2005. 293(7): p. 830-5.
94. Shapiro, A.M., et al., International trial of the Edmonton protocol for islet transplantation. N Engl J Med, 2006. 355(13): p. 1318-30.
95. Ryan, E.A., et al., Five-year follow-up after clinical islet transplantation. Diabetes, 2005. 54(7): p. 2060-9.
96. Ryan, E.A. and A.J. Shapiro, A patient with severe, recurrent hypoglycemia and glycemic lability who underwent islet transplantation. Nat Clin Pract Endocrinol Metab, 2006. 2(6): p. 349-53; quiz 354.
97. Senior, P.A. A Decade After the Edmonton Protocol. American Diabetes Association’s 71st Scientific Sessions. 2011. San Diego, CA.
98. Faradji, R.N., et al., Long-term insulin independence and improvement in insulin secretion after supplemental islet infusion under exenatide and etanercept. Transplantation, 2008. 86(12): p. 1658-65.
99. Posselt, A.M., et al., Islet transplantation in type 1 diabetics using an immunosuppressive protocol based on the anti-LFA-1 antibody efalizumab. Am J Transplant, 2010. 10(8): p. 1870-80.
100. Gangemi, A., et al., Islet transplantation for brittle type 1 diabetes: The UIC protocol. Am J Transplant, 2008. 8(6): p. 1250-61.
101. Bellin, M.D., et al., Prolonged insulin independence after islet allotransplants in recipients with type 1 diabetes. Am J Transplant, 2008. 8(11): p. 2463-70.
102. Barton, F.B., et al., Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care, 2012. 35: p. 1436-45.
103. Kendall, D.M., A.U. Teuscher, and R.P. Robertson, Defective glucagon secretion during sustained hypoglycemia following successful islet allo- and autotransplantation in humans. Diabetes, 1997. 46(1): p. 23-7.
104. Paty, B.W., et al., Intrahepatic islet transplantation in type 1 diabetic patients does not restore hypoglycemic hormonal counterregulation or symptom recognition after insulin independence. Diabetes, 2002. 51(12): p. 3428-34.
105. Gupta, V., et al., The defective glucagon response from transplanted intrahepatic pancreatic islets during hypoglycemia is transplantation site-determined. Diabetes, 1997. 46(1): p. 28-33.
106. National Institute of Diabetes and Digestive and Kidney Diseases. Pancreatic islet transplantation. 2007 [cited 2011 22 June]; Available from: http://diabetes.niddk.nih.gov/dm/pubs/pancreaticislet/.
107. Robertson, R.P., Islet transplantation a decade later and strategies for filling a half-full glass. Diabetes, 2010. 59(6): p. 1285-91.
108. Lonza, R., Langer, R., Vacanti, J., ed. Principles of Tissue Engineering. Third ed. 2007, Elsevier Academic Press: San Diego, CA.
109. Fishman, J.A., Introduction: Infection in solid organ transplant recipients. Am J Transplant, 2009. 9 Suppl 4: p. S3-6.
110. Merani, S. and A.M. Shapiro, Current status of pancreatic islet transplantation. Clin Sci (Lond), 2006. 110(6): p. 611-25.
111. Mineo, D., et al., Point: steady progress and current challenges in clinical islet transplantation. Diabetes Care, 2009. 32(8): p. 1563-9.
112. Guo, T. and M. Hebrok, Stem cells to pancreatic beta-cells: New sources for diabetes cell therapy. Endocr Rev, 2009. 30(3): p. 214-27.
113. Soria, B., Insulin-secreting cells from stem cells: How far we are? FEBS J, 2006. 273: p. 12.
114. Cardona, K., et al., Engraftment of adult porcine islet xenografts in diabetic nonhuman primates through targeting of costimulation pathways. Am J Transplant, 2007. 7(10): p. 2260-8.
115. Spring Point Project. Our work. 2008 [cited 2011 19 May]; Available from: http://www.springpointproject.org/our_work/.
