6.1 Introduction
The hunt for natural bio-active compounds having promising results for the analysis and prevention of diseases is presently a concern topic for various laboratories and industries. The ability of these bio-active compounds to appropriately combine with proteins, DNA, and other biological molecules to synthesize a suitable product would be taken advantage for crafting natural product-derived therapeutic agents (Ajikumar et al. 2008). With the advancement of technologies and evolution of advanced methods to enhance the production, detection, separation, and characterization have transformed the screening of natural bio-active compounds, which can be used efficiently for various needs (Van-Lanen and Shen 2006; Wang and Weller 2006). An array of bio-active compounds released from plants as secondary metabolites assist them to enhance their competency to survive and reduce local challenges by approving them to collaborate with their surroundings (Harborne 1993). Plants respond to the attack of pathogens, wounds, insects, and herbivores or to other biotic stresses such as malnutrition (Graham 1991) and abiotic stresses such as low temperature (Zimmerman and Cohill 1991) by stimulating a multitude of defense mechanism including induction of biosynthesis of secondary metabolites. It is very difficult to retrieve a uniform pattern of secondary metabolites in vivo by classical agriculture practices. In a bioreactor, cultivation of plant cells by in vitro which is an industrial alternative offers a precise supply of secondary metabolites with homogenous quality and yield independent of the external factors (Fowler 1985). Many complications have to be faced for acquiring secondary metabolites from plants that include environmental factors, political and labor inconstancy in the producing countries, unbounded variations in the crop quality, inefficiency of authorities to prohibit crop adulteration, and losses in storage and handling. Cell culture technology is a desirable mean for study and synthesis of plant secondary metabolites. The emerging significance of secondary metabolites has appear to be high level of concern for improving cultivation technology with the prospect of increasing their production (Zhong 2001), and researchers are now aimed in altering the production of secondary metabolites by manipulating plant cell culture. Bacteria and fungi, during the past 40 years, have been used particularly in Japan, Germany, and the USA for the production of a vast range of secondary metabolites, the same way they were used for antibiotic or amino acid production (Mulabagal and Tsay 2004).
- (i)
Polyketides – produced by the acetate-mevalonate pathway
- (ii)
Isoprenoids – produced via mevalonate pathway
- (iii)
Alkaloids – synthesized from various amino acids
- (iv)
Phenylpropanoids – produced from amino acids
- (v)
Flavonoids – produced by a combination of (i) and (iv)
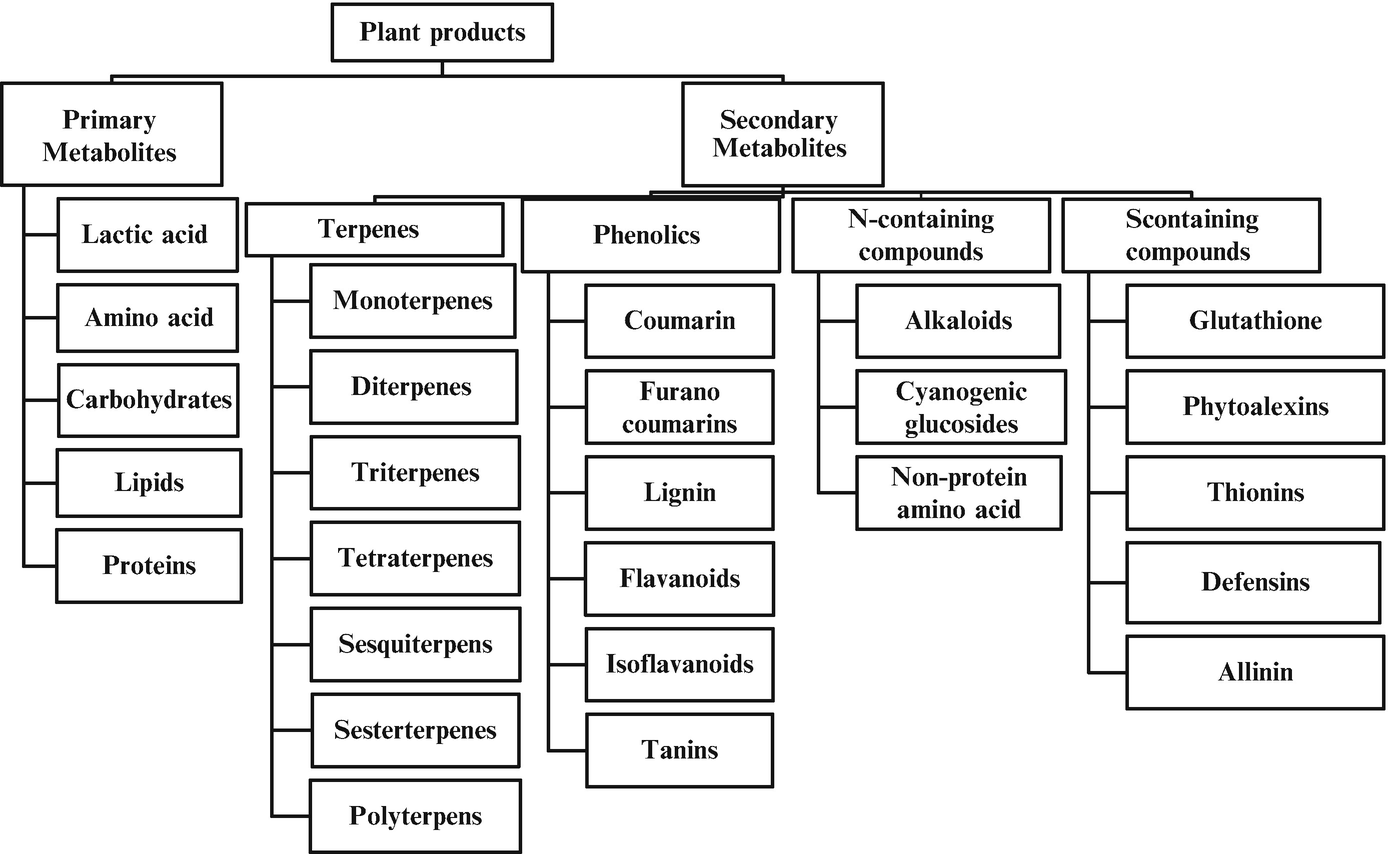
Classification of plant-derived metabolites
Studies on callus and cell culture had been done extensively for the production of secondary plant metabolites by late 1950s. The main prospect of implementing such type of technique is to synthesize secondary metabolites from the by-product of cultured cell or tissue which can be used for commercial purposes like pharmaceuticals and cosmetics, hormones, enzymes, proteins, antigens, food additives, and natural pesticides (Terrier et al. 2007). Plant biotechnology provides an excellent opportunity to manipulate cells, tissues, organs, or whole organisms by culturing them in vitro and then getting the required compounds (Rao and Ravishankar 2002). By using different biotechnological approaches, these biologically active metabolites can be developed from callus cultures, cell suspension cultures, and/or organ cultures. From various studies it was found that secondary metabolites are in great amount in differentiated plant tissue, so to harvest these metabolites for the intention to synthesize medically important compounds, various efforts are incorporated to cultivate the entire plant in in vitro conditions (Biondi et al. 2002). The organ culture has much