116. Rotenstein, L. and K.L. Close, Advances in diabetes thinking: A critical summary and analysis of Keystone 2009. Diabetes Technol Ther, 2010. 12(2): p. 167-71.
117. Hering, B.J., et al., Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med, 2006. 12(3): p. 301-3.
118. Living Cell Technology Limited. LCT’s DIABECELL trial extension approved. 2012 [cited 2012 August 27]; Available from: http://www.lctglobal.com/html/blob.php/120518 LCT to extend DIABECELL trial.pdf?attach=0&documentCode=4527&elementId=20084.
119. Living Cell Technology Limited. DIABECELL: A breakthrough treatment for type 1 diabetes. 2012 [cited 2012 August 27]; Available from: http://www.lctglobal.com/html/blob.php/LCT Diabecell fact sheet 7.pdf?attach=0&documentCode=4480&elementId=20084.
120. Revivicor. Xenotransplantation Program. 2010 [cited 2011 19 May]; Available from: http://www.revivicor.com/body_xenotransplantation.htm.
121. van der Windt, D.J., et al., Long-term controlled normoglycemia in diabetic non-human primates after transplantation with hCD46 transgenic porcine islets. Am J Transplant, 2009. 9(12): p. 2716-26.
122. Hammerman, M.R., Xenotransplantation of embryonic pig kidney or pancreas to replace the function of mature organs. J Transplant, 2011. 501749: 1-9.
123. Rogers, S.A., et al., Engraftment of cells from porcine islets of Langerhans and normalization of glucose tolerance following transplantation of pig pancreatic primordia in nonimmune-suppressed diabetic rats. Am J Pathol, 2010. 177(2): p. 854-64.
124. Rogers, S.A., et al., Engraftment of cells from porcine islets of Langerhans following transplantation of pig pancreatic primordia in non-immunosuppressed diabetic rhesus. Organogenesis, 2011. 7(3): p. 154-62.
125. Beck, J., et al., Islet encapsulation: Strategies to enhance islet cell functions. Tissue Engineering, 2007. 13(3): p. 589-99.
126. Halberstadt, C.R., et al., Subcutaneous transplantation of islets into streptozocin-induced diabetic rats. Cell Transplantation, 2005. 14(8): p. 595-605.
127. de Vos, P., et al., Alginate-based microcapsules for immunoisolation of pancreatic islets. Biomaterials, 2006. 27(32): p. 5603-17.
128. Living Cell Technologies Limited. Living Cell Technologies—World leading cell implant company. [Presentation] 2010 August/September 2010 [cited 20 May 2011]; Available from: http://www.lctglobal.com/html/blob.php/LCT in Australia Sept 2010.pdf?filetypecode=1&attach=0&document=2176.
129. Hanuman Medical Foundation. Large-Animal Studies. [cited 2012 August 27]; Available from: http://hanumanmedicalfoundation.org/type-1-diabetes-research/islet-sheet-project/large-animal-studies.html.
130. Hanuman Medical Foundation. Current & Planned Research. [cited 2012 August 27]; Available from: http://www.cercomed.com/type-1-diabetes-research/islet-sheet-project/current-and-planned-research.html.
131. Silva, A.I., et al., An overview on the development of a bio-artificial pancreas as a treatment of insulin-dependent diabetes mellitus. Med Res Rev, 2006. 26(2): p. 181-222.
132. Pileggi, A., et al., Reversal of diabetes by pancreatic islet transplantation into a subcutaneous, neovascularized device. Transplantation, 2006. 81(9): p. 1318-24.
133. ViaCyte. Cell Encapsulation. 2010 [cited 2011 29 May]; Available from: http://www.viacyte.com/tech/encapsulation.html.
134. Sernova Corporation. First Therapeutic Application—Diabetes. [cited 2011 29 April]; Available from: http://www.sernova.com/s/DiabetesProject.asp?ReportID=382934.
135. Sernova Corporation. World first—Islet transplant into man using Sernova’s Cell Pouch (TM) for treatment of diabetes. 2012 [cited 2012 August 27]; Available from: http://www.sernova.com/s/NewsReleases.asp?ReportID=542450.
136. Assmann, A., C. Hinault, and R.N. Kulkarni, Growth factor control of pancreatic islet regeneration and function. Pediatr Diabetes, 2009. 10(1): p. 14-32.
137. Levetan, C.S., et al., Discovery of a human peptide sequence signaling islet neogenesis. Endocr Pract, 2008. 14(9): p. 1075-83.
138. Reimann, M., et al., An update on preventive and regenerative therapies in diabetes mellitus. Pharmacol Ther, 2009. 121(3): p. 317-31.
139. Toso, C., et al., Effect of microcapsule composition and short-term immunosuppression on intraportal biocompatibility. Cell Transplantation, 2005. 14(2-3): p. 159-67.
140. Safley, S.A., et al., Inhibition of cellular immune responses to encapsulated porcine islet xenografts by simultaneous blockade of two different costimulatory pathways. Transplantation, 2005. 79(4): p. 409-18.
141. Miura, S., Y. Teramura, and H. Iwata, Encapsulation of islets with ultra-thin polyion complex membrane through poly(ethylene glycol)-phospholipids anchored to cell membrane. Biomaterials, 2006. 27(34): p. 5828-35.
142. Lee, D.Y., et al., A combination therapy of PEGylation and immuno suppressive agent for successful islet transplantation. J Control Release, 2006. 110(2): p. 290-5.
143. Beta-O2. Beta-O2 is Proposing a Life-Changing Benefit to Diabetes Patients. 2009 [cited 2011 19 May]; Available from: http://www.beta-o2.com/.
144. Bonner-Weir, S. and G.C. Weir, New sources of pancreatic beta-cells. Nat Biotechnol, 2005. 23(7): p. 857-61.
145. Levine, F. and P. Itkin-Ansari, Beta-cell regeneration: neogenesis, replication or both? J Mol Med, 2008. 86(3): p. 247-58.
146. Duttaroy, A., et al., The DPP-4 inhibitor vildagliptin increases pancreatic beta cell mass in neonatal rats. Eur J Pharmacol, 2011. 650(2-3): p. 703-7.
147. Xu, G., et al., Exendin-4 stimulates both beta-cell replication and neogenesis, resulting in increased beta-cell mass and improved glucose tolerance in diabetic rats. Diabetes, 1999. 48(12): p. 2270-6.
148. Thornberry, N.A. and B. Gallwitz, Mechanism of action of inhibitors of dipeptidyl-peptidase-4 (DPP-4). Best Pract Res Clin Endocrinol Metab, 2009. 23(4): p. 479-86.
149. Tian, L., et al., Reversal of new-onset diabetes through modulating inflammation and stimulating beta-cell replication innonobese diabetic mice by a dipeptidyl peptidase IV inhibitor. Endocrinology, 2010. 151(7): p. 3049-60.
150. Kim, W. and J.M. Egan, The role of incretins in glucose homeostasis and diabetes treatment. Pharmacol Rev, 2008. 60(4): p. 470-512.
151. Raman, V.S., et al., The role of adjunctive exenatide therapy in pediatric type 1 diabetes. Diabetes Care, 2010. 33(6): p. 1294-6.
152. Rother, K.I., et al., Effects of exenatide alone and in combination with daclizumab on beta-cell function in long-standing type 1 diabetes. Diabetes Care, 2009. 32(12): p. 2251-7.
153. Dupre, J., M.T. Behme, and T.J. McDonald, Exendin-4 normalized postcibal glycemic excursions in type 1 diabetes. J Clin Endocrinol Metab, 2004. 89(7): p. 3469-73.