more benefit over the conventional culture of undifferentiated cells as they are more reliable for secondary metabolite production (Rao and Ravishankar 2002). Under stress, secondary metabolite biosynthesis in plant cells can be persuaded by elicitors or precursors and/or by utilization of both. Precursors are chemical stress factors that are key substrates, intermediate products, or enzymes of secondary metabolite biosynthesis pathways. Despite, if not used at the correct stage and/or right concentration, they may have toxic or inhibitory effects on the plant cells (Gueven and Knorr 2011). Elicitors are biotic or abiotic chemicals such as heavy metals, pesticides, and detergents or physical factors such as cold shock, UV, and high pressure that induce enzymatic activity against stress (Rao and Ravishankar 2002) triggering accumulation of secondary metabolites (Zhang et al. 2002). General elicitors generate secondary metabolism in a variety of different plants, whereas specific elicitors trigger secondary metabolism in a specific plant. The magnitude of elicitation depends on the effective dose which differs depending on the plant species. Escalation of secondary metabolite production is a delicate process that relies on the dosage of environmental stress besides its stage of application during agriculture. Independent of external factors, bioreactors support a controlled supply of secondary metabolites with consistent quality and yield through in vitro plant cells cultivation (Fowler 1985). During the last five decades, secondary metabolite production employing plant cell cultures has been a scientific challenge due to insignificant cell yield, moderate growth, and genetic fluctuation of productive cell lines which makes the process inconsistent. Most of the scientific studies on feasibility of the plant cell cultures have been directed (Memelink et al. 2001; Zhong 2001; Verpoorte and Memelink 2002; Sumner et al. 2003). Thus, the aim of this chapter is to highlight the prospects of plant cell culture to produce secondary metabolites and also provide an overview on the important approaches used for the secondary metabolite production and their improvement strategies.
6.1.1 Biotechnology Engineering Coupled with Biochemistry Led to Better Yield of Secondary Metabolites
The involvement of interdisciplinary approaches like biochemistry and biotechnological techniques had managed to get a notable improvement in secondary metabolite production (Cusido et al. 2014; Dias et al. 2016). One of the best examples where biotechnology in conjugation with biochemistry led to the significant growth in production of secondary metabolites is hairy root culture. In this methodology the plant part is selected to infect with Agrobacterium rhizogenes favoring higher genetic constancy and growth, and therefore bio-active compounds released to the medium can conveniently be separated and purified to get higher yields (Anand 2010). Hence the higher yield of these bio-active compounds can efficiently be used for various applications in food and pharmaceutical industries.
6.1.2 Importance of Secondary Metabolites
The applications of plant secondary metabolites are tremendous. They may be utilized as therapeutic compounds because of their antimicrobial, anti-inflammatory, and anticancer properties. For example, vincristine (an alkaloid obtained from Catharanthus roseus) is an anticancer compound, diosgenin (a saponin obtained from Dioscorea species) is used as contraceptive, and menthol (a monoterpene obtained from oil of peppermint) is used in toothpaste. They may be used for their colors and fragrances in food and cosmetic industries and as pesticides and insecticide.
6.1.2.1 Benefits of Plant Tissue Culture Over Traditional Agricultural Practices
- (i)
Secondary metabolite biosynthesis increased with increase in the diameter of cell aggregates.
- (ii)
High mass transfer resistance caused by large aggregate size induces secondary metabolite biosynthesis due to lack of mass transfer toward the center of the cell aggregates.
- (iii)
Cell aggregate size causes diffusion resistance hindering diffusion of intracellular substrates.