154. Kielgast, U., et al., Four weeks of treatment with liraglutide reduces insulin dose without loss of glycemic control in type 1 diabetic patients with and without residual beta-cell function. Diabetes Care, 2011. 34: p. 1463-8.
155. Varanasi, A., et al., Liraglutide as additional treatment for type 1 diabetes. Eur J Endocrinol, 2011. 165: p. 77-84.
156. Kielgast, U., J.J. Holst, and S. Madsbad, Antidiabetic actions of endogenous and exogenous GLP-1 in type 1 diabetic patients with and without residual beta-cell function. Diabetes, 2011. 60(5): p. 1599-1607.
157. Froud, T., et al., The use of exenatide in islet transplant recipients with chronic allograft dysfunction: Safety, efficacy, and metabolic effects. Transplantation, 2008. 86(1): p. 36-45.
158. Ghofaili, K.A., et al., Effect of exenatide on beta cell function after islet transplantation in type 1 diabetes. Transplantation, 2007. 83(1): p. 24-8.
159. Dungan, K.M., J.B. Buse, and R.E. Ratner, Effects of therapy in type 1 and type 2 diabetes mellitus with a peptide derived from islet neogenesis associated protein (INGAP), Diabetes Metab Res Rev, 2009. 25(6): p. 558-65.
160. Drucker, D.J., The role of gut hormones in glucose homeostasis. J Clin Invest, 2007. 117(1): p. 24-32.
161. Transition Therapeutics, Gastrin based therapies. 2008 [cited 2010 31 December]; Available from: http://www.transitiontherapeutics.com/technology/diabetes.php.
162. Levetan, C., Distinctions between islet neogenesis and beta-cell replication: implications for reversal of type 1 and 2 diabetes. J Diabetes, 2010. 2(2): p. 76-84.
163. Weinzimer, S., et al., Effectiveness of continuous glucose monitoring in a clinical care environment evidence from the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring (JDRF-CGM) trial. Diabetes Care, 2010. 33(1): p. 17-22.
164. Ginsberg, B.H., The current environment of CGM technologies. J Diabetes Sci Technol, 2007. 1(1): p. 117-21.
165. Pickup, J.C. and A.J. Sutton, Severe hypoglycaemia and glycaemic control in type 1 diabetes: Meta-analysis of multiple daily insulin injections compared with continuous subcutaneous insulin infusion. Diabet Med, 2008. 25(7): p. 765-74.
166. JDRF CGM Study Group, Continuous glucose mintoring and intensive treatment of type 1 diabetes. N Eng J Med, 2008. 359: p. 1464-76.
167. Bergenstal, R.M., et al., Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med, 2010. 363(4): p. 311-20.
168. Ramchandani, N., Real-life utilization of real-time continuous glucose monitoring, 3rd International Conference on Advanced Technology & Treatments for Diabetes 2010: Basel, Switzerland.
169. Ritholz, M., Barriers to continuous glucose management use. American Diabetes Association’s 70th Scientific Sessions, 2010: Orlando, FL.
170. Price, D., et al., Performance and Reliability of the New Dexcom G4 Continuous Glucose Monitoring (CGM) System - Pivotal Trial Results. European Association for the Study of Diabetes 48th Annual Meeting, 2012: Berlin, Germany.
171. Abbott. FreeStyle Navigator continuous glucose monitoring system. 2008 [cited 2011 May 21]; Available from: http://www.freestylenavigator.com/static/cms_workspace/document/FSN-IFU-ART16072_Rev-A.pdf.
172. Dexcom. Dexcom product information. 2011 [cited 2011 May 21]; Available from: http://www.dexcom.com/products/download-product-information.
173. Minimed, Guardian REAL-Time user guide. 2006 [cited 2011 May 21]; Available from: http://www.minimed.com/pdf/guardian_real_time_user_guide.pdf.
174. Tenderich, A., Aaron Kowalski: Your questions on the artificial pancreas answered here. Diabetes Mine 2010 [cited 2010 August]; Available from: http://www.diabetesmine.com/2010/01/aaron-kowalski-your-questions-on-the-artificial-pancreas-answered-here.html.