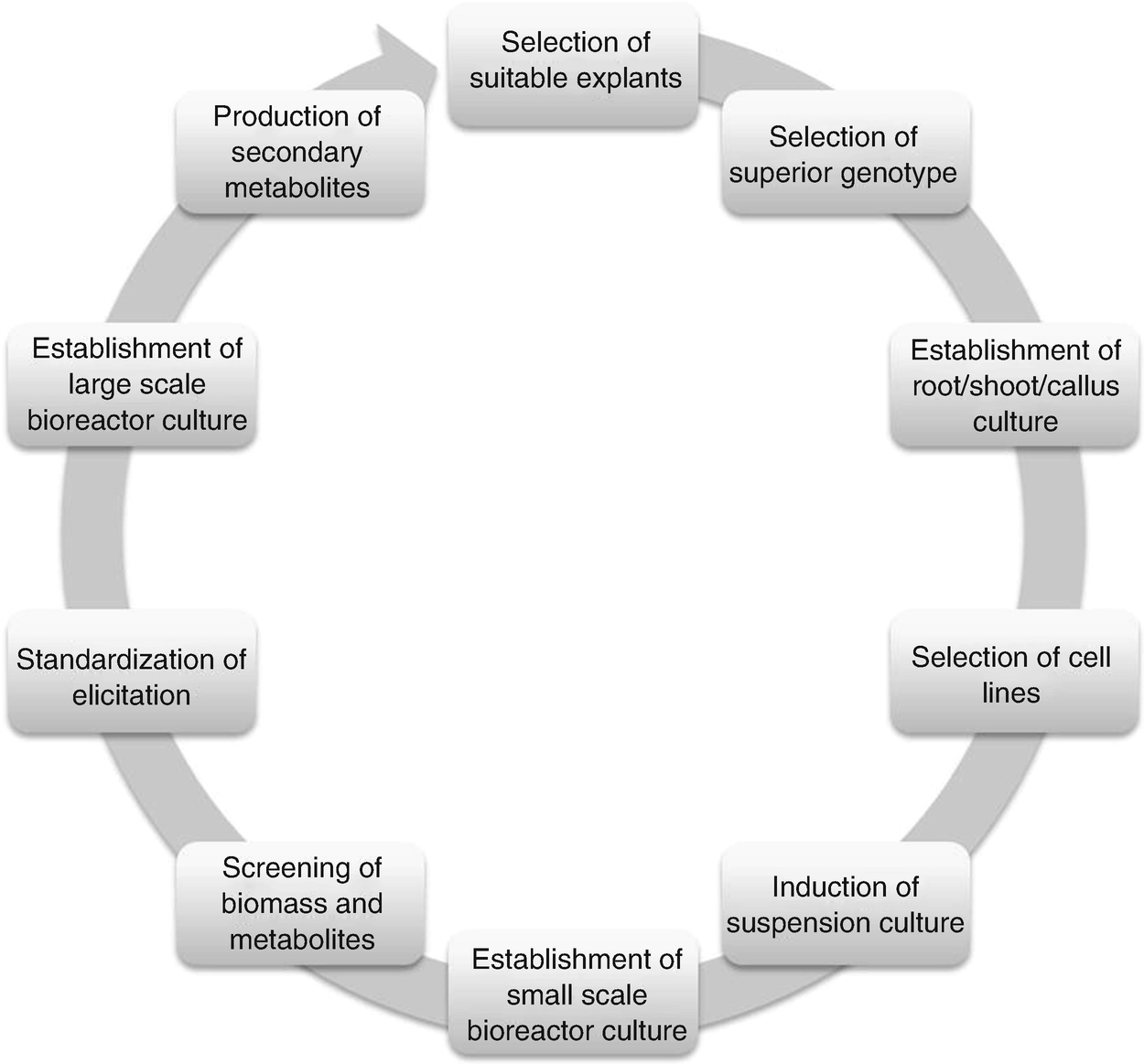
Summary of culture techniques development and production of target secondary metabolites
6.2 Plant Cell Factory-Mediated Secondary Metabolite Production
The yields of secondary metabolites are highly dependent on internal factors like physiological and developmental phase of plants. Different biotechnological methodologies have been experimented and implemented to get improved and enhanced quantity of secondary metabolites from medicinal plants. Plant tissue culture serves as an efficient substitute system to get desired natural products which are not sufficiently present in nature. Secondary metabolites produced via plant cell culture are much more favoured over the conventional agricultural production because: (i) It is independent of geographical and seasonal variations and various environmental factors; (ii) It offers a defined production system, which ensures the continuous supply of products, uniform quality and yield; (iii) It is possible to produce novel compounds that are not normally found in the parent plant (Rao and Ravishankar 2002). Secondary metabolite production from plant system includes screening of high-yielding cell line, media modification, precursor feeding, elicitation, large-scale cultivation in bioreactor system, hairy root culture, plant cell immobilization, biotransformation, and others (Rao and Ravishankar 2002; Vanishree et al. 2004).
6.2.1 Bioreactor-Mediated Secondary Metabolite Production
- (i)
Tissue composition and organization
- (ii)
Flow and mass transfer conditions in the bioreactor
- (iii)
Kinetics of cell growth and product formation
- (iv)
Genetic stability of productive cell lines
- (v)
Control of micro- and macroenvironment in the bioreactor
- (vi)
Implications of bioreactor design on downstream processing
- (vii)
Potential for process scale-up
Bioreactor operation can be batch, fed-batch, or continuous. Batch bioreactors are used to regulate optimum production conditions upon scale-up from small-scale fermentations in a flask. If the cell culture is under the impact of limiting nutrient, fed-batch operation is favored. The usual operation mode after optimization studies is the continuous mode or the chemo state which allows continuous supply of the nutrient medium and removal of the products allowing a steady state operation. If secondary metabolite biosynthesis is growth-related, a single-step bioreactor is sufficient. Elseways, stagewise fermentation is proposed where the first bioreactor is used for culture growth and the second one is used for secondary metabolite biosynthesis (Payne et al. 1993). Intracellular products usually require batch or fed-batch operations, while extracellular products allow continuous production schemes.