175. Kowalski, A.J., A JDRF research overview, Children with Diabetes Foundation Conference. 2010: Keystone, CO.
176. Ward, K., JDRF/NIDDK closed-loop control research meeting: Outpatient closed-loop study progress and panel discussion. American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
177. Kircher, R.J., Reducing postprandial hypoglycemia using fuzzy logic controller with insulin dosing governor. American Diabetes Association’s 70th Scientific Sessions. 2010. Orlando, FL.
178. Miller, S., Automatic learning algorithm for the MD-Logic artificial pancreas system. 3rd Interantional Conference on Advanced Treatments & Technologies for Diabetes. 2010. Basel, Switzerland.
179. Dassau, E., Design, validation, and clinical evaluation of a fully-automated artificial pancreatic B-cell with unannounced meal using MPMPC and IOB. 3rd International Conference on Advanced Technologies & Treatment for Diabetes. 2010. Basel, Switzerland.
180. Kovatchev, B.P., et al., In silico preclinical trials: A proof of concept in closed-loop control of type 1 diabetes. J Diabetes Sci Technol, 2009. 3(1): p. 44-55.
181. Danne, T., The low glucose suspend (LGS) function in sensor-augmented pump therapy prevents hypoglycemia in children. American Diabetes Association’s 71st Scientific Sessions. 2011. San Diego, CA.
182. Buckingham, B., JDRF/NIDDK Closed Loop Control Research Meeting. American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
183. Breton, M., et al., Fully integrated artificial pancreas in type 1 diabetes: Modular closed-loop glucose control maintains near normoglycemia. Diabetes, 2012. 61(9): p. 2230-7.
184. O’Grady, M.J. et al., The use of an automated, portable glucose control system for overnight glucose control in adolescents with type 1 diabetes (894-P). American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
185. Mackowiak, L., et al., Feasibility study assessing hypoglycemia-hyperglycemia minimizer (HHM) system in patients with type 1 diabetes (T1DM) in a clinical research center (CRC) (917-P). American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
186. Hovorka, R., AP algorithms: The Cambridge ’model predictive control’ approach. The 5th International Conference on Advanced Technologies & Treatments for Diabetes. 2012. Barcelona, Spain.
187. Hovorka, R., et al., Overnight closed loop insulin delivery (artificial pancreas) in adults with type 1 diabetes: crossover randomised controlled studies. B M J, 2011. 342: p. 1-10.
188. Weinzimer S., et al., Glucose control using closed loop insulin delivery during nights with or without antecedent afternoon exercise. American Diabetes Association’s 71st Scientific Sessions. 2011. San Diego, CA.
189. Kowalski, A., JDRF research overview. Children with Diabetes Foundation Conference. 2010. Keystone, CO.
190. Kowalski, A.J., Can we really close the loop and how soon? Accelerating the availability of an artificial pancreas: A roadmap to better diabetes outcomes. Diabetes Technol Ther, 2009. 11(S1): p. S113-9.
191. Dassau, E., et al., Enhanced 911/GPS wizard for the prevention of severe hypoglycemia-monitor, alert and locate. Diabetes, 2009. 58: p. A506.
192. Shah, R., A new generation of continuous glucose sensors- Quo vadis? The 4th International Conference on Advanced Technologies & Treatments for Diabetes. 2011: London, U.K.
193. Price, D. Impact of study design and analytic techniques on the reported accuracy of continuous glucose monitoring (CGM) systems. American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
194. Simmons, D., New results from clinical studies of a continuous glucose monitoring (CGM) system. The 5th International Conference on Advanced Technologies & Treatment for Diabetes. 2012. Barcelona, Spain.
195. Judge, K., et al., Continuous glucose monitoring using a novel glucose/galactose binding protein: results of a 12-hour feasibility study with the becton dickinson glucose/galactose binding protein sensor. Diabetes Tech Ther, 2011. 13(3): p. 309-17.