6.2.1.1 Application of Bioreactors
- (i)
Mechanical stirring
- (ii)
Airlifting bioreactors
- (iii)
Bubbling bioreactors
- (iv)
Nutrient mist bioreactors
- (v)
Temporary immersion bioreactors
All the bioreactors mentioned above have some features that they share in common which includes properly blended media and sterile air. In the case of shaking flask, shaking makes a continuous contact of media with air, whereas in bioreactors the media is agitated or supported by air bubbles or blended with air accordingly, and after that it is transferred to cultured cells. Consequently, distribution of air becomes much critical for bioreactors. In bioreactors, there are some sensors designed which regulate the change in pH, temperature, dissolved oxygen, and bubbles generated. Though mechanical bioreactors can create topmost dissolved oxygen, due to their susceptibility to shear forces, they are not generally employed for plant and tissue culture. Both airlift and bubbling bioreactor have many features in common, and they are mostly employed for plant and tissue culture. Especially for hairy root culture, bioreactors are equipped with stainless steel mesh for giving hairy roots the required support. For tissue culture, the nutrient mist and temporary immersion are used as they have common characteristics. These bioreactors consist of two components: one is for media storage and another one is for tissue culture. With the help of atomizer, the mixture of media and sterile air is sprayed in the form of very small droplet on the outer of cultured tissue; this is the mechanism for nutrient mist bioreactors. In temporary immersion bioreactors, the media is moved on the tissue culture part and there it is kept for short period of time and then later on it is pumped back to the storage tank. With the employment of these bioreactors, many potential secondary metabolites were isolated from medicinal plant cell cultures which are of great importance to industrial use.
6.2.2 Elicitors and Elicitation
Formerly, elicitor was used to describe the molecules that generate production of phytoalexins, but now it is conventionalized as a compound that improves the defense mechanism of plant (Hahn 1996; Nurnberger 1999). Elicitors can also be explained as component when added in little quality to cell system, incites the synthesis of certain important compounds. So, elicitation can be elucidated as accelerated and upgraded biosynthesis of compounds resulting from addition of elicitors in small quantity (Radman et al. 2003; Angelova et al. 2006). Among the tremendous usage of elicitors, it is also practiced for releasing the metabolites into the medium (Pitta-Alvarez et al. 2000).
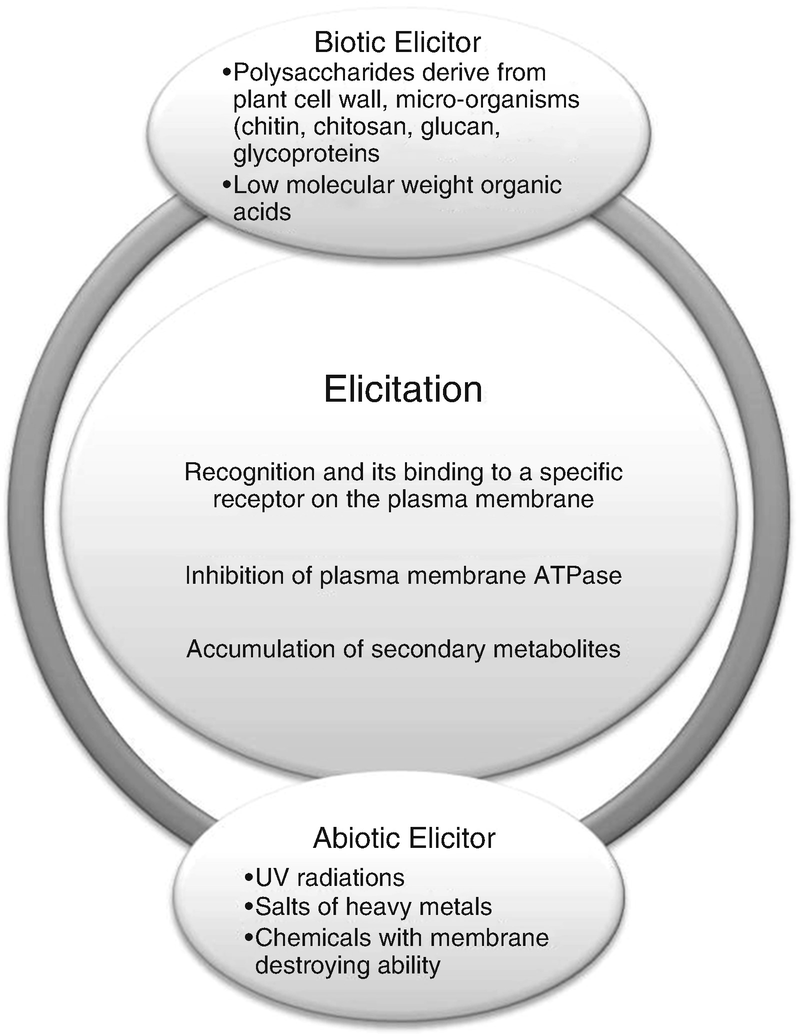
Overview of plant-derived secondary metabolite production via abiotic and biotic elicitors
Recent reports on the use of biotic and abiotic elicitors in plant cell culture to influence the production of plant-derived secondary metabolites
Plant name | Secondary metabolite | Type of culture | Elicitor | Report |
|---|---|---|---|---|
Abrus precatorius | Glycyrrhizin | Cell suspension | Fungi | Karwasara et al. (2010) |
Ajuga bracteosa | Phenols and flavonoids | Root suspension | Methyl jasmonate | Saeed et al. (2017) |
Ajuga bracteosa | Phenols and flavonoid | Shoot | Thidiazuron | Ali et al. (2018) |
Arachis hypogaea | Resveratrol | Hairy root | Sodium acetate | Condori et al. (2010) |
Artemisia absinthium | Phenols and flavonoids | Suspension | Gibberellic acid | Ali et al. (2015) |
Artemisia annua | Artemisinin | Hairy root | Fungi | Wang et al. (2009) |
Artemisia annua | Artemisinin | Cell suspension | Methyl jasmonate | Caretto et al. (2011) |
Astragalus membranaceus | Isoflavonoid | Hairy root | Methyl jasmonate | Gai et al. (2016) |
Bacopa monnieri | Bacoside A | Shoot | Methyl jasmonate | Sharma et al. (2013) |
Cannabis sativa | Tyrosol | Cell suspension | Jasmonic acid | Pec et al. (2010) |
Catharanthus roseus | Ajmalicine | Cambial cells | Cyclodextrin | Zhou et al. (2015) |