196. Hirsch, I., Human hyaluronidase + rapid analog insulin (RAI) improves postprandial glycemic control in type 1 diabetes (T1DM) compared to insulin lisopro alone (353-OR). American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
197. Muchmore, D., et al., Initial clinical experience with hyaluronidase preadministration in the treatment of type 1 diabetes by sensor-augmented analog insulin pump therapy (34-LB). American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
198. Castle, J.R., Evaluations of modified ultra-rapid acting Linjeta formulations Biod-105 and Biod-107 in patients with type 1 diabetes (350-OR). American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
199. Dassau, E., Design control and clinical evaluation of an artificial pancreas utilizing ultra-fast insulin delivery systems, The 5th International Conference on Advanced Technologies & Treatments for Diabetes. 2012: Barcelona, Spain.
200. Zisser, H., Excitation: The use of fluorescence in continuous glucose monitoring, The 3rd International Conference on Advanced Technologies & Treatments for Diabetes. 2010: Basel, Switzerland.
201. MannKind Corporation, United States Security and Exchange Commission Form 10-K. 2012 [cited 2012 September 3]; Available from: http://services.corporate-ir.net/SEC/Document.Service?id=P3VybD1hSFIwY0RvdkwyRndhUzUwWlc1cmQybDZZWEprTG1OdmJTOWtiM2R1Ykc5aFpDNXdhSEEvWVdOMGFXOXVQVkJFUmlacGNHRm5aVDA0TVRRd05URTBKbk4xWW5OcFpEMDFOdz09JnR5cGU9MiZmbj1NYW5uS2luZF8xMEtfMjAxMjAzMTUucGRm
202. Pinkos, A., Continuous glucose monitor considerations in an artificial pancreas: An FDA perspective. The 4th International Conference on Advanced Technologies & Treatments for Diabetes. 2010. Basel, Switzerland.
203. Outpatient pump shutoff pilot feasibility and efficacy study. ClinicalTrials.gov Identifier: NCT01591681. 2012 [cited 2012 September 4]; Available from: http://clinicaltrials.gov/ct2/show/NCT01591681?term=Outpatient+Pump+Shutoff+Pilot+Feasibility+and+Efficacy+Study&rank=1.
204. FDA/NIH public workshop – Innovations in technology for the treatment of diabetes: Clinical development of the artificial Pancreas (an autonomous system). 2010. Gathersburg, MD.
205. Russell, S.J., et al., Blood glucose control in type 1 diabetes with a bihormonal bionic endocrine pancreas. Diabetes Care, 2012; Available from http://www.ncbi.nlm.nih.gov/pubmed/22923666.
206. Weinzimer, S., Effect of adjuvant injected pramlintide on closed-loop insulin delivery: Preliminary findings, The 4th International Conference on Advanced Technologies & Treatments for Diabetes. 2011: London, U.K.
207. Effects of metreleptin in type 1 diabetes mellitus. ClinicalTrials.gov Identifier: NCT01268644. 2012 [cited 2012 September 4]; Available from: http://clinicaltrials.gov/ct2/show/NCT01268644?term=metreleptin&rank=5.
208. Russell, S. Case study: Automated management of blood glucose with a closed-loop bi-hormonal bionic pancreas. Phacilitate Metabolic Leaders’ Forum. 2012. San Francisco, CA.
209. JDRF Artificial Pancreas Consortium. Study Information. [cited 2011 17 May]; Available from: http://jdrfconsortium.jaeb.org/Studies.aspx.
210. Buckingham, B., et al., Preventing hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Technol Ther, 2009. 11(2): p. 93-7.
211. Renard, E. The multi-modular Model Predictive Control-To-Range (MPC2R) allows simultaneous improvement in safety and efficacy of closed-loop insulin delivery in type 1 diabetes (T1D). American Diabetes Association’s 71st Scientific Sessions. 2011. San Diego, CA.
212. Battelino, T., Overnight type 1 diabetes control under the MD-logic artificial pancreas system at a diabetes camp – the DREAM 3 study. The 5th International Conference on Advanced Technologies & Treatments for Diabetes (ATTD). 2012. Barcelona, Spain.
213. Kovatchev, B., AP platforms. The 5th International Conference on Advanced Technologies & Treatments for Diabetes. 2012. Barcelona, Spain.
214. Damiano, E. The artificial pancreas project. International Children with Diabetes Conference—Friends for Life (FFL). 2012. Orlando, FL.
215. Hovorka, R., JDRF/NIDDK closed-loop control research meeting. American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
216. Buckingham, B., et al., Prevention of nocturnal hypoglycemia using predictive alarm algorithms and insulin pump suspension. Diabetes Care, 2010. 33(5): p. 1013-7.
217. Kovatchev, B., JDRF/NIDDK closed-loop control research meeting. American Diabetes Association’s 72nd Scientific Sessions. 2012. Philadelphia, PA.
218. Doyle, F., System and control engineering for type 1 diabetes: The design of an artificial pancreas. The 5th International Conference on Advanced Technologies & Treatments for Diabetes. 2012. Barcelona, Spain.
219. Weinzimer S. The artificial pancreas and metabolic control trial aimed at preserving beta cell function. American Diabetes Association’s 71st Scientific Sessions. 2011. San Diego, CA.
220. Pink, D., Drive. 2002, New York: Riverhead Books.
221. Cheng, J.Y.C., et al., Matrix components and scaffolds for sustained islet function. Tissue Eng, 2011. 17(4): p.235-47.
Footnotes
1. The term prediabetes can be confusing since it commonly refers to people who are at high risk for developing type 2 diabetes. Prediabetes, however, is also sometimes used by scientists and healthcare providers to refer to people whose beta cells are under attack by the immune system, but who do not currently need insulin therapy. Many of these people will eventually develop type 1 diabetes. This latter definition of prediabetes is how the term is used in this report. (Return to text.)
2. As of now, there is little activity on the anti-CD20 front for type 1 diabetes, making the therapy’s timeline very uncertain. The timeline could be quite short if nobody pursues anti-CD20 use for type 1 further, or quite a bit longer if its use does continue to be explored. (Return to text.)
3. We rate this as N/A because it is currently available and works (even if not optimally). (Return to text.)
4. Because participants began these trials with little remaining functional beta cell mass and because GLP-1 therapy did not appear to increase this mass, the observed improvements in blood glucose control were thought to result from GLP-1 induced delays in gastric emptying and reductions in glucagon secretion rather than increased stimulation of insulin secretion from beta cells. (Return to text.)
5. This timeline refers to a hybrid AP system that would feature a partially or fully automated basal system, but would still require users to interact with the system at meals and other times (e.g., to inform the system that a 60 gram carbohydrate meal is about to be eaten or exercise is about to occur). An ideal AP that is fully automated and requires very limited or no user input is likely at least 10-15 years away from regulatory approval. For more, please see Section 5.3. (Return to text.)
6. It should be noted that results are not necessarily directly comparable, since the differences in study design resulted in different glucose distributions, which can affect the average error. For example, all the current systems have their highest percentage error in the hypoglycemic range. Thus, a study with a large proportion of hypoglycemic values will appear to have a higher average percentage error than a study with significantly fewer hypoglycemic values. For CGM systems still in development, we note that early-stage studies are less standardized and so the results are even less directly comparable. (Return to text.)
7. See Section 4.2 or diaTribe #27 for more on use of GLP-1 in type 1 patients. (Return to text.)
8. Until a stabilized liquid glucagon becomes available, researchers must continue the complicated reconstitution process associated with current glucagon formulations. The instability of these formulations requires that the glucagon be changed approximately every 24–36 hours. (Return to text.)