Catharanthus roseus | Lochnericine | Hairy root | Light irradiation | Binder et al. (2009) |
Calophyllum inophyllum | Inophyllum | Cell suspension | Fungi | Pawar et al. (2011) |
Centella asiatica | Asiaticoside | Hairy root | Methyl jasmonate | Kim et al. (2004) |
Datura stramonium | Hyoscyamine | Hairy root | Jasmonic acid | Amdoun et al. (2010) |
Eleutherococcus koreanum | Eleutherosides B and E | Adventitious root | Salicylic acid | Lee et al. (2015) |
Eruca sativa | Glucosinolate | Hairy root | Salicylic acid and ephephon | Kastell et al. (2018) |
Glycine max | Isoflavonoid | Cell suspension | Cold shock | Gueven and Knorr (2011) |
Gymnema sylvestre | Gymnemic acid | Cell suspension | Methyl jasmonate | Chodisetti et al. (2015) |
Hypericum perforatum | Hypericin | Cell suspension | Salicylic acid | Gadzovska et al. (2013) |
Hypericum perforatum | Hypericin | Cell suspension | Ozone exposure | Xu et al. (2011) |
Isatis tinctoria | Flavonoid | Hairy root | Aspergillus niger | Jiao et al. (2018) |
Lachenalia spp. | Caffeic and ferulic acid | Shoot | White, blue-red light | Bach et al. (2018) |
Melissa officinalis | Hydroxycinnamic acid | Suspension | Cobalt chloride | Urdova et al. (2015) |
Oldenlandia umbellata | Anthraquinones, alizaril | Adventitious root | Pectin, yeast extract, xylan | Krishnan and Siril (2018) |
Panax ginseng | Ginsenosides | Hairy root | Methyl jasmonate | Corchete and Bru (2013) |
Panax ginseng | Phenols and flavonoid | Root suspension | Salicylic acid | Ali et al. (2007) |
Plumbago indica | Plumbagin | Hairy root | Jasmonate | Gangopadhayay et al. (2011) |
Portulaca oleracea | Dopamine | Hairy root | Salicylic acid | Ahmadi et al. (2013) |
Pueraria candollei | Isoflavonoid and genistein | Hairy root | Agrobacterium and yeast | Udomsuk et al. (2011) |
Pueraria mirifica | Isoflavonoids | Hairy root | Chitosan | Korsangruang et al. (2010) |
Podophyllum hexandrum | Podophyllotoxin | Cell | Methyl jasmonate | Hazra et al. (2017) |
Polygonum multiflorum | Phenolic compound | Adventitious root | Yeast extract and chitosan | Ho et al. (2018) |
Rhodiola imbricata | Phenol and flavonoid | Callus culture | Light | Kapoor et al. (2018) |
Salvia miltiorrhiza | Tashinones | Hairy root | Methyl jasmonate | Hao et al. (2015) |
Salvia sclarea | Aethiopinone | Hairy root | Methyl jasmonate | Kuzma et al. (2009) |
Salvia miltiorrhiza | Tanshinone | Hairy root | Hyperosmotic stress | Shi et al. (2007) |
Salvia miltiorrhiza | Phenolic acid | Cell suspension | Salicylic acid | Dong et al. (2010) |
Satureja khuzistanica | Rosmarinic acid | Cell suspension | Methyl jasmonate | Khojasteh et al. (2016) |
Scutellaria lateriflora | Baicalein and scutellarin | Hairy root | Light and Cyclodextrin | Marsh et al. (2014) |
Stephania venosa | Dicentrine | Cell suspension | Salicylic acid and chitosan | Kitisripanya et al. (2013) |
Silybum marianum | Silymarin | Cell | Cyclodextrin | Almagro et al. (2011) |
Stevia rebaudiana | Phenols and flavonoids | Callus | Light | Ahmad et al. (2016) |
Taxus spp. | Taxane | Cell | Cyclodextrins | Sabater-Jara et al. (2014) |
Taxus baccata | Phenolic content | Cell suspension | Squalestatin | Jalalpour et al. (2014) |
Vitis riparia | Resveratrol | Cell suspension | Cyclodextrin | Zamboni et al. (2006) |
Vitis vinifera | Anthocyanins | Cell suspension | Pectin | Cai et al. (2011a) |
Vitis vinifera | Anthocyanins | Cell suspension | Ethephon | Cai et al. (2011b) |
6.2.2.1 Types of Elicitor
Molecules that trigger protection and stress-generated reaction in plants are collectively termed as elicitors (Radman et al. 2004). Elicitors incorporate both the pathogen-derived compounds and the substance discharged from the plants due to the activity of pathogens. Elicitors can be biotic or abiotic. The biotic elicitors as the name signifies have biological origin obtained either from pathogens or through plants themselves, whereas abiotic elicitors can be physical or chemical component (Kumar and Shekhawat 2009).
Biotic Elicitors
Carbohydrates and proteins come under the category of biotic elicitors. Biotic elicitors include different components of existing organisms like polysaccharide present in plant cell wall, namely, pectin and cellulose, as well as the excerpt of microbes particularly chitin, glucans, and glycoproteins (Nishi 1994; Benhamou 1996; Shirsau et al. 1997. In response to the invasion by pathogens along with the environmental destruction, plant releases antimicrobial compounds, i.e., phytoalexins, which are actually secondary metabolites. Nowadays in cultured cells, biotic elicitors chiefly the fungal elicitors are consider as a dynamic path for escalating secondary metabolites (Siddiqui et al. 2010).
Abiotic Elicitors
As compare to the biotic elicitors, abiotic elicitors have not been able to gain much attraction in plant cell culture (Angelova et al. 2006). Nonbiological in origin, abiotic elicitors include inorganic salts and various environmental factors chiefly UV rays, heavy metal salts like copper and cadmium ions, as well as pH. In recent times, it was concluded that the tropospheric ozone has the ability to trigger biochemical plant responses that are analogous to the compounds released during fungal attack (Zuccarini 2009). As reported by Schmeller and Wink in 1998, Taxus plant is of great importance because of its anticancer properties. Wu et al. (2001) experienced amplification of taxol synthesis when lanthanum was used as an elicitor in Taxus spp. cell culture.
6.3 Important Approaches for Production of Secondary Metabolites
6.3.1 Organ Culture-Mediated Secondary Metabolite Production
Many therapeutic compounds and other important constituents are derived from root cultures (Pence 2011; Li et al. 2002). Essential alkaloids like hyoscyamine and scopolamine and important drugs can easily be obtained by using root culture method without many efforts (Fazilatun et al. 2004). Root cultures have far more importance over the conventional higher plant root system, and it is now being explored on a high note, as root system has very slow growth rate and is much more challenging. The requirement for some secondary metabolites is increasing for commercial purpose; to cope with, plant shoot cultures are employed instead of relying on the natural plant produce (Khanam et al. 2000). Different kinds of bioreactors are employed for root and shoot cultures (Kasparova et al. 2009; Kim et al. 2002).
6.3.2 Callus Culture-Mediated Secondary Metabolite Production
Callus is an unspecialized, unorganized, growing, and dividing mass of cells. It is produced when explants are cultured in vitro on an appropriate medium, with concentration of both auxin and cytokinin in accurate ratio. Callus cultures are generally categorized into two types: embryogenic or non-embryogenic. In embryogenic type of callus culture, a single cell or a small group of competent cells follow a developmental pathway that leads to reproducible regeneration of non-zygotic embryos which are capable of producing a complete plant (Ptak et al. 2013). The major application of somatic embryogenesis include clonal propagation of genetically uniform plant material, elimination of viruses, provision of source tissue for genetic transformation, generation of whole plants from single cells called protoplasts, and development of synthetic seed technology. However in non-embryonic callus culture contains more or less similar cluster of dedifferentiated cells are taken for synthesis of secondary metabolite. Maackia amurensis has been investigated for secondary metabolites by employing callus culture (Fedoreyev et al. 2004). Biosynthetic totipotency of plant cell is the major objective behind the concept of production of secondary metabolites using cell suspension culture; hence, the genetic composition of each cell in the culture remains the same, and thus a wide range of bio-active compounds can be extracted which are available in entire plant.
6.3.3 Hairy Root Culture-Mediated Secondary Metabolite Production
In a phytohormone-deficient medium, hairy roots grow hastily with immense branching with oblique or horizontal growth (Hu and Du 2006). Hairy roots obtained from Agrobacterium rhizogenes have huge application in various commercial areas. Hairy roots have the benefit over others of not failing the genetic and biosynthetic stability; they produce secondary metabolites over subsequent generations (Giri and Narasu 2000). Hairy root cultures have been investigated abundantly in root nodule research. With the help of transformed root cultures, many possibilities of secondary metabolite biosynthesis have been examined (Kuzovkina and Schneider 2006). The substantial interrelationship between secondary metabolite production and morphological differentiation gives more momentum to utilization of cell culture technique for the production of phytochemicals on a commercial scale.
Synthesis of two different bio-active compounds synchronously is achievable through adventitious root co-cultures (Wu et al. 2008). The promising results obtained by implementing hairy root culture, now bioreactors, are incorporated to achieve much more bio-active compounds (Mehrotra et al. 2008). To obtain various valuable alkaloids and alkannins, hairy root cultures of plants, namely, Lithospermum erythrorhizon, Harpagophytum procumbens (Ludwig-Muller et al. 2008), and adventitious roots of Panax ginseng (Jeong et al. 2008) and Scopolia parviflora (Min et al. 2007) were examined in different volumes of bubble column bioreactors. Ginsenoside, which is a class of natural product steroid, glycosides, and triterpene saponins can also be synthesized by employing adventitious root culture in combination with stirred tank bioreactors (Jeong et al. 2008). To cope with the increasing demands, improved and modified bioreactors are employed having stainless steel tank plant cell growth in addition to the vessels that were also armed with specialized hangers. Among all mentioned cultures, hairy root culture has gained tremendous popularity due to its distinctive capability to achieve secondary metabolite production on a large scale.
For secondary metabolites that are released as a result of defense responses, their primary role is to protect plants, but because of its therapeutic properties, researchers have focused their attention toward it. Due to seasonal and environmental instabilities along with little knowledge about the biosynthesis and signal transduction pathway of these secondary metabolites, it becomes very challenging for pharmaceutical industries to obtain these bio-active compounds. Plant cell culture provides an excellent medium for sustainable, easily expandable production of secondary metabolites to restrict the hurdles. To boost up the yield, noticeable approaches like manipulating the supplements and bettering the culture environment and elicitation are taken into consideration (Kumar and Sopory 2008).
- (i)
It is independent to different environmental factors like soil and climatic condition.
- (ii)
Antagonistic biological impacts that disturb secondary metabolite production in the nature are excluded like microorganisms and insects.
- (iii)
Selection of suitable cultivars with the intention of achieving greater supply of secondary metabolites is possible.
- (iv)
It is cost effective.
Plant-derived secondary metabolites isolated from plant via different cell culture types
Plant name | Secondary metabolite | Type of culture | Report |
|---|---|---|---|
Adhatoda vasica | Vasine | Shoot culture | Shalaka and Sandhya (2009) |
Agastache rugosa | Rosmarinic acid | Hairy root | Lee et al. (2007) |
Aloe vera | Aloe emodin and chrysophanol | Adventitious root | Lee et al. (2013) |
Ammi majus | Umbelliferone | Shootlet | Krolicka et al. (2006) |
Andrographis paniculata | Andrographolide | Adventitious root | Parveen et al. (2009) |
Arachis hypogaea | Resveratrol | Hairy root | Condori et al. (2010) |
Artemisia | Artemisinin | Hairy root | Ikram and Simonsen (2017) |
Artemisia annua | Drimartol A | Hairy root | Abbott et al. (2010) |
Artemisia annua | Artemisinin | Callus | Baldi and Dixit (2008) |
Astragalus membranaceus | Saponins and isoflavonoids | Adventitious root | Wu et al. (2011) |
Brucea javanica | Cathin | Suspension | Wagiah et al. (2008) |
Brugmansia candida | Anisodamine | Hairy root | Cardillo et al. (2010) |
Bupleurum chinense | Saikosaponin | Adventitious root | Hao and Guan (2012) |
Bupleurum chinense | Saikosaponin | Adventitious root | Kusakari et al. (2012) |
Castilleja tenuiflora | Phenylethanoid glycosides | Adventitious root | Gomez-Aguirre et al. (2012) |
Catharanthus roseus | Catharanthine | Hairy root | Wang et al. (2010) |
Catharanthus roseus | Alkaloids | Hairy root | Li et al. (2011) |
Cayratia trifoliata | Stilbenes | Suspension | Roat and Ramawat (2009) |
Centella asiatica | Asiaticoside | Adventitious root | Mercy et al. (2012) |
Coleus blumei | Rosmarinic acid | Hairy root | Bauer et al. (2009) |
Crataegus sinaica | Flavonoid | Callus | Maharik et al. (2009) |
Datura stramonium | Hyoscyamine | Hairy root | Amdoun et al. (2010) |
Echinacea angustifolia | Caffeic acid derivatives | Adventitious root | Cui et al. (2013) |
Echinacea angustifolia | Caffeic acid derivatives | Adventitious root | Murthy et al. (2014c) |
Eleutherococcus senticosus | Eleutherosides | Suspension | Shohael et al. (2007) |
Eleutherococcus korean | Eleutherosides | Adventitious root | Lee and Paek (2012) |
Fagopyrum esculentum | Rutin | Hairy root | Lee et al. (2007) |
Gentiana macrophylla | Gentiopicroside | Hairy root | Zhang et al. (2010) |
Gentiana macrophylla | Glucoside | Hairy root | Tiwari et al. (2007) |
Gentianella austriaca | Xanthone | Multiple shoot | Vinterhalter et al. (2008) |
Glycyrrhiza glabra | Glycyrrhizin | Hairy root | Mehrotra et al. (2008) |
Glycyrrhiza uralensis | Flavonoid | Hairy root | Zhang et al. (2009) |
Glycyrrhiza uralensis | Glycyrrhizic acid | Adventitious root | Yin et al. (2014) |
Gossypium hirsutum | Gossypol | Hairy root | Verma et al. (2009) |
Gynochthodes umbellata | Anthraquinone | Callus | Anjusha and Gangaprasad (2017) |
Gynura procumbens | Phenylpropanoids | Adventitious root | Saiman et al. (2012) |
Hypericum perforatum | Phenolics, flavonoids, chlorogenic acid, and sphingoid base-1-phosphate | Adventitious root | Wu et al. (2014) |
Hypericum perforatum | Hypericin | Suspension | Hohtola et al. (2005) |
Hypericum perforatum | Hypericins | Multiple shoot | Kornfeld et al. (2007) |
Globularia trichosantha | Catalpol, aucubin, and verbascoside | Callus | Colgecen et al. (2018) |
Mentha × piperita | Menthol, pulegone | Shoot | Fejer et al. (2018) |
Momordica charantia | Flavonoid | Callus | Agarwal and Kamal (2007) |
Momordica dioica | Flavonols, hydroxycinnamic acid | Hairy root | Thiruvengadam et al. (2016) |
Morinda citrifolia | Anthraquinones | Adventitious root | Baque et al. (2012) |
Myristica fragrans | Myristin | Shoot | Indira et al. (2009) |
Ophiorrhiza rugosa | Camptothecin | Shoot | Vineesh et al. (2007) |
Panax quinquefolium | Ginsenoside | Hairy root | Mathur et al. (2010) |
Periploca sepium | Periplocin | Adventitious root | Zhang et al. (2011) |
Piper solmsianum | Piperine | Suspension | Balbuena et al. (2009) |
Pluchea lanceolata | Quercetin | Callus | Arya et al. (2008) |
Plumbago indica | Plumbagin | Hairy root | Gangopadhayay et al. (2011) |
Polygonum multiflorum | Anthraquinones, hydroxybenzoic acids, hydroxycinnamic acids, and flavonols | Hairy root | Thiruvengadam et al. (2014) |
Polygonum multiflorum | Anthraquinones, stilbenes, flavonoids, tannins, and phospholipids | Root culture | Thanh-Tam et al. (2017) |
Primula veris | Saponins | Shoot | Okrslar et al. (2007) |
Psoralea corylifolia | Daidzein | Hairy root | Shinde et al. (2010) |
Psoralea corylifolia | Isoflavones | Multiple shoot | Shinde et al. (2009) |
Rauvolfia serpentina | Reserpine | Callus | Nurchgani et al. (2008) |
Rauvolfia tetraphylla | Reserpine | Callus | Anitha and Kumari (2006) |
Rubia akane | Anthraquinone | Hairy root | Park and Lee (2009) |
Salvia miltiorrhiza | Tanshinone | Hairy root | Yan et al. (2011) |
Salvia officinalis | Flavonoid | Multiple shoot | Grzegorczyk and Wysokinska (2008) |
Salvia sclarea | Diterpenoid | Hairy root | Kuzma et al. (2009) |
Salvia viridis | Rosmarinic acid and caffeic acid | Hairy root | Grzegorczyk-Karolak et al. (2018) |
Silybum marianum | Silymarin | Hairy root | Rahnama et al. (2008) |
Spirotropis longifolia | Spirotropin A, spirotropin B, and spirotropaone | Adventitious root | Basset et al. (2012) |
Stevia rebaudiana | Steviol-glycosides | Adventitious root | Reis et al. (2011) |
Taxus × media | Paclitaxel | Hairy root | Syklowska-Baranek et al. (2009) |
Tinospora cordifolia | Berberine | Suspension | Ramarao et al. (2008) |
Tripterygium wilfordii | Triptolide, alkaloids | Adventitious root | Miao et al. (2014) |
Vitis vinifera | Resveratrol | Callus | Kin and Kunter (2009) |
Withania somnifera | Withanolides | Adventitious root | Murthy and Praveen (2013) |
Withania somnifera | Withanolide A | Hairy root | Murthy et al. (2008) |
Withania somnifera | Steroidal lactone | Callus | Mirjalili et al. (2009) |
Zataria multiflora | Rosmarinic acid | Callus | Francoise et al. (2007) |
6.4 Secondary Metabolites and Its Assimilation in Plant Cell Cultures
In order to obtain high-quality uniform product from cell culture, it is important to develop techniques that are economically feasible (Berlin and Sasse 1985). Collection of increased amount of several products in cultured cells is obtained by precise selection of productive cells and cultural conditions. For achieving higher yield of secondary metabolites for commercial demands, several strategies and efforts have been aimed for accelerating the biosynthetic activity of cultured cells (Dixon 1999; Buitelaar and Tramper 1992). Various methods are now being used to escalate the production of secondary metabolites through plant cell culture including manipulation of nutrient media and elicitation.
6.5 Yield Improvement Strategies
6.5.1 Preliminary Considerations
For the production of secondary metabolites employing plant tissue culture, information of the variety, cultivar, and species of the desired plant along with the complete profile of the bio-active compound present in them must be known (Ananga et al. 2013). Firouzi et al. (2013) destine the consequences of utilizing four ecotypes of Silybum marianum on growth method and flavonolignan production in cell culture. Particular ecotypes showed critical variation in the considered parameters. Hence selection of apt explant is an important and essential step for initiating callus culture. Usually, a good and viable explant should be small, healthy, and taken from middle part of the plant and should contain meristematic tissues.
6.5.2 Screening Cell Lines
A complete strategy for production of secondary metabolites from the desired plant cell culture must be planned before moving further. Various factors about the selection of cell line must be taken into consideration which include growth rate, culture stability, and tolerance of the culture (Shuler 1999). The term clonal selection is used for production of a population of cells having the same trait. For economic point of view, growth rate of the culture plays a crucial role. Genetic and epigenetic factors are the reason behind the fluctuation in the culture. Epigenetic factors are resulted from change in the environment and do not conclude in permanent change in cell genome.
6.5.3 Alteration of the Components of the Culture Medium
Plant tissue culture media include some or all of the following components: macronutrients, micronutrients, vitamins, amino acids, carbon source, growth regulators, solidifying agent, and undefined organic supplements (Saad and Elshahed 2012). The most frequently used media are Murashige and Skoog (MS) medium (Murashige and Skoog 1962), Linsmaier and Skoog medium (Linsmaier and Skoog 1965), Gamborg medium (Gamborg et al. 1968), and Nitsch and Nitsch medium (Nitsch and Nitsch 1969). For providing optimum growth to the desired culture and deriving required amount of secondary metabolites, some of the media are usually altered.
6.6 Conclusion and Future Prospects
Due to high concern for low yield and productivity of useful plants, with increase demand of food and health benefit products, plant tissue culture techniques are well accomplished. It was observed that plant tissue culture predicts more efficient and reliable source for most of secondary metabolite production, but on the other hand, there are only some cell cultures that can produce stable and efficient source of secondary metabolites. There were some achievements in the formulation of important secondary metabolites because of upgrading culture technique, choice of cell line, and model of bioreactor with passing time. There is no ambiguity that the in vitro culture of secondary metabolites from plant cell culture is an interesting technology for obtaining useful product. Plant tissue culture technique is an important approach for the production of those plant species which were at risk though having potential secondary metabolites which can be commercially applied for the preparation of valuable food and medicines in future.
It is the starting point for the production of valuable secondary metabolites from both plant and cell culture; therefore, there is a need to develop more research for large-scale production of compounds for economic and other purposes. Incorporation of molecular biology is the most efficient tool for handling and expression of secondary metabolite production on a large scale. There are some other studies that predict that developing the research in the area of plant tissue culture day by day results in large production of secondary metabolites. Many other examples could be presented with plant cell culture technique as this research area is developing actively to increase the production. A significant shift in the appeal of the cell culture technologies will likely come from a better understanding of the biological mechanisms that operate biosynthetic pathways and the application of this knowledge to engineering economically competitive high-value product yield.